Abstract
Rationale
Flexible behavior optimization relies on cognitive control which includes the ability to suppress automatic responses interfering with relevant goals. Extensive evidence suggests that the anterior cingulate cortex (ACC) is the central node in a predominantly frontal cortical network subserving executive tasks. Neuroimaging studies indicate that the ACC is sensitive to acute intoxication during conflict, but such evidence is limited to tasks using manual responses with arbitrary response contingencies.
Objectives
The present study was designed to examine whether alcohol's effects on top–down cognitive control would generalize to the oculomotor system during inhibition of hardwired saccadic responses.
Methods
Healthy social drinkers (N=22) underwent functional magnetic resonance imaging (fMRI) scanning and eye movement tracking during alcohol (0.6 g/kg ethanol for men, 0.55 g/kg for women) and placebo conditions in a counterbalanced design. They performed visually guided prosaccades (PS) towards a target and volitional antisaccades (AS) away from it. To mitigate possible vasoactive effects of alcohol on the BOLD (blood oxygenation level-dependent) signal, resting perfusion was quantified with arterial spin labeling (ASL) and used as a covariate in the BOLD analysis.
Results
Saccadic conflict was subserved by a distributed frontoparietal network. However, alcohol intoxication selectively attenuated activity only in the ACC to volitional AS and erroneous responses.
Conclusions
This study provides converging evidence for the selective ACC vulnerability to alcohol intoxication during conflict across different response modalities and executive tasks, confirming its supramodal, high-level role in cognitive control. Alcohol intoxication may impair top–down regulative functions by attenuating the ACC activity, resulting in behavioral disinhibition and decreased self-control.
Keywords: Anterior cingulate cortex, Cognitive control, Antisaccades, Alcohol intoxication, Error-related activity, Arterial spin labeling (ASL)
Introduction
Impaired self-control ("loss of control") is proposed to play an important role in the development and maintenance of drug and alcohol abuse (Field et al. 2010; Koob and Volkow 2010). The ability to inhibit automatic responses in favor of nonhabitual, but relevant responses is considered to be an essential aspect of cognitive control functions that are subserved by a predominantly prefrontal network, with the anterior cingulate cortex (ACC) as its central node (Botvinick 2007; Carter and van Veen 2007; Ridderinkhof et al. 2004). Its critical role is supported by the extensive anatomical connections between the ACC and lateral prefrotal association and motor cortices, spinal cord and limbic structures, allowing it to integrate top–down modulatory effects within a goal-directed context (Barbas 2000; Devinsky et al. 1995). However, the nature of the ACC involvement and the relative contributions of other areas are under continued debate. Prominent "monitoring" accounts propose that the ACC's primary role is to detect conflict and engage the lateral prefrontal cortex (PFC) to implement executive control (Botvinick et al. 2001; Kerns et al. 2004), whereas other evidence additionally supports a more direct regulatory role of the ACC in implementing motor control (Johnston et al. 2007; Nachev 2006; Roelofs et al. 2006). The ACC is also engaged on error trials, consistent with the view that performance monitoring represents a form of conflict detection (Carter and van Veen 2007). Since the lateral PFC also contributes to error processing and post-error adjustments (Danielmeier and Ullsperger 2011; Gehring and Knight 2000), the issue of the functional specialization of these principal prefrontal nodes remains unresolved. One possible means of clarifying their relative contributions is to record brain activity during conflict conditions and error processing as a function of pharmacological manipulation in the form of acute alcohol intoxication.
Even though neuroimaging evidence of acute intoxication effects on cognitive control is limited, studies using the Stroop, Eriksen flanker and Go/Nogo tasks indicate that alcohol intoxication attenuates activity in frontoparietal areas during high-conflict and error trials, most prominently in the ACC (Anderson et al. 2011; Kovacevic et al. 2012; Marinkovic et al. in press; Marinkovic et al. 2012). These findings suggest that cognitive control functions are vulnerable to acute intoxication. However, all of these studies used manual responses and probed cognitive control by introducing conflict between learned, arbitrary response contingencies. It is not known whether alcohol intoxication would exert comparable effects on cognitive control of responses that are reflexive and executed with a different motor system. Saccades are fast eye movements that help fixate and track visual stimuli in order to maintain accurate foveal image. At the cortical level, saccadic preparation and generation rely on the frontoparietal network, whereas the ACC and the lateral PFC subserve top–down inhibition (Johnston and Everling 2008; McDowell et al. 2008; Pierrot-Deseilligny et al. 2004). The antisaccade task (Hallett 1978) probes the ability to inhibit prepotent, visually guided saccades (prosaccades [PS]) and generate antisaccades (AS), eye movements toward a mirror-symmetrical position in the visual field. This conflict between the stimulus-driven PS and the volitional AS responses is reflected in more prominent activity to the AS compared to PS condition and is taken to represent saccadic inhibition in neuroimaging studies (Manoach et al. 2007; McDowell et al. 2008; Sweeney et al. 2007). Saccadic control deficits resulting from lesions of the ACC and lateral PFC confirm their role in saccadic inhibition (Gaymard et al. 1998; Milea et al. 2003; Pierrot-Deseilligny et al. 2005).
The main aim of the present study was to examine the effects of moderate alcohol intoxication on inhibitory control of eye movements and error processing during the AS task in a within-subject placebo-controlled design. Analysis of the BOLD (blood oxygenation level-dependent) activity pattern can reveal whether the effects of alcohol are selective for the oculomotor areas (e.g., frontal [FEF] and supplementary eye fields [SEF], superior parietal lobule [SPL]), top–down executive areas (e.g., ACC, PFC), or are generalized across cortical areas as a function of saccadic inhibition, error processing, and post-error adjustments. The BOLD signal depends on hemodynamic regulation and reflects neural activity only indirectly via neurovascular coupling (Buxton 2002). This presents a caveat concerning pharmacological manipulations since the BOLD magnitude differences may be partially confounded by vascular changes under alcohol (Iannetti and Wise 2007). In an effort to quantify regional cerebral blood flow (CBF) changes, we have employed a resting arterial spin labeling (ASL) scan at each scanning session. Alcohol-induced CBF changes have been described in detail elsewhere (Rickenbacher et al. 2011), and were used as covariates in the present study.
Methods
Participants
Twenty-two right-handed healthy volunteers (11 females; age (mean ± SD) = 24.8±2.5 years) participated in both alcohol and placebo sessions in a counterbalanced design, serving as their own controls. None reported any health-related problems, and none were taking any medications at the time of the study. They reported light-to-moderate alcohol use (Cahalan et al. 1969), drinking 1.8±1.0 times per week, 2.2±0.7 drinks per occasion. Subjects reported no alcoholism-related symptoms on Short Michigan Alcoholism Screening Test (SMAST; Selzer et al. 1975) and were negative for family history of alcoholism and drug abuse. All subjects gave written informed consent approved by the Human Research Committee at Massachusetts General Hospital and the Partners Healthcare Network before participating in the study. The study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki.
Task
The Antisaccade Task requires inhibition of automatic behavior on AS trials, instructing participants to look in the opposite direction from where the stimulus appears (Hallett 1978). Each 4-s trial began with a trial-type cue (Fig. 1) flanked by two dots with 10° eccentricity and was replaced by a target (Lee et al. 2011; Manoach et al. 2007). The target was presented either to the left or to the right for 1,000 ms as participants tracked and fixated it on PS trials, or looked at the dot in the opposite direction on AS trials. In the beginning of each session, the participants practiced to make saccades as quickly as possible without compromising accuracy. Stimulus presentation was synchronized with the scanner using the Presentation software (Neurobehavioral Systems). Triggers indicating trial type and saccadic direction were sent to the eye tracker system concurrently with each stimulus presentation. Subjects performed 192 trials of each type during six runs lasting 5.5 min each. Eye movements were recorded continuously during the task and analyzed offline. Fixation trials lasting 2–6 s were randomly interspersed in an event-related sequence providing temporal jitter for optimal deconvolution of the BOLD signal (Burock et al. 1998).
Fig. 1.
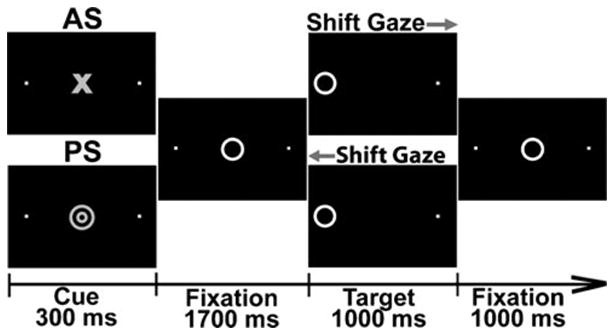
Saccadic paradigm included two types of trials. A cue was presented in the beginning of each trial for 300 ms. Red concentric circles indicate a pro-saccade (PS) trial and a blue circle signaled the anti-saccade (AS) contingency. The cue was replaced by a fixation target in the central location for 1,700 ms. The target moved either to the left or to the right (10° eccentricity) for 1,000 ms and the participants tracked it on PS trials or looked in the opposite direction on AS trials. The PS and AS trials were equiprobable, balanced for direction and were presented in a randomized order. The cue type was switched for half of the participants
Experimental design and procedure
Prior to the experiment, participants provided detailed information about their medical status, handedness, family history of alcoholism, level of response to alcohol, alcohol consumption, alcoholism-related symptoms, and filled out questionnaires probing disinhibitory and novelty seeking traits (please see Supplementary Material for details). Subjects served as their own controls by participating in both alcohol and placebo sessions in a counterbalanced order. The two sessions were scheduled 31±22 days apart on average. Upon their arrival to the laboratory, participants were asked about their compliance with the requirement to abstain from food for 3 h and from alcohol for at least 48 h before each session. All participants provided urine samples for the purpose of administering a five-drug panel test (Medimpex Inc.), and female subjects were additionally tested for pregnancy. All drug and pregnancy tests were negative. In an effort to hold constant possible hormonal influences on the imaging data (Goldstein et al. 2005), we endeavored to schedule women's scans at the same phase of the menstrual cycle based on self-reports of cycle onset days. Urine tests of luteinizing hormone (Medipmex, Inc.) were consistent with self-reports and confirmed that none of the scans were scheduled during the midcycle hormonal surge.
Breath alcohol concentration (BrAC) was measured with a breathalyzer (Draeger, Inc.) on several occasions when the subject was outside the scanner, and Q.E.D. Saliva Alcohol Test (OraSure Techn, Inc.) was used during the actual scans. Participants rated their moods and feelings with the adapted Biphasic Alcohol Effects Scale (BAES; Martin et al. 1993) three times during each session: prior to drinking, on the ascending BrAC limb, and after the scan, on the descending BrAC limb. Beverage was administered as cocktail containing vodka (Grey Goose, Bacardi), 20 % v/v and orange juice (Kovacevic et al. 2012; Marinkovic et al. 2012; Rickenbacher et al. 2011) and consumed within 10 min. Alcohol beverage contained 0.60 g/kg of ethanol for men and 0.55 g/kg for women to adjust for body mass index differences (Friel et al. 1999). Placebo beverage contained the same volume of orange juice. The task was administered 46±6 min after drinking. The average BrAC measured before the task was 0.045±0.012 % and 0.043±0.013 % following the task, suggesting that the task was administered at or near the BrAC peak. Upon completion of each experimental session, participants filled out a questionnaire asking them about various details of their experience such as perceived task difficulty, beverage dose, and intoxication self-ratings.
Data acquisition and analysis
Eye movement data
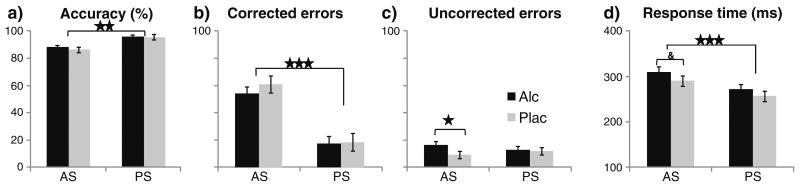
Eye movements were monitored and recorded with an infrared pupil/corneal reflection tracker system RK-826PCI (ISCAN, Inc., Woburn, MA) specifically designed for use in the MRI environment. Eye movements were tracked at 120 Hz during the functional scans and were analyzed offline with a semiautomatic MATLAB program (Mathworks, Natick, MA). Details of data acquisition and analysis are available in the Supplementary Material, and a sample tracing of the PS and AS trials is shown in Fig. S1. Performance accuracy and saccadic reaction time (SRT) were analyzed for all responses. The overall BOLD analysis includes only correct trials. Erroneous responses were mostly self-corrected, but when a saccade was performed in a wrong direction with no evidence of self-correction, they were classified as "uncorrected" (Fig. 2).
Fig. 2.
Performance measures (means ± SEM) reflected in accuracy, error rates (calculated as % of total errors), and SRTs. The overall accuracy was high (a), but participants were more accurate on visually guided PS than on AS trials involving saccadic conflict. b On most error trials, the initial reflexive glance towards the target was immediately corrected. c The saccadic errors that remained uncorrected were more frequent under alcohol than placebo on AS trials. d Saccadic RTs were slower on AS overall, with a strong trend to respond more slowly on AS trials when intoxicated. &p<0.1; *p<0.05; **p<0.001; ***p<0.0001
Imaging data
Functional and structural brain images were acquired with a 3-T whole-body scanner (Siemens, Germany) fitted with a 12-channel head coil at the Martinos Center in Boston. Special care was taken to minimize head motions with the use of padding and head positioning device. Imaging data comprised structural scans (Supplementary Material) and a series of whole-brain BOLD images collected with a T2*-weighted EPI sequence of 30 interleaved consecutive 5-mm slices in AC-PC orientation (TR=2 s, TE=30 ms, flip angle = 90°, FOV=200 mm, 64×64 matrix, 3.13×3.13 mm in-plane resolution). Brain images were analyzed with FreeSurfer and FS-FAST package (Burock and Dale 2000; Dale et al. 1999; Fischl et al. 1999a). Each subject's cortical surface was reconstructed and registered with a canonical surface for group averaging (Fischl et al. 1999b). After motion correction, smoothing and normalization, the data were analyzed with the finite impulse-response (FIR) model. Motion did not exceed 2 mm for any dataset, but motion parameters were entered into the model as regressors. Correct trials were averaged for each condition vs. fixation contrast, as well as for conflict-specific activity (i.e., AS vs. PS contrast). Random effects statistical model resulted in F-distributed group activation maps (Fischl et al. 1999b). Possible baseline shifts were removed by subtracting the average baseline HDR from the HDR waveform for both placebo and alcohol conditions, thus equating them at baseline. Voxelwise group-average maps are presented in Fig. 3. Effects of gender, beverage, and stimulus type were further explored with a region-of-interest (ROI) analysis. The ROIs were defined on a group average using the unbiased orthogonal contrast and included the voxels active at p<0.00001 at the activation peak (4–8 s). The ROI placement was blind to each individual's activity pattern as the ROIs were automatically transferred from the average onto each individual cortical surface. Percent signal changes from baseline were computed for each ROI and each subject, session and condition and presented in the form of timecourses that were also baseline normalized (Fig. 4) and used in a mixed-design analysis of variance (ANOVA) with gender as a between-group factor. Condition (AS, PS), beverage (alcohol, placebo), and gaze direction (left, right) were within-subject factors (Woodward et al. 1990).
Fig. 3.
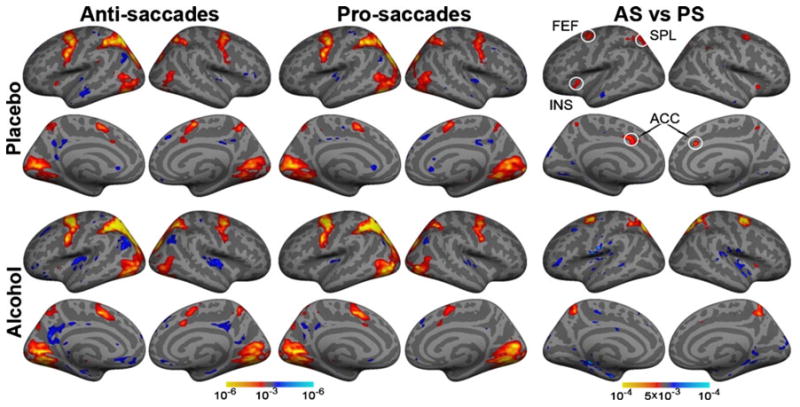
Group average statistical maps obtained with the random effects analysis of the overall activity (at 4–8 s latency) to antisaccades (AS) and prosaccades (PS) compared to fixation baseline. Conflict-specific activity (i.e., AS vs. PS difference) maps are shown in the rightmost columns. The maps are displayed on the inflated lateral and medial cortical surfaces for both hemispheres, for placebo and alcohol conditions. The observed pattern of the overall saccade-induced activity replicates other evidence and the effects of saccadic conflict are most prominent in frontal eye fields (FEF), superior parietal lobule (SPL), insula (INS), and anterior cingulate cortex (ACC). Alcohol-induced attenuation is observed only in the ACC during saccadic conflict, indicating vulnerability of cognitive control.
Fig. 4.
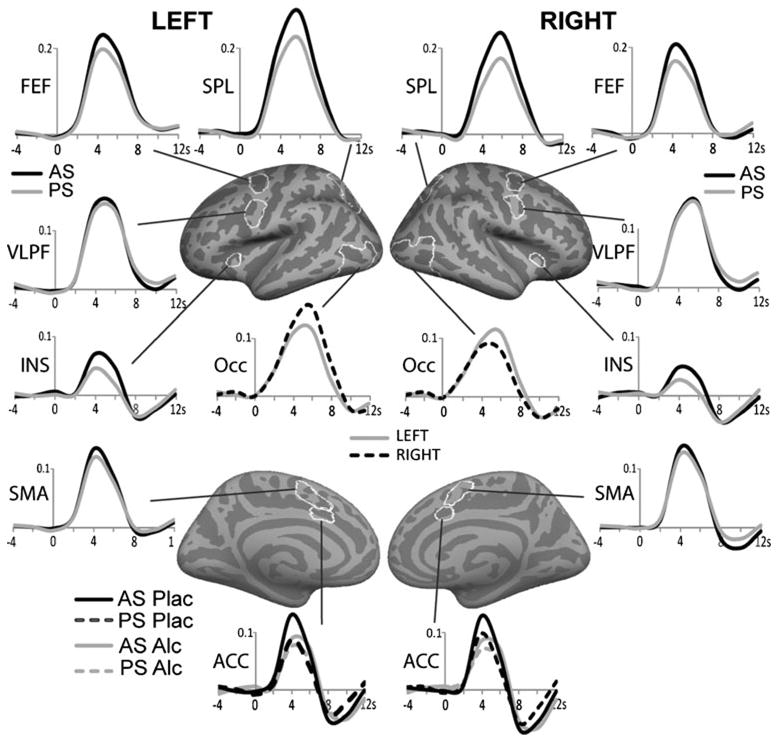
Group average timecourses (% signal change) of the BOLD signal for the effects of saccadic conflict (AS vs. PS), gaze direction (left vs. right) and alcohol. The ROIs (marked in white on the cortical surface) were based on group average voxelwise analysis of an unbiased orthogonal contrast and were baseline normalized. As shown in Table 1, saccadic conflict elicited activity in FEF, SPL, INS and marginally in SMA, but not in VLPFC. Effect of saccadic direction (left vs. right) was significant in the Occ. The only significant effect of intoxication was observed in dorsal ACC as alcohol selectively attenuated activity on AS trials. FEF frontal eye field, SPL superior parietal lobule, INS insula, SMA supplementary motor area, VLPFC ventrolateral prefrontal cortex, Occ occipital cortex, ACC anterior cingulate cortex
Error analysis
Error-related activity was investigated in 12 participants (five males) who committed >20 errors, 38±23 on average. Error trials were matched with correct trials belonging to the same AS or PS trial type and saccadic direction within each run. The matched correct trials either preceded or followed error trials evenly but were separated by intervening trials. The numbers were equated across beverage conditions to avoid potential bias. Results of the voxelwise random effects group analysis are shown in Fig. 5. Baseline-normalized percent signal change values were analyzed using a within-subject ANOVA for the factors of error (error vs. correct) and beverage (alcohol vs. placebo).
Fig. 5.
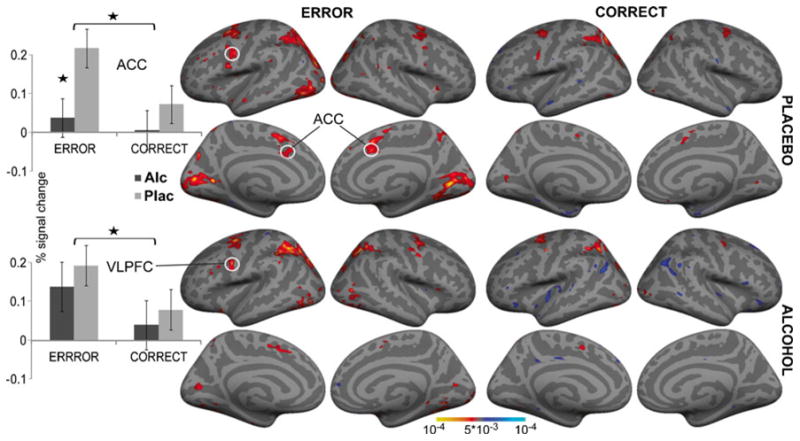
Group average voxelwise random effects analysis of the activity on erroneous and correct trials compared to fixation. Significant effects of error processing were observed only in dorsal anterior cingulate cortex (ACC) and ventrolateral prefrontal cortex (VLPFC). Histograms on the left show group average % signal change (±SEM) from baseline for the two ROIs as a function of beverage and trial type. Significant attenuation of error-related activity under intoxication is observed only in the ACC, *p<0.05
Blood flow quantification — ASL
In an attempt to mitigate possible vasoactive effects of alcohol on the BOLD signal, ASL was used to quantify resting perfusion in each session. Details of the methods and results of the ASL analysis have been reported separately (Rickenbacher et al. 2011), and a description is included as Supplementary Material. The ASL data were analyzed with the FreeSurfer analysis package for 18 participants with complete sets of resting ASL and BOLD scans. In an effort to partial out potential vascular influences, regional perfusion measures were used as covariates in the BOLD analysis of beverage effects on the ACC since the ACC was the only area showing a significant effect of beverage on the BOLD signal (Table 1).
Table 1. Summary of statistical results for ROIs including their Talairach coordinates and significance of the main effects, interactions, and saccadic conflict for alcohol and placebo.
| Area | Talairach | m.e. saccade | sacc. x bev. | AS-PS Plac | AS-PS Alc | m.e. direct. |
|---|---|---|---|---|---|---|
| L. ACC | −9 9 45 | 13.9 *** | 6.4 * | 25.0 *** | 2.1 | 0.1 |
| L. FEF | −28 -3 41 | 18.0 *** | 0.0 | 9.8 ** | 14.7 *** | 2.1 |
| L. SPL | −22 -58 47 | 21.3 *** | 0.13 | 11.8 ** | 13.5 ** | 7.7 ** |
| L. INS | −28 21 1 | 13.9 ** | 1.4 | 12.1 ** | 6.0 * | 0.0 |
| L. VLPFC | −51 1 30 | 1.8 | 0.7 | 2.0 | 0.3 | 0.9 |
| L. SMA | −11 14 30 | 4.5 * | 0.0 | 2.4 | 4.3 * | 0.6 |
| L. OCC | −43 -71 -3 | 1.0 | 0.0 | 0.9 | 0.5 | 31.0 *** |
| R. ACC | 12 13 32 | 11.03 ** | 4.0 & | 22.8 *** | 1.7 | 0.9 |
| R. FEF | 27 -4 41 | 28.5 *** | 1.0 | 11.4 ** | 25.5 *** | 0.1 |
| R. SPL | 20 -57 50 | 24.9 *** | 1.9 | 9.1 ** | 22.5 ** | 2.2 |
| R. INS | 31 19 0 | 12.6 ** | 0.2 | 8.5 ** | 8.3 ** | 0.0 |
| R. VLPFC | 54 1 34 | 0.2 | 0 | 0.1 | 0 | 0.2 |
| R. SMA | 8 6 49 | 1.3 | 0.4 | 1.5 | 0.3 | 0.2 |
| R. OCC | 42 -76 1 | 0.0 | 0.1 | 0.1 | 0.0 | 7.8 * |
Included are F-values for the main effects (m.e.) of the saccade and eye movement direction factors, saccade x beverage interaction, and AS-PS comparisons for alcohol and placebo, with p-values marked as follows:
p<0.1;
p<0.05;
p<0.01;
p<0.001
ACC anterior cingulate cortex, FEF frontal eye field, SPL superior parietal lobule, INS insula, VLPFC ventrolateral prefrontal cortex, SMA supplementary motor area, Occ occipital cortex
Behavioral results
Performance
Performance accuracy was significantly higher on PS trials (mean ± SD, 95±3.3 %) than on AS trials (87.1±12.5 %; F1,20=13.6, p<0.001), but it was not affected by alcohol (Fig. 2a). Most of the erroneous saccades were self-corrected under both beverage conditions, particularly on conflict AS compared to PS trials, F1,20=42.2, p<0.0001 (Fig. 2b). However, the saccadic response errors that remained uncorrected were more frequent under alcohol than placebo on AS trials F1,20=6.0, p<0.05 (Fig. 2c). As expected, saccadic RTs (Fig. 2d) were significantly slower on AS (300±53 ms) than on PS trials (264±54 ms), (F1,20=68.4, p<0.0001). The main effect of alcohol was marginally significant (F1,20=3.1, p<0.1), resulting from a strong trend to respond more slowly on AS trials when intoxicated (F1,20=3.8, p<0.06). These effects of alcohol on AS latency are subtle and consistent with other evidence (Blekher et al. 2002; Vorstius et al. 2008). No gender effects were observed on any of the performance measures.
BAES ratings
BAES scores were analyzed with a mixed-model ANOVA with gender as a between-group factor and beverage and phase (ascending BrAC and descending BrAC) relative to baseline as within-subject factors for the "stimulation" and "sedation" subscales. Overall, participants felt less stimulated (F1,20=26.7, p<0.0001) and more sedated (F1,20=17.4, p<0.001) at the end of the scan as compared to the baseline ratings obtained before drinking. Gender × Beverage × Phase interaction (F2,40=4.4, p<0.05) on the Stimulation scale was a result of men reporting more "stimulated" on the ascending BAL when intoxicated as compared to placebo (F1,20=6.4, p<0.05). Men's ratings of "stimulation" were also significantly higher on the ascending BAL compared to descending BAL under alcohol (F1,20=27.8, p<0.0001). Participants reported feeling more "high" under alcohol than placebo, (F1,20=7.0, p<0.01).
Post-experimental questionnaire
Likert scales ranging from 1 (not at all) to 5 (very much) were used for ratings. The task was rated as being quite easy under placebo (1.9±0.9), but alcohol intoxication rendered it somewhat more difficult (2.3±0.8; F1,20=6.6, p<0.05). Participants reported being moderately intoxicated under alcohol (2.7±0.7) and not at all under placebo (1.0±0.2). They were able to discriminate between the two beverages, estimating that they were given 2.1±0.6 "alcohol drinks" in an active dose vs. 0.1±0.4 under placebo (more details in Supplementary Material).
Neuroimaging results
Voxelwise group averages (Fig. 3) show that eye movements elicited activity in a distributed frontoparietal cortical network and occipital areas in agreement with previous studies (Ford et al. 2005; McDowell et al. 2008). Saccadic conflict (AS–PS contrast) is reflected in stronger activity to AS in FEF, SPL, insula (INS) and precuneus overall (Fig. 3). Beverage effects on AS vs. PS contrast were observed only in the ACC as it was activated by saccadic conflict under placebo but not under alcohol, indicating selective effects of alcohol on top–down functions. Additional confirmatory analyses were performed on the timecourses of the activity within ROIs (Fig. 4). Saccadic conflict resulted in stronger activity on the AS trials in FEF, SPL, INS, ACC and marginally in SMA bilaterally, as evidenced by a significant main effect of condition type, listed in Table 1. The only exception was the medial occipital area where the PS elicited stronger activity than the AS bilaterally, left, F1,21=4.8, p<0.05, and right, F1,21=4.9, p<0.05, consistent with stronger visual input on PS trials as the target is tracked (Dyckman et al. 2007). Effects of alcohol were observed only in the ACC, as the factor of beverage interacted with the saccade type on the left (F1,21=6.4, p<0.05) and with a strong trend on the right (F1,21=4.0, p<0.06). Whereas the high-conflict AS trials evoked stronger activity than PS under placebo both in the left (F1,21=25.0, p<0.0001) and the right ACC (F1,21=22.8, p<0.001), there was no difference between the AS and PS under alcohol (Table 1). These effects held up when the ASL-based measures of CBF in ACC were used as covariates for the subset of 18 participants for whom the ASL data were available. AS–PS difference remained significant under placebo both on the left (F1,16=5.6, p<0.05) and on the right (F1,16=5.0, p<0.05). Under alcohol, the ACC activity did not differ between the AS and PS (F<0.8). A main effect of saccadic direction with stronger contralateral activity was observed in the occipital cortex both on the left (F1,21=31.0, p<0.001) and on the right (F1,21=7.8, p<0.05), consistent with other evidence (McDowell et al. 2005). Greater contra-lateral activation was also evident in the left SPL overall (F1,21=7.7, p<0.01) and in the right SPL for the AS (F1,21=4.7, p<0.05). There were no effects of gender.
Error analysis
Saccadic RTs to errors were compared to SRTs on matched correct trials as a function of beverage. Significant interaction of beverage and trial type (F1,11=4.9, p<0.05) resulted from shorter SRTs to errors under placebo (249±61 ms) than alcohol (294±43 ms). Voxelwise group averages (Fig. 5) show that the activated network comprised the same predominantly frontoparietal areas as in the main analysis, justifying the use of the same ROIs as described above. Repeated-measures ANOVA indicated that only two areas were sensitive to errors. Errors elicited greater activity than correct trials (F1,11=5.6, p<0.05), which was significantly attenuated by alcohol (F1,11=7.6, p<0.05). The ACC activity to errors correlated negatively with the psychoticism (P) scale of Eysenck's Personality Questionnaire (EPQ) (Eysenck and Eysenck 1975) under alcohol (r=−0.78, p<0.01), but not placebo (r=0.19, n.s.), indicating that individuals with higher P scores are less likely to engage the ACC during performance monitoring. Moreover, the EPQ-P scores correlated with the number of weekly drinking occasions (r=0.56, p<0.01). Given that impulsivity is an important aspect of Eysenck's P scale (O'Boyle and Barratt 1993), this observation indicates that acute intoxication and consumption levels interact with impulsivity and performance monitoring. The only other area sensitive to errors was ventrolateral prefrontal cortex (VLPFC) with stronger activity on error compared to correct trials (F1,11=5.6, p<0.05). Alcohol had no effect on the VLPF activity.
In order to examine the effects of beverage on post-error slowing that is observed in tasks with relatively infrequent errors (Jentzsch and Dudschig 2009), we compared saccadic RTs on the post-error vs. post-correct trials. SRTs were longer on trials following errors (293±79 ms) than following correct responses (272±51 ms; F1,11=9.9, p<0.01). The observed post-error slowing did not interact with alcohol.
Discussion
The most notable finding of the present study is alcohol-induced decrease in dorsal ACC activity during saccadic conflict. Moderate alcohol intoxication selectively attenuated activity in the ACC to volitional AS that engage the capacity to inhibit prepotent, visually guided PS. The activity to PS was unaffected by intoxication, suggesting that alcohol affects inhibitory influences and not saccade execution. In addition, alcohol reduced the ACC activity to erroneous responses. There were no significant effects of acute alcohol intoxication on any of the other cortical areas involved in the generation of volitional saccades or error processing, indicating impairment of those aspects of cognitive control that are subserved by the ACC. These findings are compatible with our previous observation of selective alcohol-induced ACC impairment during the Stroop conflict (Marinkovic et al. 2012). The Antisaccade and the Stroop tasks rely on different effector systems and engage the oculomotor and manual motor systems, respectively. Furthermore, sac-cadic responses are hardwired and reflexive, whereas button-press manual responses are arbitrary and learned through instruction. Nevertheless, both tasks probe cognitive control functions by interfering with dominant stimulus–response mapping. Conflict-specific activity during both tasks is observed in distributed frontoparietal cortical areas, confirming previous reports (Laird et al. 2005; McDowell et al. 2008). In both studies, the BOLD activity was reduced by alcohol only in the ACC during conflict and error processing across response modalities, suggesting that alcohol primarily affects high-level cognitive control (Kovacevic et al. 2012; Marinkovic et al. 2012). However, further studies are needed to examine regionally specific effects of a range of alcohol doses and other tasks probing executive functions. The current study has employed the ASL scan and used perfusion values as covariates in the analysis of the BOLD signal. The alcohol-induced attenuation of the BOLD signal in the ACC remained significant after the changes in cerebral perfusion were partialled out, lending confidence to the observed effects of intoxication. Future studies can additionally use the perfusion values as regressors in a voxelwise analysis of the entire brain.
Behavioral studies confirm that alcohol impairs the ability to inhibit prepotent responses as it increases commission errors on different inhibitory tasks (de Wit et al. 2000; Fillmore and Vogel-Sprott 1999). Alcohol-induced response disinhibition and premature motor preparation are correlated with personality traits indexing impulsivity (Dougherty et al. 1999; Marinkovic et al. 2000). The present neuroimaging evidence extends these findings by indicating that the ACC may be the principal substrate of this alcohol-induced impairment. Indeed, the ACC activity to errors correlates negatively with EPQ-P score under alcohol, suggesting that intoxication impairs the error monitoring function especially in more impulsive individuals. Deficient self-regulation is considered to be fundamental to the ability to refrain from drinking (Field et al. 2010; Lyvers 2000), underlying the importance of these findings. Alcohol-induced ACC impairment may render the inebriated person less capable of exerting cognitive control (Zhao et al. 2012), resulting in disinhibition, dysfunctional goal-oriented behavior, etc. Indeed, numerous studies have shown that dorsal ACC is important for making decisions and guiding behavior in accordance with goals and intentions, which is the essence of top–down cognitive control (Kennerley et al. 2006; Walton et al. 2007).
In the present study, the ACC was sensitive to saccadic conflict and to error processing, in agreement with other functional magnetic resonance imaging (fMRI) studies (Ford et al. 2005) and the idea that the same neural process may underlie both error- and conflict monitoring (Carter and van Veen 2007; Mathalon et al. 2003). Alcohol attenuated the ACC activity during both, conflict AS trials and erroneous responses, consistent with previously reported sensitivity of the error monitoring system to alcohol (Ridderinkhof et al. 2002). The only other area showing increased activity to errors in the present study was the VLPFC, indicating that it may selectively contribute to error monitoring and possibly to post-error adjustments. Interestingly, this cortical region was not sensitive to saccadic conflict (Fig. 4), since the BOLD signal to AS did not differ from the activity to PS trials (Table 1). Furthermore, alcohol intoxication did not exert significant effects on the VLPFC activity either on correct trials or errors (Fig. 5). Differential sensitivity of the ACC and the VLPFC to the effects of conflict, alcohol intoxication and errors, suggests a functional dissociation between these two areas. The proposed VLPFC role in updating of task representations (Brass et al. 2005) is consistent with its contributions to working memory and attention (Corbetta and Shulman 2002), inhibitory control (Chikazoe et al. 2007), and response selection (Badre and Wagner 2007). In addition to maintaining response rules and implementing control, it may monitor for errors, supplying that information to the ACC (Gehring and Knight 2000). Since in the present study alcohol did not significantly affect the VLPFC activity, it is possible that it compensates for the alcohol-induced impairment of the ACC. In our recent study, the VLPFC was the only area showing relatively greater activity under alcohol than placebo during conflict evoked by Eriksen flanker task (Marinkovic et al. in press), in agreement with the compensatory hypothesis based on the evidence obtained in chronic alcoholics (Oscar-Berman and Marinkovic 2007; Pfefferbaum et al. 2001). On this view, cognitive control inclusive of performance monitoring and control is subserved by an interactive mediolateral network. The ACC role might be to monitor for conflict both in the early and late processing stages (Kovacevic et al. 2012; Silton et al. 2010), and to contribute to suppression of unwanted responses (Braver et al. 2001). As an essential part of this interactive circuitry, the VLPFC may monitor performance by maintaining access to task representations via working memory and attentional bias (Miller and Cohen 2001; Petrides 2005). It may also subserve post-error adjustments. In the present study, post-error slowing was not affected by alcohol, consistent with its resilience to pharmacological manipulations (Marinkovic et al. 2012; Riba et al. 2005). Given that the VLPFC activity was sensitive to errors but was not affected significantly by alcohol intoxication or even saccadic conflict, it is possible that error monitoring is carried out by the VLPFC, as suggested by other evidence (Kerns et al. 2004).
Numerous studies using the AS paradigm indicate that the ACC is recruited during the preparatory stage and is sensitive to cognitive demands (Brown et al. 2007; Johnston et al. 2007) which may be mediated by inhibitory projections to the superior colliculus (SC), suppressing its activity on AS trials (Munoz and Everling 2004). Human lesion studies show that ACC damage impairs suppression of reflexive saccades (Milea et al. 2003). Other frontoparietal areas are sensitive to saccadic conflict (Figs. 3 and 4) (McDowell et al. 2008) with the FEF controlling the AS (Connolly et al. 2002; Lee et al. 2011), SPL subserving saccadic execution (Brown et al. 2006), and SMA supporting saccadic motor programs (Curtis and D'Esposito 2003).
In conclusion, the present results support previous evidence suggesting that the ACC is essential for recruiting cognitive control during saccadic conflict and erroneous responses. More importantly, the ACC activity to volitional AS and erroneous saccades was selectively attenuated by alcohol intoxication. Taken together with our previous analogous findings with the Stroop task (Marinkovic et al. 2012), this study provides converging evidence for the selective vulnerability of the ACC to moderate alcohol intoxication during conflict across different effector systems and cognitive control tasks. By attenuating the ACC contributions during controlled processing, alcohol impairs the capacity to inhibit automatic actions in favor of non-habitual, task-relevant responses. Alcohol-induced impairment of cognitive control may result in behavioral disin-hibition, predisposing individuals to continued heavy drinking.
Supplementary Material
Acknowledgments
This work was supported by funds from the National Institutes of Health (R01-AA016624 and P41RR14075) and Medical Investigation of Neurodevelopmental Disorders (MIND) Institute. Data were collected at the MGH/MIT/HMS Athinoula A. Martinos Center for Biomedical Imaging. Authors report no financial or other conflict of interest related to this work. We thank Simon Sigalovsky, Matija Zelic, Sarah Sheldon, Roya Bagheri, Lawrence Wald, and Dara Manoach for assistance.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-013-3173-y) contains supplementary material, which is available to authorized users.
Contributor Information
Ksenija Marinkovic, Email: xenia@ucsd.edu, Department of Radiology, University of California at San Diego, 9500 Gilman Drive MC 0841, La Jolla, CA 92093-0841, USA.
Elizabeth Rickenbacher, Champalimaud Neuroscience Programme, Chapmalimaud Center for the Unknown, Lisbon, Portugal.
Sheeva Azma, Interdisciplinary Program in Neuroscience, Georgetown University, Washington, DC, USA.
Elinor Artsy, Department of Neurology, Cedars-Sinai Medical Group, Los Angeles, CA, USA.
Adrian K. C. Lee, Department of Speech and Hearing Sciences and Institute for Learning and Brain Sciences, University of Washington, Seattle, WA, USA
References
- Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, Pearlson GD. Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res. 2011;35:156–165. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Blekher T, Beard JD, O'Connor S, Orr WE, Ramchandani VA, Miller K, Yee RD, Li TK. Response of saccadic eye movements to alcohol in African American and non-Hispanic white college students. Alcohol Clin Exp Res. 2002;26:232–238. [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brown MR, Goltz HC, Vilis T, Ford KA, Everling S. Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. NeuroImage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brown MR, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. J Neurophysiol. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB. Introduction to Functional Magnetic Resonance Imaging. Cambridge University Press; New York, NY: 2002. [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. Monograph #6. Rutgers Center of Alcohol Studies; New Brunswick, NJ: 1969. American drinking practices: a national study of drinking behavior and attitudes. [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003;15:409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Ullsperger M. Post-error adjustments. Front Psychol. 2011;2:233. doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of D-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Moeller FG, Steinberg JL, Marsh DM, Hines SE, Bjork JM. Alcohol increases commission error rates for a continuous performance test. Alcohol Clin Exp Res. 1999;23:1342–1351. [PubMed] [Google Scholar]
- Dyckman KA, Camchong J, Clementz BA, McDowell JE. An effect of context on saccade-related behavior and brain activity. NeuroImage. 2007;36:774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. Hodder & Staughton; London: 1975. [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol Clin Exp Res. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychopharmacol. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. J Neurophysiol. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Friel PN, Logan BK, O'Malley D, Baer JS. Development of dosing guidelines for reaching selected target breath alcohol concentrations. J Stud Alcohol. 1999;60:555–565. doi: 10.15288/jsa.1999.60.555. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Rivaud S, Cassarini JF, Dubard T, Rancurel G, Agid Y, Pierrot-Deseilligny C. Effects of anterior cingulate cortex lesions on ocular saccades in humans. Exp Brain Res. 1998;120:173–183. doi: 10.1007/s002210050391. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal–cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging. 2007;25:978–988. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Jentzsch I, Dudschig C. Why do we slow down after an error? Mechanisms underlying the effects of posterror slowing. Q J Exp Psychol (Colchester) 2009;62:209–218. doi: 10.1080/17470210802240655. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Neurophysiology and neuroanatomy of reflexive and voluntary saccades in non-human primates. Brain Cogn. 2008;68:271–283. doi: 10.1016/j.bandc.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S. Top–down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, Marinkovic K. Theta oscillations are sensitive to both early and late conflict processing stages: effects of alcohol intoxication. PLoS One. 2012;7:e43957. doi: 10.1371/journal.pone.0043957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Hamalainen MS, Dyckman KA, Barton JJ, Manoach DS. Saccadic preparation in the frontal eye field is modulated by distinct trial history effects as revealed by magnetoencephalog-raphy. Cereb Cortex. 2011;21:245–253. doi: 10.1093/cercor/bhq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers M. "Loss of control" in alcoholism and drug addiction: a neuroscientific interpretation. Exp Clin Psychopharmacol. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Cain MS, Polli FE, Edelman JA, Fischl B, Barton JJ. Neural activity is modulated by trial history: a functional magnetic resonance imaging study of the effects of a previous antisaccade. J Neurosci. 2007;27:1791–1798. doi: 10.1523/JNEUROSCI.3662-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E, Klopp J, Maltzman I. Alcohol effects on movement-related potentials: a measure of impulsivity? J Stud Alcohol. 2000;61:24–31. doi: 10.15288/jsa.2000.61.24. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S. Effects of alcohol intoxication on response conflict in a flanker task. Journal of Addiction Research & Therapy. doi: 10.4172/2155-6105.S3-002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E. Acute alcohol intoxication impairs top–down regulation of Stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Hum Brain Mapp. 2012;33:319–333. doi: 10.1002/hbm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biol Psychol. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68:255–270. doi: 10.1016/j.bandc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JE, Kissler JM, Berg P, Dyckman KA, Gao Y, Rockstroh B, Clementz BA. Electroencephalography/magnetoencephalography study of cortical activities preceding prosaccades and antisaccades. Neuroreport. 2005;16:663–668. doi: 10.1097/00001756-200505120-00002. [DOI] [PubMed] [Google Scholar]
- Milea D, Lehericy S, Rivaud-Pechoux S, Duffau H, Lobel E, Capelle L, Marsault C, Berthoz A, Pierrot-Deseilligny C. Antisaccade deficit after anterior cingulate cortex resection. Neuroreport. 2003;14:283–287. doi: 10.1097/00001756-200302100-00026. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nachev P. Cognition and medial frontal cortex in health and disease. Curr Opin Neurol. 2006;19:586–592. doi: 10.1097/01.wco.0000247609.36482.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Boyle M, Barratt ES. Impulsivity and DSM-III-R personality disorders. Pers Indiv Differ. 1993;14:3. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. NeuroImage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17:17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Nyffeler T, Milea D. The role of the human dorsolateral prefrontal cortex in ocular motor behavior. Ann N Y Acad Sci. 2005;1039:239–251. doi: 10.1196/annals.1325.023. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodriguez-Fornells A, Munte TF, Barbanoj MJ. A neu-rophysiological study of the detrimental effects of alprazolam on human action monitoring. Brain Res Cogn Brain Res. 2005;25:554–565. doi: 10.1016/j.cogbrainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Rickenbacher E, Greve DN, Azma S, Pfeuffer J, Marinkovic K. Effects of alcohol intoxication and gender on cerebral perfusion: an arterial spin labeling study. Alcohol. 2011;45:725–737. doi: 10.1016/j.alcohol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GP. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Roelofs A, van Turennout M, Coles MG. Anterior cingulate cortex activity can be independent of response conflict in Stroop-like tasks. Proc Natl Acad Sci U S A. 2006;103:13884–13889. doi: 10.1073/pnas.0606265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, Van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Studies Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Silton RL, Heller W, Towers DN, Engels AS, Spielberg JM, Edgar JC, Sass SM, Stewart JL, Sutton BP, Banich MT, Miller GA. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top–down attentional control. NeuroImage. 2010;50:1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Keedy SK, McDowell JE, Clementz BA. fMRI studies of eye movement control: investigating the interaction of cognitive and sensorimotor brain systems. NeuroImage. 2007;36(Suppl 2):T54–T60. doi: 10.1016/j.neuroimage.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstius C, Radach R, Lang AR, Riccardi CJ. Specific visuomotor deficits due to alcohol intoxication: evidence from the pro- and antisaccade paradigms. Psychopharmacology (Berl) 2008;196:201–210. doi: 10.1007/s00213-007-0954-1. [DOI] [PubMed] [Google Scholar]
- Walton ME, Croxson PL, Behrens TE, Kennerley SW, Rushworth MF. Adaptive decision making and value in the anterior cingulate cortex. NeuroImage. 2007;36(Suppl 2):T142–T154. doi: 10.1016/j.neuroimage.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JA, Bonett DG, Brecht ML. Introduction to linear models and experimental design. Harcourt Brace Jovanovich; San Diego, CA: 1990. [Google Scholar]
- Zhao LY, Tian J, Wang W, Qin W, Shi J, Li Q, Yuan K, Dong MH, Yang WC, Wang YR, Sun LL, Lu L. The role of dorsal anterior cingulate cortex in the regulation of craving by reappraisal in smokers. PLoS One. 2012;7:e43598. doi: 10.1371/journal.pone.0043598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.