Abstract
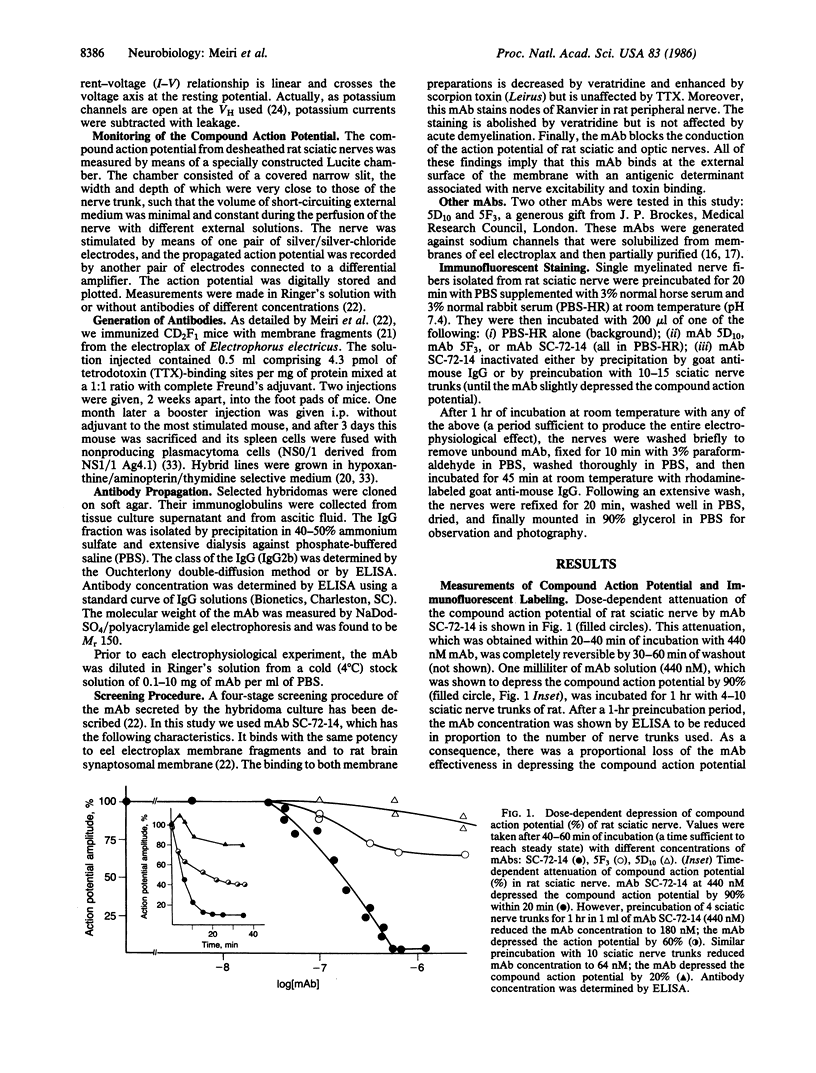
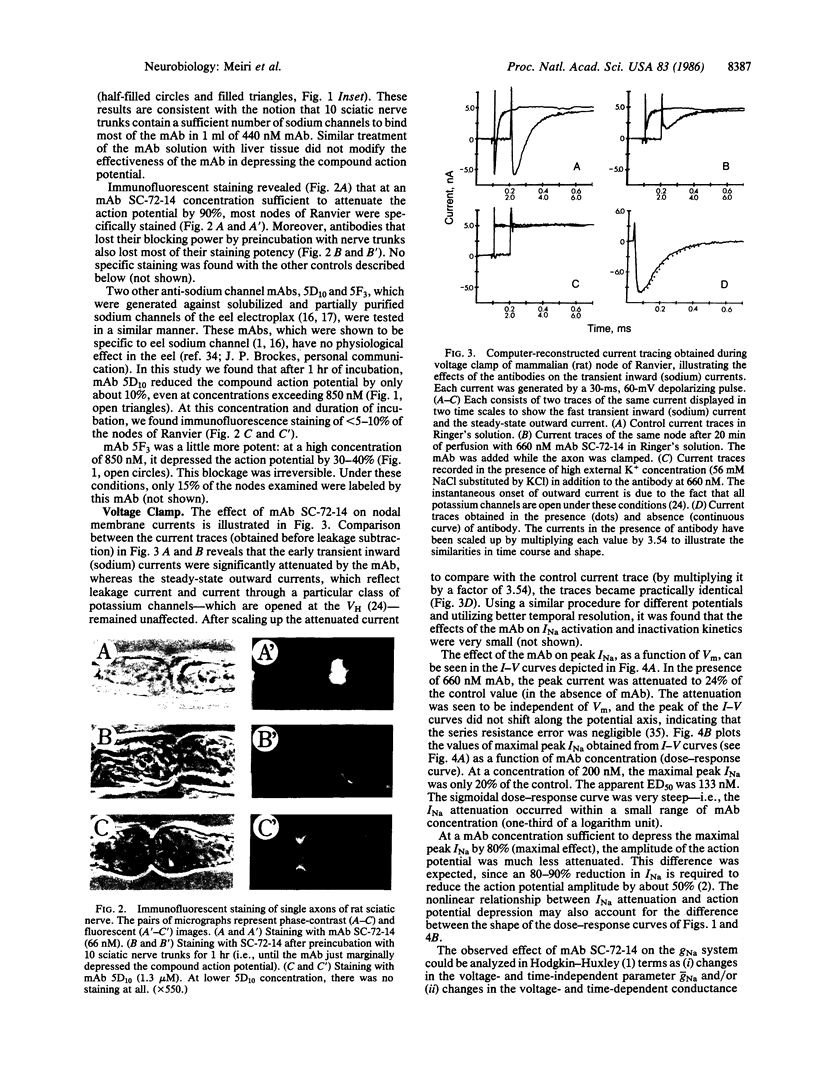
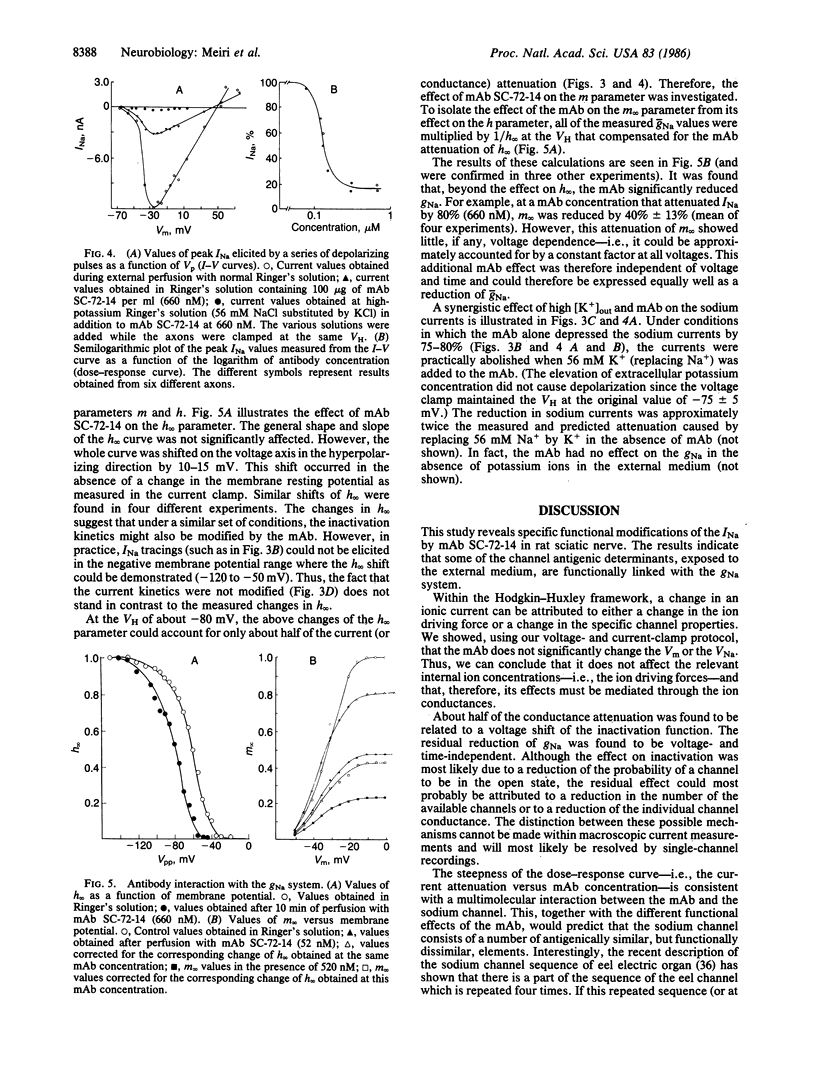
Monoclonal antibodies (mAbs) were generated against the sodium channels in the intact membrane of the eel electroplax. These antibodies bind to nodes of Ranvier, as indicated by immunofluorescence. When externally applied to rat nerve fibers one of these mAbs blocks impulse conduction. In voltage-clamp experiments, this mAb was found to attenuate sodium current amplitude without affecting the time course. The dose-response curve was very steep and had an ED50 of 133 nM. About half of the mAb effect was shown to be due to a shift, in the hyperpolarizing direction, of the steady-state sodium inactivation versus membrane potential curve. The remaining effect was voltage- and time-independent. This mAb had no effect on the potassium or leakage currents. The results indicate that on the external surface of the sodium channel, there are a number of antigenically similar determinants, which are functionally linked to specific elements of the sodium conductance system. These functionally related determinants were preserved through the course of evolution.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973 Apr 13;242(5398):459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Barhanin J., Meiri H., Romey G., Pauron D., Lazdunski M. A monoclonal immunotoxin acting on the Na+ channel, with properties similar to those of a scorpion toxin. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1842–1846. doi: 10.1073/pnas.82.6.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binah O., Palti Y. Potassium channels in the nodal membrane of rat myelinated fibres. Nature. 1981 Apr 16;290(5807):598–600. doi: 10.1038/290598a0. [DOI] [PubMed] [Google Scholar]
- Brismar T. Potential clamp analysis of membrane currents in rat myelinated nerve fibres. J Physiol. 1980 Jan;298:171–184. doi: 10.1113/jphysiol.1980.sp013074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadei J. M., Gordon R. D., Lampson L. A., Schotland D. L., Barchi R. L. Monoclonal antibodies against the voltage-sensitive Na+ channel from mammalian skeletal muscle. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6227–6231. doi: 10.1073/pnas.81.19.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. The molecular basis of neuronal excitability. Science. 1984 Feb 17;223(4637):653–661. doi: 10.1126/science.6320365. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y. Asymmetry currents in the mammalian myelinated nerve. J Physiol. 1980 Dec;309:499–519. doi: 10.1113/jphysiol.1980.sp013523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Hille B., Neumcke B., Nonner W., Stämpfli R. Conductance of the sodium channel in myelinated nerve fibres with modified sodium inactivation. J Physiol. 1976 Nov;262(3):729–742. doi: 10.1113/jphysiol.1976.sp011617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Segal D. M. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1975;44:639–667. doi: 10.1146/annurev.bi.44.070175.003231. [DOI] [PubMed] [Google Scholar]
- Ellisman M. H., Agnew W. S., Miller J. A., Levinson S. R. Electron microscopic visualization of the tetrodotoxin-binding protein from Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4461–4465. doi: 10.1073/pnas.79.14.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H., Levinson S. R. Immunocytochemical localization of sodium channel distributions in the excitable membranes of Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6707–6711. doi: 10.1073/pnas.79.21.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H., Miller J. A., Agnew W. S. Molecular morphology of the tetrodotoxin-binding sodium channel protein from Electrophorus electricus in solubilized and reconstituted preparations. J Cell Biol. 1983 Dec;97(6):1834–1840. doi: 10.1083/jcb.97.6.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz L. C., Brockes J. P. Immunochemical properties and cytochemical localization of the voltage-sensitive sodium channel from the electroplax of the eel (Electrophorus electricus). J Neurosci. 1983 Nov;3(11):2300–2309. doi: 10.1523/JNEUROSCI.03-11-02300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Grünhagen H. H., Dahl G., Reiter P. Tetrodotoxin receptors in membrane fragments: purification from Electrophorus electricus electroplax and binding properties. Biochim Biophys Acta. 1981 Apr 6;642(2):267–285. doi: 10.1016/0005-2736(81)90445-4. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich B., Bonilla E., Casadei J., Barchi R. Immunocytochemical localization of the mammalian voltage-dependent sodium channel using polyclonal antibodies against the purified protein. J Neurosci. 1984 Sep;4(9):2259–2268. doi: 10.1523/JNEUROSCI.04-09-02259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The receptor for tetrodotoxin and saxitoxin. A structural hypothesis. Biophys J. 1975 Jun;15(6):615–619. doi: 10.1016/S0006-3495(75)85842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Levinson S. R., Ellory J. C. Molecular size of the tetrodotoxin binding site estimated by irradiation inactivation. Nat New Biol. 1973 Sep 26;245(143):122–123. doi: 10.1038/newbio245122a0. [DOI] [PubMed] [Google Scholar]
- Meiri H., Zeitoun I., Grunhagen H. H., Lev-Ram V., Eshhar Z., Schlessinger J. Monoclonal antibodies associated with sodium channel block nerve impulse and stain nodes of Ranvier. Brain Res. 1984 Sep 17;310(1):168–173. doi: 10.1016/0006-8993(84)90023-4. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Fritz L. C., Raftery M. A., Brockes J. P. Isolation and characterization of a monoclonal antibody against the saxitoxin-binding component from the electric organ of the eel Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1673–1677. doi: 10.1073/pnas.79.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Nonner W. A new voltage clamp method for Ranvier nodes. Pflugers Arch. 1969;309(2):176–192. doi: 10.1007/BF00586967. [DOI] [PubMed] [Google Scholar]
- Palti Y., Gold R., Stämpfli R. Diffusion of ions in myelinated nerve fibers. Biophys J. 1979 Jan;25(1):17–31. doi: 10.1016/S0006-3495(79)85275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palti Y., Moran N., Stämpfli R. Potassium currents and conductance. Comparison between motor and sensory myelinated fibers. Biophys J. 1980 Dec;32(3):955–966. doi: 10.1016/S0006-3495(80)85029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- TAYLOR R. E., MOORE J. W., COLE K. S. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960 Nov;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G., Foster R. E. Ionic channel distribution and heterogeneity of the axon membrane in myelinated fibers. Brain Res. 1980 Oct;203(2):205–234. doi: 10.1016/0165-0173(80)90008-9. [DOI] [PubMed] [Google Scholar]