Abstract
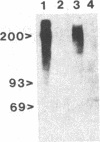
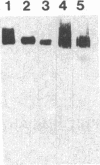
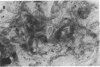
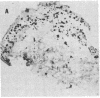
The neural cell adhesion molecule (NCAM) has been detected in regenerating limb bud of adult newts in addition to brain and peripheral nerves. In the regenerating tissue, NCAM was found primarily on mesenchymal cells and also in wound epidermis. Infusion of Fab fragments of antibodies to NCAM into limb buds at the early blastema stage delayed the regenerative process. Previous studies have indicated that NCAM serves as a homophilic ligand for adhesion among cells that express this molecule and, in doing so, can influence the interaction of nerves with their environment. The expression of NCAM in regenerating limb and the effects of antibody infusion are therefore consistent with the observation that limb regeneration requires interactions among axons and mesenchymal cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockes J. P. Mitogenic growth factors and nerve dependence of limb regeneration. Science. 1984 Sep 21;225(4668):1280–1287. doi: 10.1126/science.6474177. [DOI] [PubMed] [Google Scholar]
- Covault J., Sanes J. R. Neural cell adhesion molecule (N-CAM) accumulates in denervated and paralyzed skeletal muscles. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4544–4548. doi: 10.1073/pnas.82.13.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J., Finne U., Deagostini-Bazin H., Goridis C. Occurrence of alpha 2-8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983 Apr 29;112(2):482–487. doi: 10.1016/0006-291x(83)91490-0. [DOI] [PubMed] [Google Scholar]
- Finne J., Mäkelä P. H. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J Biol Chem. 1985 Jan 25;260(2):1265–1270. [PubMed] [Google Scholar]
- Grumet M., Rutishauser U., Edelman G. M. Neural cell adhesion molecule is on embryonic muscle cells and mediates adhesion to nerve cells in vitro. Nature. 1982 Feb 25;295(5851):693–695. doi: 10.1038/295693a0. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hoffman S., Sorkin B. C., White P. C., Brackenbury R., Mailhammer R., Rutishauser U., Cunningham B. A., Edelman G. M. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982 Jul 10;257(13):7720–7729. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maier C. E., Grimm R. A., Singer M. Neurotrophic and neuronotrophic effects in the regenerating newt limb bud after electrical stimulation of brachiospinal nerves. Brain Res. 1984 Jun 3;301(2):363–369. doi: 10.1016/0006-8993(84)91105-3. [DOI] [PubMed] [Google Scholar]
- Maier C. E., McQuarrie I. G., Singer M. A nerve-conditioning lesion accelerates limb regeneration in the newt. J Exp Zool. 1984 Nov;232(2):181–186. doi: 10.1002/jez.1402320205. [DOI] [PubMed] [Google Scholar]
- Maier C. E., McQuarrie I. G., Singer M. Parent and daughter axon density in the limb stump and regenerating limb bud of the newt. Exp Neurol. 1984 Feb;83(2):443–447. doi: 10.1016/S0014-4886(84)90113-4. [DOI] [PubMed] [Google Scholar]
- Maier C. E., Singer M. Gangliosides stimulate protein synthesis, growth, and axon number of regenerating limb buds. J Comp Neurol. 1984 Dec 10;230(3):459–464. doi: 10.1002/cne.902300313. [DOI] [PubMed] [Google Scholar]
- Maier C. E., Singer M. The effect of light on forelimb regeneration in the newt. J Exp Zool. 1977 Nov;202(2):241–244. doi: 10.1002/jez.1402020213. [DOI] [PubMed] [Google Scholar]
- Mudge A. W. Schwann cells induce morphological transformation of sensory neurones in vitro. Nature. 1984 May 24;309(5966):367–369. doi: 10.1038/309367a0. [DOI] [PubMed] [Google Scholar]
- Neufeld D. A. Formation of prechondrogenic mesenchymal cell aggregates in the regenerating newt limb. Dev Biol. 1982 Sep;93(1):36–42. doi: 10.1016/0012-1606(82)90236-6. [DOI] [PubMed] [Google Scholar]
- Rutishauser U. Developmental biology of a neural cell adhesion molecule. Nature. 1984 Aug 16;310(5978):549–554. doi: 10.1038/310549a0. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Grumet M., Edelman G. M. Neural cell adhesion molecule mediates initial interactions between spinal cord neurons and muscle cells in culture. J Cell Biol. 1983 Jul;97(1):145–152. doi: 10.1083/jcb.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U. Influences of the neural cell adhesion molecule on axon growth and guidance. J Neurosci Res. 1985;13(1-2):123–131. doi: 10.1002/jnr.490130109. [DOI] [PubMed] [Google Scholar]
- SINGER M. The influence of the nerve in regeneration of the amphibian extremity. Q Rev Biol. 1952 Jun;27(2):169–200. doi: 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Silver J., Rutishauser U. Guidance of optic axons in vivo by a preformed adhesive pathway on neuroepithelial endfeet. Dev Biol. 1984 Dec;106(2):485–499. doi: 10.1016/0012-1606(84)90248-3. [DOI] [PubMed] [Google Scholar]
- Singer M., Maier C. E., McNutt W. S. Neurotrophic activity of brain extracts in forelimb regeneration of the urodele, Triturus. J Exp Zool. 1976 May;196(2):131–150. doi: 10.1002/jez.1401960202. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Tosney K. W., Watanabe M., Landmesser L., Rutishauser U. The distribution of NCAM in the chick hindlimb during axon outgrowth and synaptogenesis. Dev Biol. 1986 Apr;114(2):437–452. doi: 10.1016/0012-1606(86)90208-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., McCoy R. D., Vollger H. F., Wilkison N. C., Troy F. A. Use of prokaryotic-derived probes to identify poly(sialic acid) in neonatal neuronal membranes. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1971–1975. doi: 10.1073/pnas.81.7.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace H. The components of regrowing nerves which support the regeneration of irradiated salamander limbs. J Embryol Exp Morphol. 1972 Oct;28(2):419–435. [PubMed] [Google Scholar]
- Zanetti N. C., Solursh M. Epithelial effects on limb chondrogenesis involve extracellular matrix and cell shape. Dev Biol. 1986 Jan;113(1):110–118. doi: 10.1016/0012-1606(86)90113-2. [DOI] [PubMed] [Google Scholar]