Abstract
Palinacousis is an auditory illusion consisting of perseveration or echoing of an external auditory stimulus after it has ceased. This rare clinical symptom has been reported in ictal (seizure), postictal, and nonictal states, and causative lesions have been most consistently found in or near the temporal lobes. It is distinct from the auditory hallucinations seen in psychiatric illness. We report the case of a 61-year-old man who experienced several days of palinacousis while undergoing treatment for newly diagnosed metastatic lung adenocarcinoma. Palinacousis was presumed to be triggered by intracranial metastases near the auditory cortex. An electroencephalogram showed bilateral theta slowing over the left greater than right temporal lobes without epileptiform activity. Palinacousis remitted with corticosteroid and whole brain radiation therapy.
Keywords: neuroanatomy, techniques, brain neoplasms, nervous system neoplasms, neuroradiology, clinical specialty, neurooncology, clinical specialty
Introduction
Palinacousis was first described in 1971 as a paroxysmal auditory phenomenon, where an individual perceives a sound to be persisting or echoing after cessation of the auditory stimulus.1 A PubMed search with the keyword “palinacousis” reveals that since the initial description of this phenomenon, there have been only 15 reported cases comprised predominantly of single case reports. This literature provides a spectrum of features and descriptions of the auditory phenomenon itself, although there is resolute consistency in the features that distinguish palinacousis from other types of auditory hallucinations. For instance, palinacousis is triggered by an easily identifiable external auditory stimulus, whereas psychiatric hallucinations often involve thought echoing where there is no actual sensory trigger. The content of hallucinations in palinacousis is characteristically neutral which is opposed to the often disturbing quality of the hallucinations and behaviors associated with psychotic disorders.
Palinacousis has a visual analog, palinopsia, which was described in 19512 and whose precise mechanism remains disputed. This phenomenon is thought to result from abnormal visual processing leading to perception of persisting or recurring visual images after a visual stimulus has been removed. Most causative lesions are in the temporo-occipital area. Palinopsia has been observed both during a seizure and spontaneously; in the latter case, it is believed to represent loss of suppression of normal visual input.3 Like palinacousis, there is a clear distinction between sensory illusion and hallucination, and there is no correlation with underlying psychiatric illness.
Although palinacousis is uniformly described as a symptom of a structural brain lesion, there is wide variability among the types of lesions that may be associated with the phenomenon (ie, tumors, vascular malformations, and intracerebral hemorrhages) as well as variability in the proposed causative mechanisms. There is a strong suspicion based on several case reports that palinacousis is a peri-ictal phenomenon. In most instances, the causative lesion resides in the auditory cortex of the dominant temporal lobe, temporoparietal junction, or temporo-occipital junction; however, there are exceptions to this in the literature. We report a case of a 61-year-old man who experienced several days of palinacousis while undergoing treatment for multiple intracranial metastases, including large metastases near both auditory cortices.
Case Description
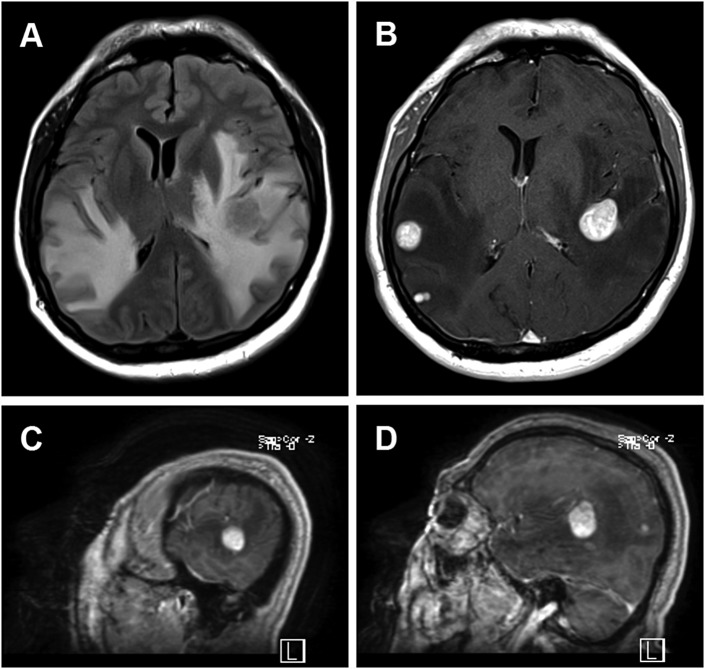
A 61-year-old ambidextrous man with history of tobacco use presented with 5 months of progressively worsening frontal and left temporal headaches, bilateral tinnitus, gait instability, confusion and impulsivity, receptive dysphasia, and an episode of hemoptysis. He was diagnosed with right upper lobe lung adenocarcinoma as well as multiple intracerebral metastases. The 2 largest metastases were located in the left peri-insular temporoparietal lobe and the right posterior superior temporal lobe (Figure 1).
Figure 1.
Brain MRI of patient with multiple intracerebral metastases from lung adenocarcinoma. A, Axial T2-FLAIR MRI shows extensive abnormal hyperintensity within the bilateral temporoparietal subcortical white matter and left internal and external capsules, with effacement of overlying cortical sulci and underlying lateral ventricles as well as rightward shift of midline structures. B, Axial T1-postgadolinium MRI shows multiple homogenously avidly enhancing round metastases. Sagittal T1-postgadolinium MRI shows the proximity of the large right-sided (C) and left-sided (D) metastases to the superior temporal gyri. FLAIR indicates fluid attenuated inversion recovery; MRI, magnetic resonance imaging.
On examination, he was afebrile, alert, and oriented, with fair memory for recent events. His behavior was mildly impulsive and disinhibited. Speech was fluent without dysarthria though tangential and perseverative with mildly impaired comprehension. He made frequent paraphasic errors. He was able to repeat a short phrase and named most common objects accurately. Visual fields were full to confrontational testing, pupils were reactive, extraocular movements were conjugate, and facial movements were symmetric. Extremity power and sensation were normal, he could walk without imbalance, and bilateral extensor plantar responses were noted.
In addition to chronic tinnitus, the patient reported a new auditory problem that he first noticed after hearing a name spoken on television. The name “rattled around” in his head, repeating itself many times and for many minutes. The episode consisted of spontaneous repetitions of the original sound. The patient was uncertain whether the sound appeared to come from the left or right or whether the repetitions followed a rhythm. The patient’s baseline tinnitus did not change during the episode nor did his level of alertness or ability to speak or comprehend language. The patient found the episode bothersome and distracting and noted that he could relieve the symptom by walking or initiating some other distracting activity. He could not make the repetitions stop by manually occluding his external auditory meatus.
Over the next few days, he experienced additional episodes that began after hearing other names, words, and sounds. At its worst, there were several episodes of palinacousis per day. There were never any spontaneous or persecutory auditory hallucinations; the symptom always followed a natural external sound stimulus. He had never experienced auditory illusions or hallucinations in the past and had no history of psychiatric illness.
An electroencephalogram (EEG) revealed occasional bilateral theta slowing most pronounced over the left temporal region, without epileptiform abnormalities. The patient did not experience symptoms of palinacousis during the EEG recording. The patient had occasional episodes of right arm tingling which were not coincident with the episodes of palinacousis. Given concern for focal sensory seizures, lacosamide 100 mg twice a day was initiated. A trial of quetiapine 25 mg did not alleviate the auditory symptoms.
Because of the extensive and symptomatic vasogenic edema surrounding the brain metastases, intravenous dexamethasone 4 mg every 6 hours was started at the time the patient was admitted. Palliative whole brain radiation was initiated on hospital day 4, with a planned total dose of 3000 cGy over 10 treatments. By hospital day 8, palinacousis and episodes of right arm tingling had completely resolved and dysphasia had mildly improved.
Discussion
The semiology of this patient’s auditory illusions is concordant with previous descriptions of palinacousis. As in previous reports, the perseverated sound was often a human voice speaking individual words, short phrases, or fragments of words that had been true external stimuli as opposed to thought echoing in schizophrenia.4 There was no variation in the quality of the sound over many repetitions. According to the literature, episodes can last from several seconds to several hours, and this was true for our patient as well, with most episodes beginning immediately or soon after the stimulus.1 As in previous cases, the symptoms either terminated spontaneously or were extinguished by another stimulus.
Although there has been suggestion that people localize the perceived auditory sensation to the ear contralateral to the lesion, this has not been a consistent finding in the literature and our patient did not experience any lateralization of the sound. The content of the sound is reported to be invariably neutral. A variety of speech and language deficits have been described in association with palinacousis, and these include receptive or expressive dysphasia, paraphasic errors, neologisms, and perseveration of speech. These language disturbances have been attributed to seizure activity in some patients and to focal lesional effects in others. Many of these features, particularly the paraphasic errors, were prominent early in our patient’s hospital course when his auditory disturbances were most severe. In reviewing previous cases in the literature, this is not a consistent finding. Mohamed et al describe a patient with a left inferior parietal metastasis who developed palinacousis once her dysphasia resolved,5 while Di Dio et al describe a patient with a left parietal hemorrhage whose palinacousis began at the onset of her aphasia.6
Palinacousis is a disorder of cortical auditory processing, and the pathologies associated with the phenomenon are varied. Of the 15 previously reported cases of palinacousis, 7 patients were found to have left temporal or parietal lesions, and 9 were found to have left temporal or parietal focal abnormalities on EEG. Palinacousis in the present case was most likely triggered by either one or both of the large posterior temporal lobe metastases, although the EEG in our patient was more abnormal on the left. The episodes resolved with initiation of steroids and radiation therapy which resulted in considerable decrease in the size and conspicuity of the metastatic lesions and perilesional edema as seen on follow-up imaging 3 months later. Presumably this improvement related to the decreased irritation of the temporal cortex. Any effect of the antiepileptic medication is less certain, as it was given at the same time as the steroids and radiation. Repeat EEG was not performed, although the patient was ultimately taken off the antiepileptic medication in follow-up and his auditory symptoms did not recur.
Whether palinacousis results from ictal discharges is unsettled.6 Favoring this hypothesis is the concept that auditory inputs can be abnormally retrieved from memory due to electrical excitation,1,7–9 and that in some cases palinacousis has remitted with antiepileptic medications.10,11 There are also early reports of palinacousis being followed directly by a grand mal seizure.9 However, there are no case reports that capture electrographic seizures correlating with clinical events. In the case described by Mohamed et al,5 a patient with a left parietal tumor underwent positron emission tomography during an episode of auditory perseveration. The scan showed hypometabolism outside of the hypermetabolic region of the tumor itself, which fails to support an ictal phenomenon as an explanation for the symptoms.
Conversely, a postictal mechanism involving a loss of normal suppression of auditory perseveration has been proposed.5 In our case, the EEG showed left temporal slowing but no epileptiform activity, although symptoms of palinacousis did not occur during recording. We believe the patient had focal sensory seizures involving the right arm; these seizures did not occur prior to, or in conjunction with, palinacousis.
It remains unclear whether palinacousis is a negative release phenomenon resulting from loss of function of a region of auditory cortex that normally suppresses auditory perseveration, or whether it is related to transient hyperexcitation of supplementary auditory pathways. Rare reports of perseverative speech associated with palinacousis have raised the question of whether the symptoms relate to an inability to suppress previous thoughts, although the possibility that this too is related to hyperexcitability of certain speech pathways is unexplored. It has also been suggested that palinacousis may be seen with lesions that do not severely damage the primary auditory or speech cortices.12 Whether auditory cortex involvement is a necessary feature or not, patients with temporal lobe lesions with fixed language deficits may not be able to express the symptoms of palinacousis; and thus, the phenomenon may be underreported.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Jacobs L, Feldman M, Bender MB. Palinacousis or persistent auditory sensations. Trans Am Neurol Assoc. 1971;96:123–126 [PubMed] [Google Scholar]
- 2. Critchley M. Types of visual perseveration: “paliopsia” and “illusory visual spread”. Brain. 1951;74(3):267–299 [DOI] [PubMed] [Google Scholar]
- 3. Cummings JL, Syndulko K, Goldberg Z, Treiman DM. Palinopsia reconsidered. Neurology. 1982;32(4):444–447 [DOI] [PubMed] [Google Scholar]
- 4. Prueter C, Waberski TD, Norra C, Podoll K. Palinacousis leading to the diagnosis of temporal lobe seizures in a patient with schizophrenia. Seizure. 2002;11(3):198–200 [DOI] [PubMed] [Google Scholar]
- 5. Mohamed W, Ahuja N, Shah A. Palinacousis—evidence to suggest a post-ictal phenomenon. J Neurol Sci. 2012;317(1-2):6–12 [DOI] [PubMed] [Google Scholar]
- 6. Di Dio AS, Fields MC, Rowan AJ. Palinacousis—auditory perseveration: two cases and a review of the literature. Epilepsia. 2007;48(9):1801–1806 [DOI] [PubMed] [Google Scholar]
- 7. Penfield W, Perot P. The brain’s record of auditory and visual experience. Brain. 1963;86:595–696 [DOI] [PubMed] [Google Scholar]
- 8. Bender MB, Diamond SP. An analysis of auditory perceptual defects with observations on the localization of dysfunction. Brain. 1965;88(4):675–686 [Google Scholar]
- 9. Jacobs L, Feldman M, Diamond SP, Bender MB. Palinacousis: persistent or recurring auditory sensations. Cortex. 1973;9(3):275–287 [DOI] [PubMed] [Google Scholar]
- 10. Malone GL, Leiman HI. Differential diagnosis of palinacousis in a psychiatric patient. Am J Psychiatry. 1983;140(8):1067–1068 [DOI] [PubMed] [Google Scholar]
- 11. Patterson MC, Tomlinson FH, Stuart GG. Palinacousis: a case report. Neurosurgery. 1988;22(6):1088–1090 [DOI] [PubMed] [Google Scholar]
- 12. Kim JS, Kwon M, Jung JM. Palinacousis in temporal lobe intracerebral hemorrhage. Neurology. 2007;68(16):1321. [DOI] [PubMed] [Google Scholar]