Abstract
The growing tumor employs various strategies to establish its growth, progression and spread in the host. Angiogenesis or formation of new blood vessels from existing ones and escape from immune surveillance are the two critical steps that ensure proper establishment and growth of the newly formed tumor. Thus understanding the novel pathways associated with tumor angiogenesis and immunity may lead to the development of newer therapeutic strategies using the regulators of these pathways to improve patient outcomes. These two pivotal steps in the process of tumorigenesis are governed by plethora of endogenous factors. The neuroendocrine molecules, which include the catecholamine neurotransmitters, dopamine, norepinephrine and epinephrine are of growing interest considering their varied and diverse regulatory roles both in the process of tumor angiogenesis and tumor immunity. This review focuses on the emerging roles of catecholamines in modulating tumor angiogenesis and immunity, and also discusses the probable molecular mechanisms of their actions. Understanding of this new group of endogenous regulators of tumor growth may lead to the development of newer therapeutic approaches for the treatment of cancer.
Keywords: Catecholamine, Tumor, Angiogenesis, Immunity
Introduction
Catecholamine (CA) neurotransmitters, dopamine (DA), norepinephrine (NE) and epinephrine (E), are biogenic amines derived from the amino acid tyrosine and possess a catechol group with an attached amino group (Ganong 2005; Goldstein et al. 2003). CA are synthesized both in the brain as well as in the peripheral organs and cells such as adrenal medulla, non neuronal gut cells, platelets and lymphocytes (Axelsson 1971; Basu et al. 1993, 1995a; Basu and Dasgupta 2000a; Sarkar et al. 2010; Bergquist et al. 1994; Eisenhofer et al. 1996, 1997; Ganong 2005; Goldstein et al. 2003). These molecules play vital physiological roles particularly in response to stress (Glaser and Kiecolt-Glaser 2005). CA also have profound effects in the brain, cardiovascular system and also regulate carbohydrate and fat metabolisms in the body (Barth et al. 2007; Havel 1968; Laverty 1978; Mayer 1974; Chakroborty et al. 2011). NE and E act through α (α1 and α2) and β (β1 and β2) adrenoceptors in target cells. α1 adrenoceptor mediates its functions by increasing the intracellular calcium level and α2 adrenoceptor downregulates adenylate cyclase and thus inhibits intracellular cyclic AMP. β1 and β2 adrenoceptors activate adenylate cyclase to increase intracellular cAMP (Robison et al. 1967; Schuller 2007; Thaker et al. 2007). DA acts through its D1 (D1 and D5) and D2 (D2, D3 and D4) classes of receptors present in target cells (Missale et al. 1998; Sarkar et al. 2010). The D1 class of receptors on activation increases intracellular cAMP, whereas the D2 class inhibits intracellular cAMP (Missale et al. 1998). The current review addresses the emergent roles of catecholamine neurotransmitters in two very important aspects of tumor growth, tumor angiogenesis and immunity, which will help to have a better understanding of the disease.
Angiogenesis and its importance in cancer
Angiogenesis , a physiological process by which new blood vessels are formed from the existing blood vessels is essential for the growth of the developing fetus as well as wound healing, tissue repair and reproduction in adults (Dvorak 2005), is a multistep process involving division and migration of endothelial cells, degradation of old and synthesis of new basement membrane, organization into tubes, and coverage by pericytes, each of which is intricately regulated at the molecular level by a set of pro and antiangiogenic molecules (Asahara et al. 1999; Battegay 1995; Folkman 2007). Deregulation at any step leads to disorders (Tandle and Libutti 2003). Cancer is the most common pathological condition where the fine balance between pro and antiangiogenic factors is lost resulting in uncontrolled angiogenesis with aberrant, non-uniformly distributed, irregularly branched blood vessels that lack a clear hierarchal arrangement (Folkman 2007; Tandle and Libutti 2003; Chakroborty et al. 2009, 2011). It is now well established that antiangiogenic therapy targeting the structural and functional abnormalities of tumor blood vessels is a promising feature of cancer therapy that can retard growth and progression of tumors (Jain 2005; Folkman 2007).
Catecholamines and tumor angiogenesis
The molecular determinants regulating the angiogenic cascade are subject of great interest for their potential to be developed as newer therapeutic targets to combat cancer (Folkman 2007). Therefore identifying and validating various endogenous molecules that control the process of angiogenesis in a tumor is of interest. Among the endogenous molecules that have been identified as potential targets for future therapies, CA are of recent interest owing to their distinct actions in the regulation of the angiogenic process (Basu et al. 2001, 2004; Chakroborty et al. 2004, 2009; Cole and Sood 2012; Sarkar et al. 2004).
Dopamine and tumor angiogenesis
Recent reports from our laboratory have established that endogenous DA secreted by different peripheral organs is an important inhibitor of tumor angiogenesis and hence growth (Chakroborty et al. 2004, 2009). Ablation of peripheral dopaminergic nerves significantly increases angiogenesis, microvascular permeability and growth of malignant tumors in mice (Basu et al. 2004) and treatment of tumor bearing animals with exogenous DA inhibits tumor growth and angiogenesis (Sarkar et al. 2008). DA acts through its D2 receptors present in endothelial cells to inhibit VEGF induced phosphorylation of VEGFR2, mitogen-activated protein (MAP) kinase and focal adhesion kinase (FAK), thereby blocking the critical signaling pathways required to mediate the endothelial functions of VEGF (Basu et al. 2001; Sarkar et al. 2004). Also, DA D2 receptor knockout mice show increased tumor growth due to increased angiogenesis which correlates well with the striking increase in VEGFR2 phosphorylation in tumor endothelial cells collected from these animals (Basu et al. 2004). Moreover, rats with hyperactive dopaminergic system have decreased tumor growth, angiogenesis and metastasis, which further support the importance of DA in regulating tumor angiogenesis and growth (Teunis et al. 2002). In experimental mouse model of schizophrenia that had a faulty DA uptake system resulted in an elevated DA level in plasma, the growth of Lewis lung carcinoma was considerably slow (Asada et al. 2008). DA also reduces chronic stress mediated angiogenesis and hence tumor growth in ovarian cancer models (Moreno-Smith et al. 2011).
In addition to its role in regulation of the VEGF induced angiogenic process, DA also plays a prominent role in vasculogenesis, the process by which endothelial progenitor cells (EPCs) are recruited from the bone marrow (BM) to developing vessels within the tumor (Chakroborty et al. 2008). Tumor growth is associated with depletion of DA in the BM niche followed by increased mobilization of EPC from the BM to tumor site (Chakroborty et al. 2008). DA treatment significantly decreases VEGF-induced EPC mobilization from the BM, which is associated with a decrease in BM MMP-9 expression due to inhibition of ERK1/ERK2 phosphorylation (Chakroborty et al. 2008).
DA as a potent anti-VEGF agent improves the efficacy and acts synergistically with anticancer drugs, doxorubicin and 5 FU to significantly inhibit growth of human breast (MCF-7) and colon (HT29) cancer transplanted into nude mice (Sarkar et al. 2008). Moreover DA treatment helps to normalize the structure of aberrant tumor blood vessels and restore their normal functions which help to improve the concentration of anticancer drug in tumor tissues (Chakroborty et al. 2011). DA normalizes abnormal tumor blood vessels by targeting the two prime cellular components that build up blood vessels: pericytes and endothelial cells. By acting through its D2 receptors DA directly up-regulates the expression of angiopoietin 1 (Ang1) in pericytes and the expression of the zinc finger transcriptional factor, Krüppel-like factor-2 (KLF2) in tumor endothelial cells (Chakroborty et al. 2011).
NE, E and tumor angiogenesis
The actions of NE and E are mediated via α1, α2, and β-adrenergic receptor families that exhibit discrete tissue expressions and also signal through definite pathways (Lutgendorf et al. 2011; Tilan and Kitlinska 2010). Signaling through β adenoreceptors regulate functions of epithelial cells, vascular myocytes and pericytes, which play important roles in tumor angiogenesis and its progression (Baker et al. 2011; Daly and McGrath 2011; Cole and Sood 2012). NE and E act through β adrenergic receptors to upregulate VEGF expression in several human tumors (Moreno-Smith et al. 2010) that acts through VEGFR2 to increase tumor angiogenesis and growth (Dvorak 2005). Highly invasive ovarian carcinoma with a greater tumor burden was observed in experimental mouse tumor models using HeyA8 and SKOV3ip1 ovarian cancer cells where chronic behavioral stress led to elevated levels of tissue NE and E (Sood et al. 2006). Chronic stress resulted in enhanced expressions of angiogenic cytokines, VEGF, MMP2, MMP9 and proinflammatory molecules, IL6 and IL 8 in these tumor models (Thaker et al. 2006; Yang et al. 2009). The signaling steps involved were the activation of cyclic AMP-PKA pathway and Src by β adrenergic receptor (Thaker et al. 2006; Nilsson et al. 2007; Moreno-Smith et al. 2011). However inhibition of this receptor by β adrenergic blocker, propranolol was associated with reduced angiogenesis and tumor growth (Thaker et al. 2006; Nilsson et al. 2007; Moreno-Smith et al. 2011). In nasopharyngeal cell lines, HONE-1, HNE-1and CNE-1, NE upregulated the expressions of VEGF, MMP2 and MMP9 suggesting NE could possibly affect nasopharyngeal tumor progression by controlling the expressions of angiogenic cytokines (Yang et al. 2006). NE through the same mechanism also stimulates the expression of VEGF in human melanoma, multiple myeloma and pancreatic cancer cell lines (Yang et al. 2009; Guo et al. 2009).
A recent report shows that NE upregulates VEGF expression in cancer cells by inducing hypoxia inducible factor 1α and this action of NE not only involves β adrenergic receptors, but also α adrenergic receptors (Park et al. 2011). This report further demonstrates that propanolol, a β adrenergic receptor blocker could abolish VEGF synthesis in these cells (Park et al. 2011). Propranolol also potentiates the anti-cancer effects of chemotherapeutic drugs in breast cancer (Powe et al. 2010; Pasquier et al. 2011; Melhem-Bertrandt et al. 2011). Furthermore, stimulation of α adrenergic receptors have trophic effects on endothelial cells i.e. on proliferation, migration and its ability to form capillaries (Vinci et al. 2007; Tilan and Kitlinska 2010). Additionally, NE and E modulate cells in the tumor microenviroment such as macrophages, which regulate tumor angiogenesis (Lutgendorf et al. 2008).
Tumor immunity and effector cells
Tumor immunity or how a growing tumor elicits or escapes an immune response within the host body is a very interesting aspect of tumor progression owing to the fact that tumors originate from transformation of normal cells and the immune system fails to recognize these cells as non self (Zitvogel et al. 2006; Schreiber et al. 2011). Although spontaneous rejection of established tumors in the body is of rare occurrence, growing evidence suggest that both innate and adaptive immunity play important roles in tumor immune response, which might promote or inhibit the growth of tumor. A growing tumor is infiltrated by T lymphocytes, macrophages, dendritic cells and occasionally by B cells, the functions of which are regulated by a number of endogenous molecules (von Kleist et al. 1987; Coronella et al. 2001; Whiteside 2006).
Catecholamines in tumor immunity
Sympathetic nervous system (SNS) lying at the interface between the brain and immune system plays an integral role in maintaining cross talk between them and thereby regulating different physiological functions to maintain homeostasis and deregulation of SNS is associated with different diseases like cancer where altered immunity is noted (Elenkov et al. 2000). Therefore the roles of DA, E and NE, the main effectors of SNS in the regulation of tumor growth and progression have drawn immense attentions due to their dual nature of actions, which can be either immunosuppressive or immune mediated anti-tumor responses (Chambers et al. 1993; Basu and Dasgupta 2000a; Sarkar et al 2010).
Dopamine and tumor immunity
The existence of different subtypes of dopamine receptors in the primary immune organ, thymus (Mignini et al. 2009), in circulating immune effector cells lymphocytes, monocytes, neutrophils, dendritic cells and innervation of secondary lymphoid organs with sympathetic nerves and production of DA by lymphocytes suggest the possible role of DA in the regulation of immune system (Basu et al. 1993; Basu and Dasgupta 2000a, b; Ferrari et al. 2004; Kirillova et al. 2008; McKenna et al. 2002; Nakano et al. 2008, 2009; Sarkar et al. 2010). Many studies indicate that both central and peripheral DA can influence the growth and progression of tumors by affecting the functions of immune competent cells within the body (Basu et al. 1995a, b; Basu and Dasgupta 2000b; Barnes and Gordon 2008; Rubí and Maechler 2010). 1-methyl-4-phenyl 1,2,3,6 tetrahydropyridine (MPTP)-induced striatal dopaminergic depletion in mice showed increased incidence and growth of Ehrlich carcinoma due to decreased T cell proliferation, IgG and IgM secretion by B-cells, NK and cytotoxic T cell activities suggesting significant depression of the immune system (Basu et al. 1995a, b). The anti-tumoral action of DA has also been reported to be manifested by stimulation of peritoneal macrophages, NK cells and cytotoxic T cells (Basu et al. 1992a, b; Dasgupta and Lahiri 1987; Basu and Dasgupta 2000a, b; Sarkar et al. 2010). Alternatively, DA can indirectly influence the growth of tumors by regulating the growth and release of prolactin (Ganong et al. 1985; Ben-Jonathan 1985; Lissoni et al. 2004) as prolactin also regulates the functions of NK cells and lymphokine activated killer cells (Redelman et al. 2008; Souberbielle and Dalgleish 1994). Recently in another interesting study it has been shown that CD4+CD25+ regulatory T lymphocytes (Tregs) which play a vital role in the control of immune homeostasis also contain substantial amounts of DA (Cosentino et al. 2007). DA on release acts via its D1 receptors present in these cells to suppress IL-10 and TGF-β synthesis (Cosentino et al. 2007). The frequency and functions of Tregs in growing tumor are important because increase in the number of these cells may favor tumor development or growth, and therefore influence the course of the disease (Beyer and Schultze 2006).
NE, E and tumor immunity
A growing number of studies have suggested that chronic stress has specific effects on the immune systems of cancer patients (Price et al. 2001; Reiche et al. 2004; Spiegel and Giese-Davis 2003). Although stress cannot directly cause cancer, the increased secretion of NE and E, the two important sympathetic mediators (Herman et al. 1996; McEwen 2007) which usually act as immunosuppressors promote favorable environment for tumor cells to grow and metastasize (Inbar et al. 2011). Signaling through β adrenergic receptors regulates tumor growth, progression and metastasis by influencing a number of cellular and molecular processes among which regulation of immune response is an important parameter (Antoni et al. 2006). β adrenergic receptors are present in both helper and T suppressor lymphocytes, B lymphocytes, NK cells, monocyte/macrophage and dendritic cells (Nance and Sanders 2007). Stimulation of β adrenergic receptors usually inhibits lymphocyte responses, NK cell cytotoxicity and dendritic cell functions (Marino and Cosentino 2011). In ovarian cancer cells, NE and E by acting through β receptors increase the production and activities of the proinflammatory cytokines, IL6 and IL8 that in turn stimulate the growth of tumors (Nilsson et al. 2007; Shahzad et al. 2010). Animal studies have also shown that β-AR stimulation suppresses NK-cell activity and compromises resistance to tumor metastases (Shakhar and Ben-Eliyahu 1998; Ben-Eliyahu et al. 2000). There is also a report which suggests that endogenous E together with prostaglandins can suppress cytotoxic T-lymphocyte and NK cell responses and thereby promote leukemia progression in rats (Inbar et al. 2011). Impaired β adrenergic receptor expression in the circulating cells of chronic lymphocytic leukemia (CLL) patients lead to the loss of adenylate cycle activity, which in turn is associated with disease progression (Kamp et al. 1997). In addition it has also been reported that long acting β2 adrenergic receptor agonists such as salmeterol and formoterol can promote apoptosis in leukemic cells independent of β2 adrenergic activation (Mamani-Matsuda et al. 2004). It has been shown that stress-induced neuroendocrine activation leading to NE secretion could increase the metastasis of breast cancer cells to distant sites including the lymph nodes and lung without effecting the growth of primary tumors by increased recruitment of CD11b+F4/80+ macrophages into primary tumors and induced prometastatic gene expression with M2 macrophage differentiation (Sloan et al. 2010). Modulation of adrenergic receptors may also be important for cancer vaccine strategies. Botta and Maestroni (2008) evaluated the role of β2 adrenergic receptors in the outcome of a dendritic cell (DC) based cancer vaccination in murine E.G7-ovalbumin (OVA) model and have reported that the adrenergic modulation by blocking β2 adrenergic receptors together with the activation of toll-like receptor 2 at the site of dendritic cell inoculation could either have enhanced antitumor effects or be tolerogenic depending on the maturation state of the transferred DCs.
Conclusion
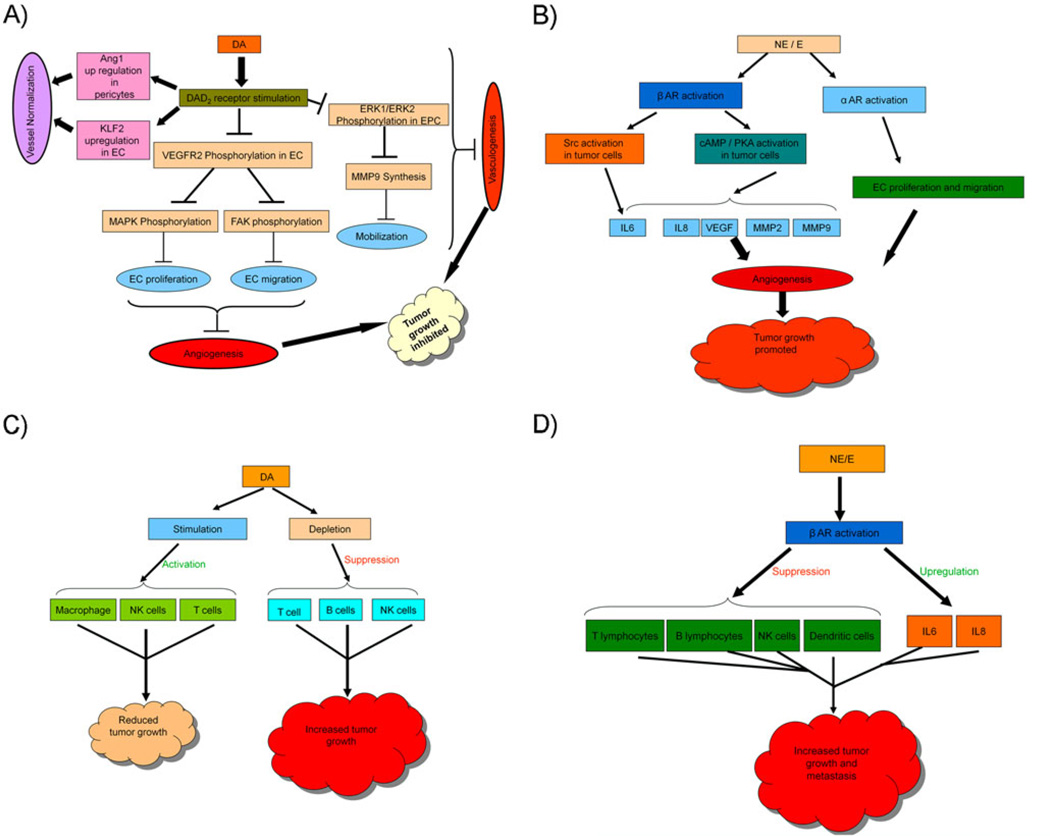
In a growing tumor, the switch to the ‘angiogenic phase’ as a result of loss of equilibrium between the positive and negative endogenous angiogenic regulators is considered as the key step that ultimately decides the fate of the tumor in terms of its ability to grow further and metastasize (Hanahan and Folkman 1996; Bergers and Benjamin 2003). Also, only when a growing tumor acquires the ability to evade the host immune surveillance it can thrive in the host body (Schreiber et al. 2011). Therefore the regulators of both these two processes are deemed important for the development of new therapies for the treatment of cancer. The studies outlined in our review highlight the important distinct roles of CA neurotransmitters (Fig. 1); DA inhibits tumor angiogenesis and stimulates tumor immunity whereas NE and E stimulate angiogenesis and inhibit immune functions in cancer.
Fig. 1.
Schematic overview of signaling pathways involved in catecholamine mediated regulation of angiogenesis in the process of tumor development (a & b). Schematic overview of signaling pathways involved in catecholamine mediated regulation of tumor immunity (c & d) Dopamine (DA), D2 receptors (DAD2), VEGF-A receptor 2 (VEGFR2), MAP kinase (MAPK), focal adhesion kinase (FAK), endothelial cells (EC), endothelial progenitor cells (EPC), matrix metalloproteinase 9 (MMP9), angiopoietin 1 (Ang1), Kruppel like-factor 2 (KLF2), norepinephrine (NE), epinephrine (e), β adrenergic receptors (β AR), α adrenergic receptors (α AR)
It will be also important to mention here that several epidemiological studies have demonstrated increased incidence of melanoma, breast and thyroid cancers in Parkinson’s syndrome, a hypodopaminergic disease (Rubí and Maechler 2010). In contrast, schizophrenic patients with probable hyperactive dopaminergic system do not suffer from higher cancer rates in spite of being exposed to cancer causing risk factors (Seeman and Kapur 2000; Bushe and Hodgson 2010). These reports thus suggest that DA may have a protective role in cancer, which is further strengthened by another report where the investigators observed increased numbers of breast cancer in patients treated with DA D2 receptor antagonists (Wang et al. 2002). Similarly other clinical reports have indicated reduced progression and mortality in melanoma and breast cancer patients treated with β2 adrenergic antagonists (Barron et al. 2011; Melhem-Bertrandt et al. 2011). However, there is also a report, which suggests no effects of β2 adrenergic antagonists on cancer progression and mortality (Shah et al. 2011).
Finally, since cancer is a multifactorial disease (Lyman 1992; Zabaleta 2012) drugs which can control more than one factor regulating its growth and progression and metastasis may be critical for the successful treatment of this disease (Hopkins 2008). These studies suggest that the CAs and/or their agonists/antagonists hold considerable promise as new drugs in cancer therapy. The β blockers and DA D2 receptor agonists are in clinical use at present for the treatment of other diseases (Alvarez et al. 2007; Perron et al. 2004). These inexpensive drugs already approved for clinical uses, safe and effective with manageable side effects can be considered for future clinical trials for the treatment of cancer.
Acknowledgment
This work was supported in part by the National Institutes of Health grant CA124763 to S.B. We acknowledge Dr. Partha Sarathi Dasgupta for his valuable suggestion.
Footnotes
Disclosure The authors have no conflicts of interest or financial disclosures to make.
Contributor Information
Chandrani Sarkar, Department of Pathology, The Ohio State University, Columbus, OH 43210, USA.
Debanjan Chakroborty, Department of Pathology, The Ohio State University, Columbus, OH 43210, USA.
Sujit Basu, Email: sujit.basu@osumc.edu, Department of Pathology, The Ohio State University, Columbus, OH 43210, USA; Arthur G. James Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
References
- Alvarez C, Martí-Bonmatí L, Novella-Maestre E, Sanz R, Gómez R, Fernández-Sánchez M, Simón C, Pellicer A. Dopamine agonist cabergoline reduces hemoconcentration and ascites in hyperstimulated women undergoing assisted reproduction. J Clin Endocrinol Metab. 2007;92:2931–2937. doi: 10.1210/jc.2007-0409. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada M, Ebihara S, Numachi Y, Okazaki T, Yamanda S, Ikeda K, Yasuda H, Sora I, Arai H. Reduced tumor growth in a mouse model of schizophrenia, lacking the dopamine transporter. Int J Cancer. 2008;123:511–518. doi: 10.1002/ijc.23562. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Axelsson J. Catecholamine functions. Annu Rev Physiol. 1971;33:1–30. doi: 10.1146/annurev.ph.33.030171.000245. [DOI] [PubMed] [Google Scholar]
- Baker JG, Hill SJ, Summers RJ. Evolution of beta-blockers: from anti-anginal drugs to ligand-directed signalling. Trends Pharmacol Sci. 2011;32:227–234. doi: 10.1016/j.tips.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Gordon J. Harnessing serotonergic and dopaminergic pathways for lymphoma therapy: evidence and aspirations. Semin Cancer Biol. 2008;18:218–225. doi: 10.1016/j.semcancer.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, Radermacher P, Calzia E. Glucose metabolism and catecholamines. Crit Care Med. 2007;35:S508–S518. doi: 10.1097/01.CCM.0000278047.06965.20. [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol. 2000a;102:113–124. doi: 10.1016/s0165-5728(99)00176-9. [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS. Role of dopamine in malignant tumor growth. Endocrine. 2000b;12:237–241. doi: 10.1385/ENDO:12:3:237. [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS, Ray MR, Lahiri T. Stimulation of NK activity in Ehrlich ascites carcinoma –bearing mice following dopamine treatment. Biogenic Amines. 1992a;8:191–197. [Google Scholar]
- Basu S, Banerjee S, Dasgupta PS, Roy Chowdhury J. Stimulation of splenic lymphocyte proliferation and increased life span of solid Ehrlich carcinoma-bearing mice following dopamine treatment. Biogenic Amines. 1992b;9:177–181. [Google Scholar]
- Basu S, Dasgupta PS, Lahiri T, Chowdhury JR. Uptake and biodistribution of dopamine in bone marrow, spleen and lymph nodes of normal and tumor bearing mice. Life Sci. 1993;53:415–424. doi: 10.1016/0024-3205(93)90645-j. [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS, Roy Chowdhury J. Altered plasma level and uptake of dopamine by platelets in some human malignant tumors. Biogenic Amines. 1995a;11:31–38. [Google Scholar]
- Basu S, Dasgupta PS, Chowdhury JR. Enhanced tumor growth in brain dopamine-depleted mice following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment. J Neuroimmunol. 1995b;60:1–8. doi: 10.1016/0165-5728(95)00044-3. [DOI] [PubMed] [Google Scholar]
- Basu S, Nagy JA, Pal S, Vasile E, Eckelhoefer IA, Bliss VS, Manseau EJ, Dasgupta PS, Dvorak HF, Mukhopadhyay D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med. 2001;7:569–574. doi: 10.1038/87895. [DOI] [PubMed] [Google Scholar]
- Basu S, Sarkar C, Chakroborty D, Nagy J, Mitra RB, Dasgupta PS, Mukhopadhyay D. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 2004;64:5551–5555. doi: 10.1158/0008-5472.CAN-04-1600. [DOI] [PubMed] [Google Scholar]
- Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med (Berl) 1995;73:333–346. doi: 10.1007/BF00192885. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8:154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocr Rev. 1985;6:564–589. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bergquist J, Tarkowski A, Ekman R, Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci U S A. 1994;91:12912–12916. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- Botta F, Maestroni GJ. Adrenergic modulation of dendritic cell cancer vaccine in a mouse model: role of dendritic cell maturation. J Immunother. 2008;31:263–270. doi: 10.1097/CJI.0b013e318160995e. [DOI] [PubMed] [Google Scholar]
- Bushe CJ, Hodgson R. Schizophrenia and cancer: in 2010 do we understand the connection? Can J Psychiatry. 2010;55:761–767. doi: 10.1177/070674371005501203. [DOI] [PubMed] [Google Scholar]
- Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–4356. doi: 10.1158/1078-0432.CCR-04-0059. [DOI] [PubMed] [Google Scholar]
- Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Invest. 2008;118:1380–1389. doi: 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009;69:3727–3730. doi: 10.1158/0008-5472.CAN-08-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroborty D, Sarkar C, Yu H, Wang J, Liu Z, Dasgupta PS, Basu S. Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc Natl Acad Sci U S A. 2011;108:20730–20735. doi: 10.1073/pnas.1108696108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers DA, Cohen RL, Perlman RL. Neuroimmune modulation: signal transduction and catecholamines. Neurochem Int. 1993;22:95–110. doi: 10.1016/0197-0186(93)90002-m. [DOI] [PubMed] [Google Scholar]
- Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronella JA, Telleman P, Kingsbury GA, Truong TD, Hays S, Junghans RP. Evidence for an antigen-driven humoral immune response in medullary ductal breast cancer. Cancer Res. 2001;61:7889–7899. [PubMed] [Google Scholar]
- Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F, Lecchini S. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109:632–642. doi: 10.1182/blood-2006-01-028423. [DOI] [PubMed] [Google Scholar]
- Daly CJ, McGrath JC. Previously unsuspected widespread cellular and tissue distribution of beta-adrenoceptors and its relevance to drug action. Trends Pharmacol Sci. 2011;32:219–226. doi: 10.1016/j.tips.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Dasgupta PS, Lahiri T. Stimulation of tumoricidal activity of peritoneal macrophage by dopamine treatment. Med Sci Res. 1987;15:1301–1302. [Google Scholar]
- Dvorak HF. Angiogenesis: update 2005. J Thromb Haemost. 2005;3:1835–1842. doi: 10.1111/j.1538-7836.2005.01361.x. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Aneman A, Hooper D, Rundqvist B, Friberg Mesenteric organ production, hepatic metabolism, and renal elimination of norepinephrine and its metabolites in humans. J Neurochem. 1996;66:1565–1573. doi: 10.1046/j.1471-4159.1996.66041565.x. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Aneman A, Friberg P, Hooper D, Fåndriks L, Lonroth H, Hunyady B, Mezey E. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab. 1997;82:3864–3871. doi: 10.1210/jcem.82.11.4339. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Ferrari M, Cosentino M, Marino F, Bombelli R, Rasini E, Lecchini S, Frigo G. Dopaminergic D1-like receptor-dependent inhibition of tyrosine hydroxylase mRNA expression and catecholamine production in human lymphocytes. Biochem Pharmacol. 2004;67:865–873. doi: 10.1016/j.bcp.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Ganong WF. Synaptic and junctional transmission. In: Ganong WF, editor. Review of medical physiology. New York: McGraw-Hill; 2005. pp. 85–120. [Google Scholar]
- Ganong WF, Alper RH, Steels MK. In: Catecholamines as hormone regulator. Jonathon NB, Bahrs JM, Weiners RI, editors. New York: Raven; 1985. pp. 13–18. [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D, Zhang M. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep. 2009;22:825–830. doi: 10.3892/or_00000505. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Havel RJ. The autonomic nervous system and intermediary carbohydrate and fat metabolism. Anesthesiology. 1968;29:702–713. doi: 10.1097/00000542-196807000-00014. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Hopkins A. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One. 2011;6:e19246. doi: 10.1371/journal.pone.0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Kamp T, Liebl B, Haen E, Emmerich B, Hallek M. Defects of beta 2-adrenergic signal transduction in chronic lymphocytic leukaemia: relationship to disease progression. Eur J Clin Invest. 1997;27:121–127. doi: 10.1046/j.1365-2362.1997.700623.x. [DOI] [PubMed] [Google Scholar]
- Kirillova GP, Hrutkay RJ, Shurin MR, Shurin GV, Tourkova IL, Vanyukov MM. Dopamine receptors in human lymphocytes: radioligand binding and quantitative RT PCR assays. J Neurosci Methods. 2008;174:272–280. doi: 10.1016/j.jneumeth.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty R. Catecholamines: role in health and disease. Drugs. 1978;16:418–440. doi: 10.2165/00003495-197816050-00003. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Vaghi M, Pescia S, Rovelli F, Ardizzola A, Valtulina F, Malugani F, Gardani G, Tancini G. Biological response modifiers of cancer-related neuroendocrine disorders: efficacy of the long-term dopaminergic agonist cabergoline in the treatment of breast cancer-induced hyperprolactinemia. J Biol Regul Homeost Agents. 2004;18:291–294. [PubMed] [Google Scholar]
- Lutgendorf SK, Lamkin DM, Jennings NB, Arevalo JM, Penedo F, DeGeest K, Langley RR, Lucci JA, 3rd, Cole SW, Lubaroff DM, Sood AK. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14:6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, Goodheart M, Buekers TE, Mendez L, Krueger G, Clevenger L, Lubaroff DM, Sood AK, Cole SW. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2011;25:250–255. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman GH. Risk factors for cancer. Prim Care. 1992;19:465–479. [PubMed] [Google Scholar]
- Mamani-Matsuda M, Moynet D, Molimard M, Ferry-Dumazet H, Marit G, Reiffers J, Mossalayi MD. Long-acting beta2-adrenergic formoterol and salmeterol induce the apoptosis of B-chronic lymphocytic leukaemia cells. Br J Haematol. 2004;124:141–150. doi: 10.1046/j.1365-2141.2003.04746.x. [DOI] [PubMed] [Google Scholar]
- Marino F, Cosentino M. Adrenergic modulation of immune cells: an update. Amino Acids. 2011 doi: 10.1007/s00726-011-1186-6. [DOI] [PubMed] [Google Scholar]
- Mayer SE. Effect of catecholamines on cardiac metabolism. Circ Res. 1974;35:129–137. [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ. Dopamine receptor expression on human T-and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol. 2002;132:34–40. doi: 10.1016/s0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignini F, Tomassoni D, Traini E, Amenta F. Dopamine, vesicular transporters and dopamine receptor expression and localization in rat thymus and spleen. J Neuroimmunol. 2009;206:5–13. doi: 10.1016/j.jneuroim.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863–1881. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Smith M, Lu C, Shahzad MM, Pena GN, Allen JK, Stone RL, Mangala LS, Han HD, Kim HS, Farley D, Berestein GL, Cole SW, Lutgendorf SK, Sood A. Dopamine blocks stress-mediated ovarian carcinoma growth. Clin Cancer Res. 2011;17:3649–3659. doi: 10.1158/1078-0432.CCR-10-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun. 2008;373:286–291. doi: 10.1016/j.bbrc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Takagi R, Hashimoto K, Tanaka Y, Matsushita S. Dopamine released by dendritic cells polarizes Th2 differentiation. Int Immunol. 2009;21:645–654. doi: 10.1093/intimm/dxp033. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y, Jennings N, Arevalo J, Lutgendorf SK, Gallick GE, Sanguino AM, Lopez-Berestein G, Cole SW, Sood AK. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282:29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- Park SY, Kang JH, Jeong KJ, Lee J, Han JW, Choi WS, Kim YK, Kang J, Park CG, Lee HY. Norepinephrine induces VEGF expression and angiogenesis by a hypoxiainducible factor-1α protein-dependent mechanism. Int J Cancer. 2011;128:2306–2316. doi: 10.1002/ijc.25589. [DOI] [PubMed] [Google Scholar]
- Pasquier E, Ciccolini J, Carre M, Giacometti S, Fanciullino R, Pouchy C, Montero MP, Serdjebi C, Kavallaris M, André N. Propranolol potentiates the anti-angiogenic effects and antitumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2011;2:797–809. doi: 10.18632/oncotarget.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control. 2004;15:535–541. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;7:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MA, Tennant CC, Butow PN, Smith RC, Kennedy SJ, Kossoff MB, Dunn SM. The role of psychosocial factors in the development of breast carcinoma: part II. Life event stressors, social support, defense style, and emotional control and their interactions. Cancer. 2001;91:686–697. [PubMed] [Google Scholar]
- Redelman D, Welniak LA, Taub D, Murphy WJ. Neuroendocrine hormones such as growth hormone and prolactin are integral members of the immunological cytokine network. Cell Immunol. 2008;252:111–121. doi: 10.1016/j.cellimm.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- Robison GA, Butcher RW, Sutherland EW. Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci. 1967;139:703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- Rubí B, Maechler P. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let’s seek the balance. Endocrinology. 2010;151:5570–5581. doi: 10.1210/en.2010-0745. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1554–H1560. doi: 10.1152/ajpheart.00272.2004. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. 2008;14:2502–2510. doi: 10.1158/1078-0432.CCR-07-1778. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: an update. Brain Behav Immun. 2010;24:525–528. doi: 10.1016/j.bbi.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Schuller HM. Neurotransmitter receptor-mediated signaling pathways as modulators of carcinogenesis. Prog Exp Tumor Res. 2007;39:45–63. doi: 10.1159/000100045. [DOI] [PubMed] [Google Scholar]
- Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci U S A. 2000;97:7673–7675. doi: 10.1073/pnas.97.14.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG. Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol. 2011;72:157–161. doi: 10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M, Nishimura M, Lee JW, Jennings NB, Bottsford-Miller J, Vivas-Mejia P, Lutgendorf SK, Lopez-Berestein G, Bar-Eli M, Cole SW, Sood AK. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem. 2010;285:35462–35470. doi: 10.1074/jbc.M110.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160:3251–3258. [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souberbielle B, Dalgleish AG. In: The psychoimmunology of cancer: mind and body in the fight for survival. Lewis CE, Sullivan CO, Baraclough J, editors. New York: Oxford University Press; 1994. [Google Scholar]
- Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- Tandle A, Libutti SK. Antiangiogenic therapy: targeting vascular endothelial growth factor and its receptors. Clin Adv Hematol Oncol. 2003;1:41–48. [PubMed] [Google Scholar]
- Teunis MA, Kavelaars A, Voest E, Bakker JM, Ellenbroek BA, Cools AR, Heijnen CJ. Reduced tumor growth, experimental metastasis formation, and angiogenesis in rats with a hyperreactive dopaminergic system. FASEB J. 2002;16:1465–1467. doi: 10.1096/fj.02-0145fje. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 100.Thaker PH, Lutgendorf SK, Sood AK. The neuroendocrine impact of chronic stress on cancer. Cell Cycle. 2007;6:430–433. doi: 10.4161/cc.6.4.3829. [DOI] [PubMed] [Google Scholar]
- 101.Tilan J, Kitlinska J. Sympathetic neurotransmitters and tumor angiogenesis-link between stress and cancer progression. J Oncol. 2010:539706. doi: 10.1155/2010/539706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vinci MC, Bellik L, Filippi S, Ledda F, Parenti A. Trophic effects induced by alpha1D-adrenoceptors on endothelial cells are potentiated by hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H2140–H2147. doi: 10.1152/ajpheart.00390.2007. [DOI] [PubMed] [Google Scholar]
- 103.von Kleist S, Berling J, Bohle W, Wittekind C. Immunohistological analysis of lymphocyte subpopulations infiltrating breast carcinomas and benign lesions. Int J Cancer. 1987;40:18–23. doi: 10.1002/ijc.2910400105. [DOI] [PubMed] [Google Scholar]
- 104.Wang PS, Walker AM, Tsuang MT, Orav EJ, Glynn RJ, Levin R, Avorn J. Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry. 2002;59:1147–1154. doi: 10.1001/archpsyc.59.12.1147. [DOI] [PubMed] [Google Scholar]
- 105.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 106.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 107.Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, Barsky SH, Glaser R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267–275. doi: 10.1016/j.bbi.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zabaleta J. Multifactorial etiology of gastric cancer. Methods Mol Biol. 2012;863:411–435. doi: 10.1007/978-1-61779-612-8_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]