Abstract
Deficiencies in rod-specific cyclic guanosine monophosphate (cGMP) phosphodiesterase-6 (PDE6) are the third most common cause of autosomal recessive retinitis pigmentosa (RP). Previously, viral gene therapy approaches on pre-clinical models with mutations in PDE6 have demonstrated that the photoreceptor cell survival and visual function can be rescued when the gene therapy virus is delivered into the subretinal space before the onset of disease. However, no studies have currently been published that analyze rescue effects after disease onset, a time when human RP patients are diagnosed by a clinician and would receive the treatment. We utilized the AAV2/8(Y733F)-Rho-Pde6α gene therapy virus and injected it into a pre-clinical model of RP with a mutation within the alpha subunit of PDE6: Pde6αD670G. These mice were previously shown to have long-term photoreceptor cell rescue when this gene therapy virus was delivered before the onset of disease. Now, we have determined that subretinal transduction of this rod-specific transgene at post-natal day (P) 21, when approximately half of the photoreceptor cells have undergone degeneration, is more efficient in rescuing cone than rod photoreceptor function long term. Therefore, AAV2/8(Y733F)-Rho-Pde6α is an effective gene therapy treatment that can be utilized in the clinical setting, in human patients who have lost portions of their peripheral visual field and are in the mid-stage of disease when they first present to an eye-care professional.
INTRODUCTION
Retinitis pigmentosa (RP) is the most common cause of hereditary blindness worldwide, and mutations within the rod-specific cyclic guanosine monophosphate (cGMP) phosphodiesterase 6 (PDE6) is the third most common cause of autosomal recessive RP (1–4). The early clinical symptoms of this disease include night blindness, rod cell degeneration and secondary cone cell death (5–8). There are several mouse models with mutations in the beta subunit of PDE6 that have been utilized for studying gene therapy for RP, and only one published manuscript for gene therapy in a mouse model with a mutation in the alpha subunit of PDE6 (9–15). Recently, with advances in gene therapy, such as the use of the AAV2/8(Y733F) virus to efficiently transduce the photoreceptor cells, long-term rescue effects have been provided for two mouse models of RP caused by mutations within PDE6, one with the mutation in the beta subunit of PDE6 and the other with a mutation in the alpha subunit of PDE6 (10,15).
For several reasons, these gene therapy studies have been conducted by delivering the virus before the onset of degeneration in the pre-clinical model. This allows for the greatest efficacy of the gene therapy virus to be shown in order to prove it is effective for RP treatment. Additionally, cryoanesthesia can be used for young mice, which is a safer alternative to ketamine/xylazine or isoflurane anesthetics (16). Lastly, the transduction of the gene therapy virus has been found to be limited to the location and size of the subretinal bleb during the surgical procedure (15). The maturation of the subretinal extracellular matrix in older mice can block viral spread after subretinal injection, limiting the rescue effect to a smaller number of transduced cells (17–19). Now that gene therapy viruses have proved to have long-term rescue effects in mouse models with PDE6 deficiencies when delivered prior to the onset of photoreceptor degeneration, it will be important to study whether or not these gene therapy viruses can promote cell survival and rescue visual function when delivered after the onset of disease.
Human patients with retinal dystrophic diseases, such as RP, may not be diagnosed by a clinician until they have mid-stage disease. This includes the loss of their peripheral visual field, when rod photoreceptor cells have already begun to degenerate. A recent publication studying both human patients and canine models of Leber congenital amaurosis (LCA) after subretinal injection of an AAV2-CMV-RPE65 virus indicated that despite functional rescue of vision, the photoreceptor cells continue to degenerate at the same rate (20). This would suggest that gene therapy treatment after the onset of disease may not be effective for use in human clinical trials.
Only two studies have been published that examined the effects of gene therapy delivered after disease onset (21,22). The first study tested AAV5 gene therapy in a murine model of LCA, caused by a mutation in a retinal pigment epithelium (RPE)-specific gene. This study was able to provide restoration of cone function delivering the gene therapy vector after disease onset. The second study provided AAV5 gene therapy to a canine model of achromatopsia caused by a mutation within the beta subunit of the cyclic nucleotide-gated photoreceptor channels (CNGB3). However, gene therapy delivered after disease onset to this cone-specific mutation was unable to restore function unless the vector was delivered in combination with ciliary neurotrophic factor.
Therefore, we tested whether it is feasible to restore both rod and cone cell function in the Pde6α mutant mice, as no studies have shown cone restoration in models with mutations within the rod photoreceptors. We proposed to transduce our previously utilized AAV2/8(Y733F)-Rho-Pde6α gene therapy virus in the Pde6αD670G mutant mouse model after the onset of photoreceptor degeneration. This AAV2/8(Y733F) gene therapy vector has been shown to be more effective in photoreceptor cell transduction than the AAV5 serotype (23). Additionally, this mouse model was shown to have long-term rescue of the photoreceptor cell nuclei, visual function and prevention of the secondary migration of the RPE when the gene therapy virus was delivered at post-natal day (P) 5, before the disease onset (15). We utilized the same mouse model and gene therapy vector, but transduced the virus into the subretinal space at P21, after approximately half of the photoreceptor cells in the eye have undergone degeneration. We compared results from gene therapy treatment in this pre-clinical model of RP before the disease onset to treatment using the same vector in the same model during mid-stage of disease (i.e. after the loss of approximately half of the photoreceptor cells).We found that even when the gene therapy virus is delivered at mid-stage of disease (i.e. after the onset of disease), we can promote photoreceptor cell survival and a rescue of visual function long-term in the pre-clinical model of RP.
RESULTS
AAV2/8(Y733F)-Rho-Pde6α delivery at approximate time of human diagnosis
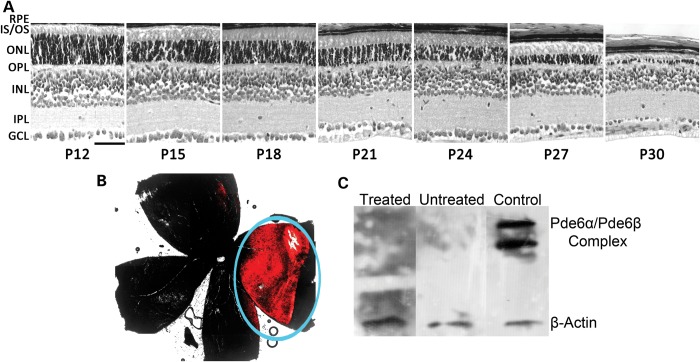
Human RP patients typically present to an eye care professional after loss of their peripheral visual field, suggesting a mid-stage of RP disease where rod photoreceptors have already begun to undergo degeneration. We gave a single subretinal injection of AAV2/8(Y733F)-Rho-Pde6α into the right eyes of the Pde6αD670Gmice at post-natal (P) day 21. This corresponds to mid-stage of RP in the Pde6αD670G mice, as seen in a longitudinal histological analysis from P12 to P30 (Fig. 1A). At the time of injection, P21, the mice have lost approximately half of their photoreceptor outer nuclear layer (ONL) thickness. Expression of the transgene and protein of interest, PDE6α, will occur by P24 in the already diseased retina, when further loss of the photoreceptor cells has continued after delivery of the AAV virus.
Figure 1.
Viral spread and lack of immune response in late-degeneration treated mutant eyes. A single subretinal injection of the AAV2/8(Y733F)-Rho-Pde6α was delivered at post-natal (P) day 21, in mid-stage photoreceptor neurodegeneration as matches human patients at the time of diagnosis. Scale bar: 600 µm (A). A single subretinal injection of AAV2/8-TurboRFP into the right eye of P21 Pde6αD670G mice and visualized at P55. Red fluorescence was overlayed onto a bright-field image of the whole mount for localization of fluorescence in respect to the entire retina (B). Immunoblot analysis for antibodies against PDE6α and β-actin on retinal lysates from a C57BL/6J (B6) mouse retina. Nine micrograms of C57BL/6J (B6) retinal lysate was loaded per lane, and lanes were incubated with blood serum from a Pde6αD670G mouse 1 month after a single subretinal injection of AAV2/8(Y733F)-Rho-Pde6α (treated lane), blood serum from an untreated Pde6αD670G mouse (untreated lane), and commercial anti-PDE6α (control lane). β-Actin was used as a loading control. PDE6α antibody was not specific, and cross-reacted with the highly homologous PDE6β subunit. N = 5 mice (C).
Subretinal injections of AAV2/8(Y733F)-Rho-Pde6α at P5, as previously studied in the Pde6αD670G mutant mouse model (15), occurred before the full development of the extracellular matrix of the eye. To determine that the transduction efficiency of AAV2/8(Y733F) within the retina is the same at both P5 and P21, and not lessened by the developed extracellular matrix, we administered a subretinal injection of AAV2/8-TurboRFP to a litter of homozygous Pde6αD670G mutant mice at P21. This vector will express red fluorescent protein (RFP), which allows for the visualization of the cells transduced by the virus. After a single subretinal injection at P21, RFP expression remained visible within the retina at 2 months of age, indicating that the virus was taken up by the retinal cells and survived for at least 5 weeks post-injection (Fig. 1B). As expected, RFP expression was detected within the portion of the retina that most likely correlated with the subretinal bleb, but was found to be similar in size and expression to that of the subretinal bleb after gene therapy delivery at P5, affecting ∼20–30% of the retina (Fig. 1B; blue circle; 15).
AAV2/8(Y733F)-Rho-Pde6α does not induce an inflammatory reaction in Pde6αD670G mice
The continual expression of RFP after a single subretinal injection of AAV2/8-TurboRFP at P21 suggests that the AAV2/8(Y733F)-Rho-Pde6α will be able to survive within the diseased retina and express PDE6α. However, one main concern when this gene therapy approach is studied is the potential for antibody production in the host after transduction of the AAV2/8(Y733F) virus. We collected serum from five transduced Pde6αD670G mice and five untreated Pde6αD670G mice at 2 months of age. We then used this serum on a western blot to test for antibodies against PDE6α in a C57BL/6J (B6) mouse retina. Both the serum from the Pde6αD670G mouse transduced with the gene therapy vector (treated lane) and the serum from an untreated Pde6αD670G mouse (untreated lane) did not react against PDE6α in the B6 retinal lysate (Fig. 1C). In contrast, a commercial PDE6α antibody was able to detect the presence of the PDE6αβ complex from the same B6 retinal lysate on the western blot gel (control lane). Beta-actin was used as a loading control to confirm the presence of the B6 retinal lysate at similar levels for each lane. Therefore, no antigens were produced 1 month after injection with the AAV2/8(Y733F)-Rho-Pde6α virus in the Pde6αD670G mouse.
Despite gene delivery after disease onset, increased PDE6α protein improved photoreceptor survival in Pde6αD670G mice
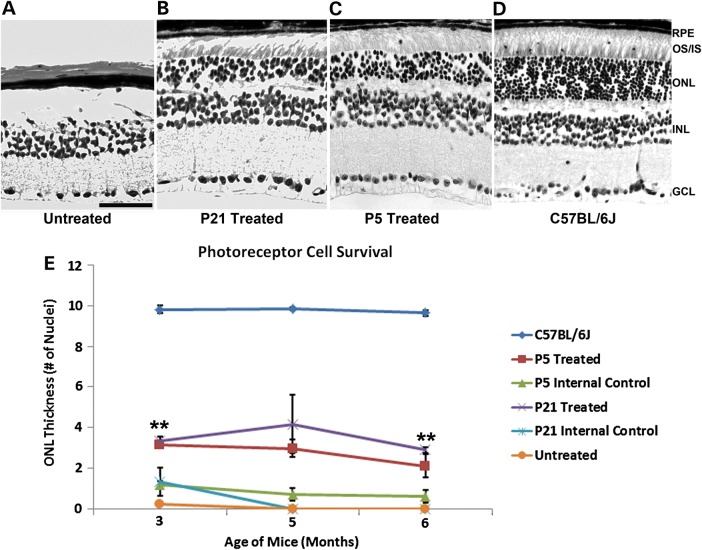
Since AAV2/8(Y733F)-Rho-Pde6α virus delivery at P21 does not cause antigen production and may protect the photoreceptor cells in the diseased retina, we examined histological sections from both treated and untreated Pde6αD670G mutant eyes in a longitudinal study and compared these to injections of the gene therapy virus at P5, before and after disease onset. As expected, the untreated Pde6αD670G fellow eye had no detectable photoreceptor cells remaining at 6 months of age (Fig. 2A). In contrast, the P21-treated Pde6αD670G eye (Fig. 2B) displayed photoreceptor cell survival, at a similar thickness to the ONL of a P5-treated Pde6αD670G eye (Fig. 2C). The ONL thickness of the P21-treated Pde6αD670G eyes was approximately one to two cell layers less than the P5-treated eyes, consistent with the degeneration between P5 and P21 before the delivery of the gene therapy virus (Fig. 2B and C). In comparison with a wild-type B6 mouse, P21-treated Pde6αD670G eyes had a ∼60–70% loss of the ONL layer and IS/OS (Fig. 2D).
Figure 2.
Improved photoreceptor survival after AAV2/8(Y733F)-Rho-Pde6α transduction at mid-stage of disease. H&E stained retinal section of an untreated Pde6αD670G mouse retina (A), the fellow P21-treated Pde6αD670G mouse retina (B), a P5-treated Pde6αD670G mouse retina (C) and B6 control mouse (D) at 6 months of age. Scale bar: 600 µm. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; IS, inner segments; OS, outer segments; RPE, retinal pigment epithelium. Quantification of the thickness of the ONL by counting the columns of photoreceptor nuclei in the rescued half of the treated retina, the un-rescued half of the treated retina (labeled internal control) and fellow-untreated eyes from 3 to 6 months of age (E). Error bars show SEM for each time-point and the significance was calculated for the rescued half of the P21-treated retina compared with the untreated fellow eyes using paired t-test analysis. N ≥ 3 mice. **P < 0.01.
The rescue at 6 months of age in the Pde6αD670G-treated eyes suggested that gene therapy delivery may halt the degeneration in transduced cells at the time the wild-type PDE6α protein is expressed by the virus. To determine the extent of this rescue, we quantified the thickness of the ONL of the rescued portion of the treated retina compared with both the un-rescued opposing side of the treated retina that did not receive the subretinal bleb (as an internal control), and the fellow-untreated Pde6αD670G eyes from 3 to 6 months of age, when photoreceptor cells would typically undergo full photoreceptor neurodegeneration in the mutant Pde6αD670G mice (Fig. 2E).
Compared with a B6 mouse control, there is an ∼60% loss of the photoreceptor cell ONL thickness by 3 months of age in the P21-treated eyes, as would be expected when the gene therapy is delivered after the onset of photoreceptor degeneration. However, there is significantly more photoreceptor cells surviving in the retinas treated with the gene therapy virus than in the untreated fellow eye at both 3 and 6 months of age, a quarter of the mouse lifespan (Fig. 2E). Additionally, this photoreceptor cell survival in the P21-treated mutant eyes is similar to the cell survival in the P5-treated mutant eyes, suggesting that the diseased retina does not hinder the effectiveness of the gene therapy virus (Fig. 2E). The opposing side of the treated retinas, which did not receive the subretinal bleb, showed a similar ONL thickness to the untreated fellow eye, with all photoreceptor cells degenerating by 4 months of age, as would be expected with little to no rescue (Fig. 2E).
Additionally, although there was increased variability in the photoreceptor cell survival when the gene therapy virus is delivered after the onset of degeneration, there is a stable number of photoreceptor cells surviving over the 3–6 month testing period, at ∼60–70% of the ONL thickness of a healthy wild-type B6 retina (Fig. 2E). Thus, at 6 months of age, the untreated Pde6αD670G eyes had no detectable photoreceptor cells, whereas the treated fellow eyes had significant photoreceptor cell survival even when the gene therapy virus is delivered to a degenerating retina.
Despite gene delivery after disease onset, global functional rescue is observed in Pde6αD670G mice
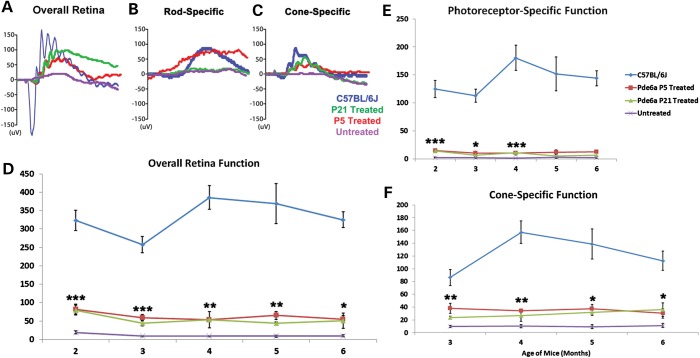
Since the Pde6αD670G mutant photoreceptors survived after AAV2/8(Y733F)-Rho-Pde6α transduction, even in a degenerating retina, we tested whether these remaining, rescued photoreceptor cells were functional. We measured electroretinogram (ERG) recordings in the Pde6αD670G mice after a single subretinal injection of AAV2/8(Y733F)-Rho-Pde6α at P21 and compared them with a wild-type B6 mouse, P5-treated Pde6αD670G mice and the untreated Pde6αD670G mutant eyes (Fig. 3). By 5 months of age, over 4 months post-injection, a representative ERG trace for the maximum rod–cone scotopic ERG setting for an untreated mutant eye presents a full loss of all visual response (Fig. 3A). However, the treated eye retained a strong visual response (Fig. 3A), although with a lower b-wave amplitude (∼100 µV) than that of a control wild-type B6 mouse, but comparable with the amplitude of the P5-treated Pde6αD670G eye (Fig. 3A). There was a loss of the a-wave amplitude in the treated eyes as well, suggesting a loss of some of the photoreceptor cells.
Figure 3.
Rescued visual function after AAV2/8(Y733F)-Rho-Pde6α transduction. Representative scotopic maximum ERG traces for a B6 control mouse (blue), a P5-treated eye (red), a P21-treated eye (green) and an untreated eye (purple) of a Pde6αD670G mouse at 5 months of age (A). Representative scotopic dim light rod-specific ERG traces of a B6 control mouse (blue), a P5-treated eye (red), a P21-treated eye (green) and an untreated eye (purple) of a Pde6αD670G mouse at 5 months of age (B), and representative photopic single flash cone-mediated ERG traces for a B6 control mouse (blue), a P5-treated eye (red), a P21-treated eye (green) and an untreated eye (purple) of a Pde6αD670G mouse at 5 months of age (C). Maximum scotopic b-wave amplitudes in a B6 mouse, the treated Pde6αD670G eyes and fellow-untreated eyes monthly between 2 and 6 months of age (D). Maximum scotopic photoreceptor-mediated a-wave amplitudes (shown as positive values) in a B6 mouse, the treated Pde6αD670G eyes and fellow-untreated eyes monthly between 2 and 6 months of age (E). Photopic cone-specific b-wave amplitudes in a B6 mouse, the treated Pde6αD670G eyes and fellow-untreated eyes from 3 to 6 months of age (F). Error bars show SEM for each time-point and the significance was calculated for the P21-treated eyes compared with untreated fellow eyes using the ratio paired t-test analysis. N ≥ 3 mice. *P < 0.05; **P < 0.01; ***P < 0.001.
Quantification of the cohort of mice detected rod–cone maximum scotopic visual responses for the Pde6αD670G-treated eyes at approximately one-third of the response of wild-type B6 mice, but the treated eyes had significantly increased visual responses compared with the untreated fellow eyes (Fig. 3D). The significant increase in visual response for the treated eyes remained stable over 6 months, the longest time analyzed in this study (Fig. 3D).
Furthermore, since PDE6α mutations lead to a rod–cone dystrophic disease, we examined the loss of the a-wave amplitude seen in the maximum rod–cone scotopic ERG traces for the Pde6αD670G-treated mice compared with wild-type B6 mice (Fig. 3A). Dim light, scotopic ERGs, recording responses from rod photoreceptor cells, did not display visual responses in either the P21 Pde6αD670G-treated or untreated eyes compared with a B6 control mouse (Fig. 3B). We found that only three P21 Pde6αD670G-treated eyes had rod-only ERG recordings, and the later delivery of the gene therapy virus may prevent any rod cell functional response detected by the ERG. However, quantification from 2 to 6 months of age for Pde6αD670G mice given a single subretinal injection of AAV2/8(Y733F)-Rho-Pde6α at both P5 and P21 displayed a-wave amplitudes at approximately one-sixth of the B6 mouse a-wave response, but significantly increased compared with the untreated fellow eyes for 4 months of age, although this visual response was barely increased over the untreated fellow eyes at both 5 and 6 months of age (Fig. 3E).
For human RP patients, the loss of the peripheral visual field has already begun when they first present to an eye care professional. The loss of the cone photoreceptor cells, giving the central visual field, is important for performing daily activities. Therefore, even if only a relatively small amount of rod cells survive, and these cells are able to provide a barely detectable visual response, cone cells still have the possibility of surviving and remaining healthy in the treated eyes. We tested for cone cell function in the Pde6αD670G mice given a single subretinal injection of AAV2/8(Y733F)-Rho-Pde6α at P21 using the photopic ERG setting. Representative photopic ERG responses at 5 months of age did not detect visual function in the untreated Pde6αD670G eyes (Fig. 3C). In contrast, the treated Pde6αD670G eyes had a similar cone-specific ERG response to that of the B6 control eyes (Fig. 3C). Quantification of the cone cell ERG responses were significantly improved in the treated Pde6αD670G eyes compared the untreated fellow eyes for the 6 months examined (Fig. 3F). Additionally, cone cell visual function is sustained in the treated Pde6αD670G mutant eyes, at approximately half the response of wild-type B6 eyes, for all 6 months examined (Fig. 3F). Taken together, our data suggest that treatment with AAV2/8(Y733F)-Rho-Pde6α, even after the onset of degeneration, rescues cone photoreceptor cell survival and both cone-specific and maximum visual response for at least 6 months in the Pde6αD670G mutant mice.
Despite gene delivery at mid-stage of disease, there is a long-term delay of RPE degeneration in treated Pde6αD670G mutant eyes
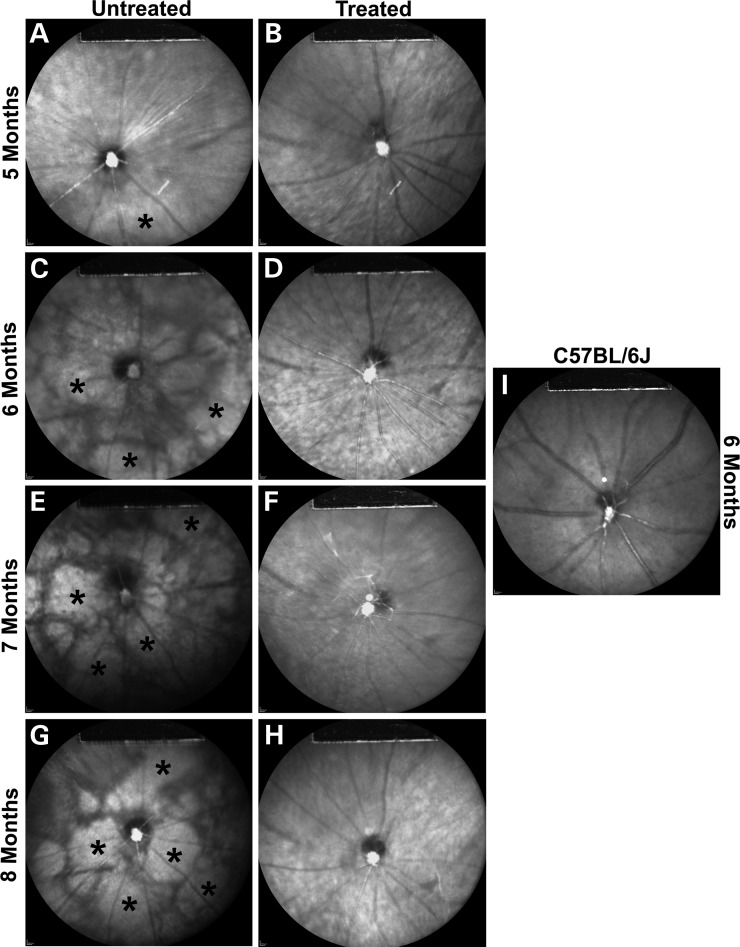
Since a single subretinal injection of AAV2/8(Y733F)-Rho-Pde6α after disease onset can protect the survival and visual function of the cone photoreceptor cells, we tested whether it can rescue the secondary RPE degeneration visible upon fundus examination in RP patients. We examined infrared (IR) images of P21-treated and fellow-untreated Pde6αD670G mutant eyes from 5 to 8 months of age. Representative IR images of a mutant Pde6αD670G eye over time displays a relatively normal fundus at 5 months of age (Fig. 4A). However, a mottled, degenerative appearance begins at 6 months of age and continues to substantially increase through 8 months of age, the last time analyzed (Fig. 4C, E and G; asterisks). In comparison, the gene therapy treated Pde6αD670G mutant eyes, after the onset of disease, display a normal, healthy retinal image from 5 to 8 months of age (Fig. 4B, D, F and H) similar to a B6 control at 6 months of age (Fig. 4I).
Figure 4.
Delay of retinal pigment epithelial atrophy after AAV2/8(Y733F)-Rho-Pde6α transduction. Representative infrared (IR) images of an untreated Pde6αD670G mutant eye at 5 (A), 6 (C), 7 (E) and 8 (G) months of age compared with the fellow-treated Pde6αD670G mutant eye at 5 (B), 6 (D), 7 (F) and 8 (H) months of age. Images for all time-points are taken from the same mouse. Representative IR image of a C57BL/6J control mouse at 6 months of age (I). Increased IR reflectance represents RPE atrophy (*). IR imaging was obtained at 790 nm absorption and 830 nm emission using a 55° lens. Images were taken of the central retina, with the optic nerve located at the center of the image and the site of the subretinal bleb along the lower left-hand quadrant.
DISCUSSION
Our previous studies have proved efficacy of the AAV2/8(Y733F)-Rho-Pde6α virus after subretinal injection in the pre-clinical model of RP before the onset of degeneration (15). By genetically enhancing rod PDE6α activity, we were able to restore rod function and therefore prevent the progressive cell-autonomous loss of the rods for up to 11 months of age. Additionally, we prevented the subsequent non-cell-autonomous loss of the cone photoreceptors and the secondary disease effects found in human RP patients. We also provided evidence that the visual rescue noted in our previous manuscript was due to the Pde6α transgene delivery after subretinal injection of the AAV2/8(Y733F)-Rho-Pde6α virus, as mice given subretinal injections of AAV2/8-TurboRFP, without the Pde6α transgene, did not show any visual rescue at 6 weeks of age, and had a reduced response in comparison with their uninjected fellow eyes (15). In the majority of the current pre-clinical animal models for RP gene therapy, the delivery of the virus has been performed before the onset of disease (9–15). This proves the efficacy of the gene therapy vector in halting the presentation of disease, but it does not mimic human scenarios. Since most newly diagnosed RP patients already exhibit significant loss of rods and cones in their peripheral retinas, gene therapy intervention must be able to rescue photoreceptor cells after degeneration onset.
Therefore, we proposed experiments to address the clinically important question of whether visual function can be rescued in retinas with grossly advanced disease. Human patients are typically diagnosed during mid-stage photoreceptor degeneration, and we injected the AAV2/8(Y733F)-Rho-Pde6α virus at post-natal (P) day 21 to match this time-point in the pre-clinical model. In the mouse, approximately half of the ONL layer has been lost by P21 (Fig. 1A). Retinal whole mount imaging displayed AAV2/8(Y733F) uptake into the retina localized at the site of the subretinal bleb, as would be expected (Fig. 1B). The diseased retina allows for a similar spread, accounting for the uptake of the virus in ∼20–30% of the retinal photoreceptor cells, as was seen in the mouse eye after a P5 injection of the AAV2/8-TurboRFP (15). Additionally, studies have shown that the full development of the extracellular matrix could block the viral spread from transducing as many photoreceptor cells as it can affect when injected at 2 weeks of age or earlier (17–19). The size of the RFP expression in our study suggests that the development of the extracellular matrix does not block the viral spread after subretinal injection at P21.
Human clinical trials for LCA, utilizing the AAV2 serotype for gene therapy, are currently ongoing (NCT00999609; NCT00821340; NCT01496040; NCT00481546). Although these trials have been effective in restoring visual responses, it has been a concern that the human body will produce antibodies after it detects the presence of the AAV vector. However, in our study, western blot analysis for the presence of antibodies against PDE6α 1 month after delivery of the gene therapy virus did not suggest an immune response in the host (Fig. 1C). Phase I safety trials typically enroll three patients to test for adverse safety events, and, therefore, we used a sample size of five mice to test for this potential immune reaction. We propose that although systemic delivery of AAV2/8(Y733F) may be able to induce neutralizing antigens, subretinal injection of the gene therapy virus allowed for the host immune response to remain deactivated. However, it is possible that the immune response may induce neutralizing antibodies after subretinal injection of the gene therapy virus, but it is not at the threshold response that can be detected with our immunoblot analysis. It is still necessary to conduct a Phase I safety trial when delivering gene therapy to human patients, along with a dosage trial, in case of species-related differences in the immune response between the mice and the humans.
Current clinical trials suggest that the eye is immune-privileged enough that a secondary injection into the contralateral eye is safe and effective and does not provoke the immune system (24–27). Whether or not a second injection into the same eye would be possible without rejection of the gene therapy virus is something that still needs to be determined. These data suggest that AAV2/8(Y733F) may be used in human patients without an immune reaction for at least a single injection procedure and is a useful gene therapy virus for future clinical studies.
Since the AAV2/8(Y733F)-Rho-Pde6α safely functioned in the diseased retinal environment, the rescued photoreceptor cells and visual function is promising for patients who have been diagnosed after mid-stage of RP. However, rod photoreceptor cell visual function was barely detectable upon ERG analysis in our study, for both P5- and P21-treated mutant eyes. P5-treated eyes had a significant increase in rod cell function, although at approximately one-sixth of the response of a wild-type B6 mouse for the 6-month study period, as previously published (15). However, P21-treated mutant eyes had a significant increase in rod cell function for 4 months of age, but no significant difference at both 5 and 6 months of age (Fig. 3). This may be due to the delivery of the gene therapy virus into a retina where rod cells have reached the stage of no return. There may be a threshold of rod cells remaining in the retina for the ERG to be able to detect a response, and the amount transduced by the viral vector at the site of the subretinal bleb may not be strong enough for a detectable visual response. Not achieving a minimum number of rescued rods is likely to cause non-autonomous death of rescued rods (28–30). However, even in the worst-case scenario, if the AAV2/8(Y733F)-Rho-Pde6α virus is unable to rescue rod photoreceptor cell function after delivery during degeneration, we still found significant cone cell survival and visual responses (Fig. 2–3). The cone cells contribute to the maximal mixed scotopic ERG responses, which explain their significant rescue in the P21-treated eyes over the 6-month study period. Furthermore, cone cell visual function persisted without decline through the 6-month study period, suggesting that this long-term rescue is as beneficial when the gene therapy virus is delivered during the mid-stage of photoreceptor neurodegeneration as it is when delivered before the onset of disease (15).
Overall, the AAV2/8(Y733F)-Rho-Pde6α virus is able to protect cone cell function, however, it appears that the protection of the rod cells will depend on how many rod photoreceptors remain at the time of gene therapy delivery. Since many RP patients initially present with considerable degeneration, earlier treatment may produce more favorable outcomes. It still remains to be determined when a threshold may be met where there is no longer the possibility to rescue the cone cells, but this study shows that at the point when patients are diagnosed by clinicians, mid-stage degeneration, it is still possible to halt secondary cone cell degeneration.
Although the rod cell survival and function declined compared with a B6 wild-type mouse, the gene therapy treatment with the AAV2/8(Y733F)-Rho-Pde6α virus at P21 is able to protect against the secondary disease effects of RP. RP is characterized as a rod–cone dystrophic disease, where the rod cells undergo degeneration and this is followed by the secondary loss of the cone cells. Furthermore, secondary effects to the loss of the photoreceptors, both the rods and the cones, occur in RP patients, such as the atrophy of the RPE cell layer and remodeling of the retina. The protection of the cone cell survival and overall function with the AAV2/8(Y733F)-Rho-Pde6α virus treatment at P21 is able to provide protection against the later secondary effects of RP, such as the RPE atrophy, for at least 8 months of age.
The first human gene therapy trial for LCA reported enhanced visual function for at least 3 years in ∼33–50% of patients (31–33). This was profoundly encouraging given that these four phase-1 safety trials only enrolled patients with end-stage disease (no visual function). However, a follow-up study revealed that the gene therapy failed to halt, or even slow, photoreceptor degeneration (20). Treatment failure may be due to insufficient or inefficient vector transduction due to the AAV2 serotype. Our current report is the first study to analyze gene therapy treatment after disease onset in a model with a mutation within the rod photoreceptor cell. Our study suggests that the AAV2/8(Y733F) vector has the ability to efficiently transduce the photoreceptor cells (20–30% transduced) after subretinal injection, even within an already diseased retina. However, in agreement with the follow-up study, we found that there may be a point of no return for the rod cell rescue (20). Rod cells are present at 3 weeks of age, the age of treatment with the gene therapy virus, but these cells appear to lose their visual function as shown in the ERG results of this study (Fig. 3).
When first seen by a healthcare professional, PDE6 patients have peripheral vision loss—both night and daytime forms, which are manifestations of peripheral rod and cone degeneration, respectively. PDE6 is expressed in rods, but not cones. Cone loss, on the other hand, is due to secondary, non-cell-autonomous effects. Mid-stage gene therapy administration cannot halt the progressive cell-autonomous degeneration of rods but can prevent the secondary subsequent, non-cell-autonomous cone loss.
Therefore, we found that although rod photoreceptor cells may succumb to degeneration, the AAV2/8(Y733F)-Rho-Pde6α virus is able to provide long-term rescue and function of the cone cells and delay secondary effects of RP disease, such as the atrophy of the RPE. This lasted for at least a quarter of the mouse lifespan, the longest time-point studied in this analysis. Furthermore, cone cell survival and visual function were similar to the rescue effects found when gene therapy is delivered prior to disease onset. Thus, even when delivered after degeneration onset, the AAV2/8(Y733F)-Rho-Pde6α virus can be a useful gene therapy vector to provide long-term cone cell survival and function for human patients with RP caused by mutations in PDE6A.
MATERIALS AND METHODS
Mouse lines and husbandry
C57BL/6J-Pde6αnmf363/nmf363, with a D670G mutation, herein referred to as Pde6αD670G, mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained in the Columbia University Pathogen-free Eye Institute Annex Animal Care Services Facility under a 12/12-h light/dark cycle. All experiments were approved by the local Institutional Animal Care and Use Committee (IACUC) under protocol #AAAF-2107. Mice were used in accordance with the Statement for the Use of Animals in Ophthalmic and Vision Research of the Association for Research in Vision and Ophthalmology, as well as the Policy for the Use of Animals in Neuroscience Research of the Society for Neuroscience. Pde6αD670G mice used in this study were bred from a colony of mice that has been previously reported (34). Pde6αD670G are coisogenic in the C57BL/6J (B6) background; therefore, age-matched B6 mice were used as experimental controls (The Jackson Laboratory).
Construction of AAV vectors
AAV serotype 2/8 capsids containing a point mutation in surface-exposed tyrosine residues, AAV2/8(Y733F), exhibit higher transduction efficiency in photoreceptors and a faster onset of expression than other AAV serotypes when delivered into the subretinal space of the eye (10). Therefore, these vectors were used for packaging our Pde6α construct. Vector plasmids were constructed by inserting 1.1 kb of the murine rhodopsin promoter region [Ensembl, rhodopsin, chromosome 6: (115, 930, 881–115, 931, 988); Ensembl, rhodopsin, ATG = 1: (−1125, −17)] and a full-length murine Pde6α cDNA fragment into the pZac2.1 plasmid to generate pZac2.1.mRhodopsin.mPde6α.SV40 (35). AAV vectors were created, packaged and purified at the Penn Vector Corporation, to become AAV2/8(Y733F).mRhodopsin.mPde6α.SV40 (University of Pennsylvania, PA, USA). The AAV2/8(Y733F).mRhodopsin.mPde6α.SV40 vector will be referred to as AAV2/8(Y733F)-Rho-Pde6α in this publication. AAV2/8.CMV.TurboRFP.RBG was purchased from the Penn Vector Corporation as the AAV serotype control and will be referred to as AAV2/8-TurboRFP in this publication.
Transduction of AAV vectors
To increase levels of wild-type PDE6α, we injected AAV2/8(Y733F)-Rho-Pde6α [0.7 µl, 1.26e13 genome copy (GC)/ml] into the subretinal space of the right eye of Pde6αD670G mice at either post-natal day (P) 5 or P21. AAV2/8-TurboRFP (0.7 µl, 7.28e12 GC/ml) was injected into the subretinal space of the right eye of Pde6αD670G mice at P21 for control experiments. Virus particles were injected at the 6 o’clock position of the eye, ∼1.5 mm from the limbus, to produce a subretinal bleb in the mid-periphery of the retina (36). The left eyes of all mice were kept as a matched control for experimental analyses. Anesthesia and surgery were performed as previously described (12).
Whole mount analysis
Mice were euthanized according to the established IACUC guidelines. Eyes were enucleated and placed in 2% paraformaldehyde for 1 h at room temperature. The optic nerve, cornea and lens were removed. The whole eyecup was then flattened by means of four radial cuts extending out from the optic nerve and mounted with mounting medium (Vectashield, Burlingame, CA, USA). RFP visualization was achieved by fluorescence microscopy, and bright-field imaging was used to visualize the whole retina (Leica DM 5000B microscope). Pictures were taken at ×2.5 magnification using the Leica Application Suite Software (Leica Microsystems Inc., Germany).
Immunoblot analysis
A B6 retina was homogenized in 10% sodium dodecyl sulfate (SDS) by brief sonication and denatured at 100°C for 5 min. Following centrifugation, the total protein content per sample was measured using the DC protein assay method (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were separated by SDS polyacrylamide gel electrophoresis. The samples were then transferred to nitrocellulose membranes, which were blocked in 3% bovine serum albumin (BSA; Santa Cruz Biotechnology, Santa Cruz, CA, USA), 150 mmol/l NaCl, 100 mmol/l Tris (pH 7.4) and 0.5% Tween-20 (BSA-TTBS). Membranes were incubated with either blood serum from a treated Pde6αD670G mouse (1:15), blood serum from an untreated Pde6αD670G mouse (1:15) or anti-PDE6α (1:300, Abcam) as primary antibodies in BSA-TTBS. After washing in TTBS, filters were incubated with goat anti-rabbit or goat anti-mouse-conjugated horseradish peroxidase secondary antibodies (1:20 000, Santa Cruz Biotechnology). After washing, antibody complexes were visualized by a chemiluminescence detection kit (Immobilon Western, Millipore Corporation, Billerica, MA, USA) and a Kodak BioMax film (Kodak, Rochester, NY, USA).
Infrared imaging
IR fundus imaging was obtained with the Spectralis scanning laser confocal ophthalmoscope (Heidelberg Engineering, Carlsbad, CA, USA). IR imaging was obtained at 790 nm absorption and 830 nm emission using a 55° lens. Images were taken of the central retina, with the optic nerve located in the center of the image and the site of the subretinal bleb along the lower left-hand quadrant.
Histochemical analyses
Mice were sacrificed and the eyes enucleated as previously described (37). Excalibur Pathology, Inc. prepared Hematoxylin and Eosin retinal sections. The morphology of photoreceptors and the amount of photoreceptor cell nuclei of AAV2/8(Y733F)-Rho-Pde6α-treated eyes were compared with the untreated fellow eyes. Quantification of photoreceptor nuclei was conducted on several sections that contained the optic nerve, as follows: the distance between the optic nerve and the ciliary body was divided into four, approximately equal, quadrants. Three columns of nuclei (how many cell nuclei thick) were counted within each single quadrant. These counts were then used to determine the average thickness of the ONL for each individual animal at each time. For the treated eyes, the rescued half of the treated retina between the optic nerve and the ciliary body was quantified in this manner, with the opposite side of the retina, between the optic nerve and the ciliary body, being quantified similarly and considered the unrescued half of the treated mutant eye (the internal control). Averages and standard deviations were calculated from animals for each time-point using paired t-test statistical analyses with statistical significance set at P < 0.05. Sectioning proceeded along the long axis of the segment, so that each section contained upper and lower retina as well as the posterior pole.
Electroretinograms
Mice were dark-adapted overnight, manipulations were conducted under dim red light illumination, and recordings were made using Espion ERG Diagnosys equipment (Diagnosys LLL, Littleton, MA, USA). Adult B6 control mice were tested at the beginning of each session to ensure equal readouts from the electrodes for both eyes before testing the experimental mice. Pupils were dilated using topical 2.5% phenylephrine hydrochloride and 1% tropicamide (Akorn, Inc., Lakeforest, IL, USA). Mice were anesthetized by intraperitoneal injection of 0.1 ml/10 g body weight of anesthesia [1 ml ketamine 100 mg/ml (Ketaset III, Fort Dodge, IA, USA) and 0.1 ml xylazine 20 mg/ml (Lloyd Laboratories, Shenandoah, IA, USA) in 8.9 ml PBS]. Body temperature was maintained at 37°C using a heating pad during the procedure. Hand-made electrodes were placed upon the corneas and gonioscopic prism solution (Alcon Labs, Inc., Fort Worth, TX, USA) was applied to each eye. Both eyes were recorded simultaneously. A total of 40–60 responses were averaged for each trial. All further detail on the ERG method has been described previously (38,39). We measured scotopic dim light maximal b-wave ERG responses to assess rod-only responses, scotopic maximal b-wave ERG responses to assess inner retina function, scotopic maximal a-wave ERG responses to assess photoreceptor-specific function and photopic maximal b-wave ERG responses to assess cone-specific function. Maximal responses were taken from the Espion readout in microvolts and quantified using ratio paired t-test statistical analyses with statistical significance set at P < 0.05.
AUTHORS' CONTRIBUTIONS
K.J.W. and S.H.T. designed research; K.J.W. and J.S.P. performed research; K.J.W. and S.H.T. analyzed data; K.J.W. and S.H.T. wrote the paper.
FUNDING
This work was supported by the National Institute of Health Core (5P30EY019007), National Cancer Institute Core (5P30CA013696) and unrestricted funds from Research to Prevent Blindness, New York, NY, USA. S.H.T. is a member of the RD-CURE Consortium and is supported by Tistou and Charlotte Kerstan Foundation, the National Institute of Health (R01EY018213), the Research to Prevent Blindness Physician-Scientist Award, the Barbara and Donald Jonas Family Fund, the Schneeweiss Stem Cell Fund, New York State (N09G-302) and the Foundation Fighting Blindness New York Regional Research Center Grant (C-NY05-0705-0312), the Joel Hoffman Fund, Gale and Richard Siegel Stem Cell Fund, Charles Culpeper Scholarship, Irma T. Hirschl Charitable Trust, Bernard and Anne Spitzer Stem Cell Fund, Professor Gertrude Rothschild Stem Cell Foundation and Gebroe Family Foundation. K.J.W. is supported by the National Institute of Health (5T32EY013933, 5T32DK007647-20).
ACKNOWLEDGEMENTS
We are grateful for Dr Chyuan-Sheng Lin's pulled capillary needles for use in this study. We are also grateful for Dr Patsy Nishina's contribution of the mouse model used in this study.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Dryja T.P., Berson E.L. Retinitis pigmentosa and allied diseases: Implications of genetic heterogeneity. Invest. Ophthalmol. Vis. Sci. 1995;36:1197–1200. [PubMed] [Google Scholar]
- 2.Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 3.Berson E.L. Retinitis pigmentosa. The friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 1993;34:1659–1676. [PubMed] [Google Scholar]
- 4.Daiger S.P., Bowne S.J., Sullivan L.S. Perspective on genes and mutations causing retinitis pigmentosa. Arch. Ophthalmol. 2007;125:151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curcio C.A., Owsley C., Jackson G.R. Spare the rods, save the cones in aging and age-related maculopathy. Invest. Ophthalmol. Vis. Sci. 2000;41:2015–2018. [PubMed] [Google Scholar]
- 6.Owsley C., Jackson G.R., Cideciyan A.V., Huang Y., Fine S.L., Ho A.C., Maguire M.G., Lolley V., Jacobson S.G. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2000;41:267–273. [PubMed] [Google Scholar]
- 7.Owsley C., Jackson G.R., White M., Feist R., Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001;108:1196–1202. doi: 10.1016/s0161-6420(01)00580-2. [DOI] [PubMed] [Google Scholar]
- 8.Jackson G.R., Owsley C., Curcio C.A. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res. Rev. 2002;1:381–396. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 9.Petrs-Silva H., Dinculescu A., Li Q., Min S.H., Chiodo V., Pang J.J., Zhong L., Zolotukhin S., Srivastava A., Lewin A.S., et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 2009;17:463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang J.J., Dai X., Boye S.E., Barone I., Boye S.L., Mao S., Everhart D., Dinculescu A., Liu L., Umino Y., et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011;19:234–242. doi: 10.1038/mt.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tosi J., Sancho-Pelluz J., Davis R.J., Hsu C.W., Wolpert K.V., Sengillo J.D., Lin C.S., Tsang S.H. Lentivirus-mediated expression of cDNA and shRNA slows degeneration in retinitis pigmentosa. Exp. Biol. Med. 2011;236:1211–1217. doi: 10.1258/ebm.2011.011053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis R., Tosi J., Janisch K.M., Kasanuki J.M., Wang N.K., Kong J., Tsui I., Cillufo M., Woodruff M.L., Fain G.L., et al. Functional rescue of degenerating photoreceptors in mice homozygous for a hypomorphic cGMP phosphodiesterase 6 allele (Pde6bH620Q) Invest. Ophthalmol. Vis. Sci. 2008;49:5067–5076. doi: 10.1167/iovs.07-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang J.J., Boye S.L., Kumar A., Dinculescu A., Deng W., Li J., Li Q., Rani A., Foster T.C., Chang B., et al. AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEbeta mutation. Invest. Ophthalmol. Vis. Sci. 2008;49:4278–4283. doi: 10.1167/iovs.07-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang B., Hawes N.L., Hurd R.E., Davisson M.T., Nusinowitz S., Heckenlively J.R. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 15.Wert K.J., Davis R.J., Sancho-Pelluz J., Nishina P.M., Tsang S.H. Gene therapy provides long-term visual function in a pre-clinical model of retinitis pigmentosa. Hum. Mol. Genet. 2013;22:558–567. doi: 10.1093/hmg/dds466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirate J., Horikoshi I., Watanabe J., Ozeki S. Effect of hypothermia and ether anesthesia on the dispositions of creatinine and urea in mice. J. Pharmacobiodyn. 1984;7:883–890. doi: 10.1248/bpb1978.7.883. [DOI] [PubMed] [Google Scholar]
- 17.Tawara A., Varner H.H., Hollyfield J.G. Proteoglycans in the mouse interphotoreceptor matrix. II. Origin and development of proteoglycans. Exp. Eye Res. 1989;48:815–839. doi: 10.1016/0014-4835(89)90066-3. [DOI] [PubMed] [Google Scholar]
- 18.Blanks J.C., Johnson L.V., Hageman G.S. Stage-specific binding of peanut agglutinin to aggregates of degenerating photoreceptor cells in the rd mouse retina. Exp. Eye Res. 1993;57:265–273. doi: 10.1006/exer.1993.1124. [DOI] [PubMed] [Google Scholar]
- 19.Gruter O., Kostic C., Crippa S.V., Perez M.T., Zografos L., Schorderet D.F., Munier F.L., Arsenijevic Y. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther. 2005;12:942–947. doi: 10.1038/sj.gt.3302485. [DOI] [PubMed] [Google Scholar]
- 20.Cideciyan A.V., Jacobson S.G., Beltran W.A., Sumaroka A., Swider M., Iwabe S., Roman A.J., Olivares M.B., Schwartz S.B., Komaromy A.M., et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc. Natl Acad. Sci. USA. 2013;110:E517–E525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Li W., Dai X., Kong F., Zheng Q., Zhou X., Lu F., Chang B., Rohrer B., Hauswirth W.W., et al. Gene therapy rescues cone structure and function in the 3-month-old rd12 mouse: a model for midcourse RPE65 Leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2011;52:7–15. doi: 10.1167/iovs.10-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komaromy A.M., Rowlan J.S., Corr A.T., Reinstein S.L., Boye S.L., Cooper A.E., Gonzalez A., Levy B., Wen R., Hauswirth W.W., et al. Transient photoreceptor deconstruction by CNTF enhances rAAV-mediated cone functional rescue in late stage CNGB3-achromatopsia. Mol. Ther. 2013;21:1131–1141. doi: 10.1038/mt.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boye S.L., Conlon T., Erger K., Ryals R., Neeley A., Cossette T., Pang J., Dyka F.M., Hauswirth W.W., Boye S.E. Long-term preservation of cone photoreceptors and restoration of cone function by gene therapy in the guanylate cyclase-1 knockout (GC1KO) mouse. Invest. Ophthalmol. Vis. Sci. 2011;52:7098–7108. doi: 10.1167/iovs.11-7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steiger K., Lorenz B. Gene therapy for vision loss: recent developments. Discov. Med. 2010;10:425–433. [PubMed] [Google Scholar]
- 25.Bainbridge J.W., Tan M.H., Ali R.R. Gene therapy progress and prospects: the eye. Gene Ther. 2006;13:1191–1197. doi: 10.1038/sj.gt.3302812. [DOI] [PubMed] [Google Scholar]
- 26.Dudus L., Anand V., Acland G.M., Chen S.J., Wilson J.M., Fisher K.J., Maguire A.M., Bennett J. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vision Res. 1999;39:2545–2553. doi: 10.1016/s0042-6989(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 27.Amado D., Mingozzi F., Hui D., Bennicelli J.L., Wei Z., Chen Y., Bote E., Grant R.L., Golden J.A., Narfstrom K., et al. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci. Transl. Med. 2010;2:21ra16. doi: 10.1126/scitranslmed.3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kedzierski W., Bok D., Travis G.H. Non-cell-autonomous photoreceptor degeneration in RDS mutant mice mosaic for expression of a rescue transgene. J. Neurosci. 1998;18:4076–4082. doi: 10.1523/JNEUROSCI.18-11-04076.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streichert L.C., Birnbach C.D., Reh T. A diffusible factor from normal retinal cells promotes rod photoreceptor survival in an in vitro model of retinitis pigmentosa. J. Neurobiol. 1999;39:475–490. [PubMed] [Google Scholar]
- 30.Delyfer M.N., Forster V., Neveux N., Picaud S., Leveillard T., Sahel J.A. Evidence for glutamate-mediated excitotoxic mechanisms during photoreceptor degeneration in the rd1 mouse retina. Mol. Vis. 2005;11:688–696. [PubMed] [Google Scholar]
- 31.Bainbridge J.W., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., Tyler N., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 32.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M., et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson S.G., Cideciyan A.V., Aleman T.S., Sumaroka A., Windsor E.A., Schwartz S.B., Heon E., Stone E.M. Photoreceptor layer topography in children with Leber congenital amaurosis caused by RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2008;49:4573–4577. doi: 10.1167/iovs.08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto K., McCluskey M., Wensel T.G., Naggert J.K., Nishina P.M. New mouse models for recessive retinitis pigmentosa caused by mutations in the Pde6a gene. Hum. Mol. Genet. 2009;18:178–192. doi: 10.1093/hmg/ddn327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baehr W., Champagne M., Lee A.K., Pittler S.J. Complete cDNA sequences of mouse rod photoreceptor cGMP phosphodiesterase alpha- and beta-subunits, and indentification of beta’-subunit gene. FEBS Lett. 1991;278:107–114. doi: 10.1016/0014-5793(91)80095-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wert K.J., Skeie J.M., Davis R.J., Tsang S.H., Mahajan V.B. Subretinal injection of gene therapy vectors and stem cells into the perinatal mouse eye. J. Vis. Exp. 2012;69 doi: 10.3791/4286. doi:pii:4286.10.3791/4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsang S.H., Burns M.E., Calvert P.D., Gouras P., Baylor D.A., Goff S.P., Arshavsky V.Y. Role of the target enzyme in deactivation of photoreceptor G protein in vivo. Science. 1998;282:117–121. doi: 10.1126/science.282.5386.117. [DOI] [PubMed] [Google Scholar]
- 38.Hood D.G., Birch D.G. Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest. Ophthalmol. Vis. Sci. 1994;35:2948–2961. [PubMed] [Google Scholar]
- 39.Hood D.C., Birch D.G. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis. Neurosci. 1992;8:107–126. doi: 10.1017/s0952523800009275. [DOI] [PubMed] [Google Scholar]