Abstract
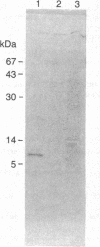
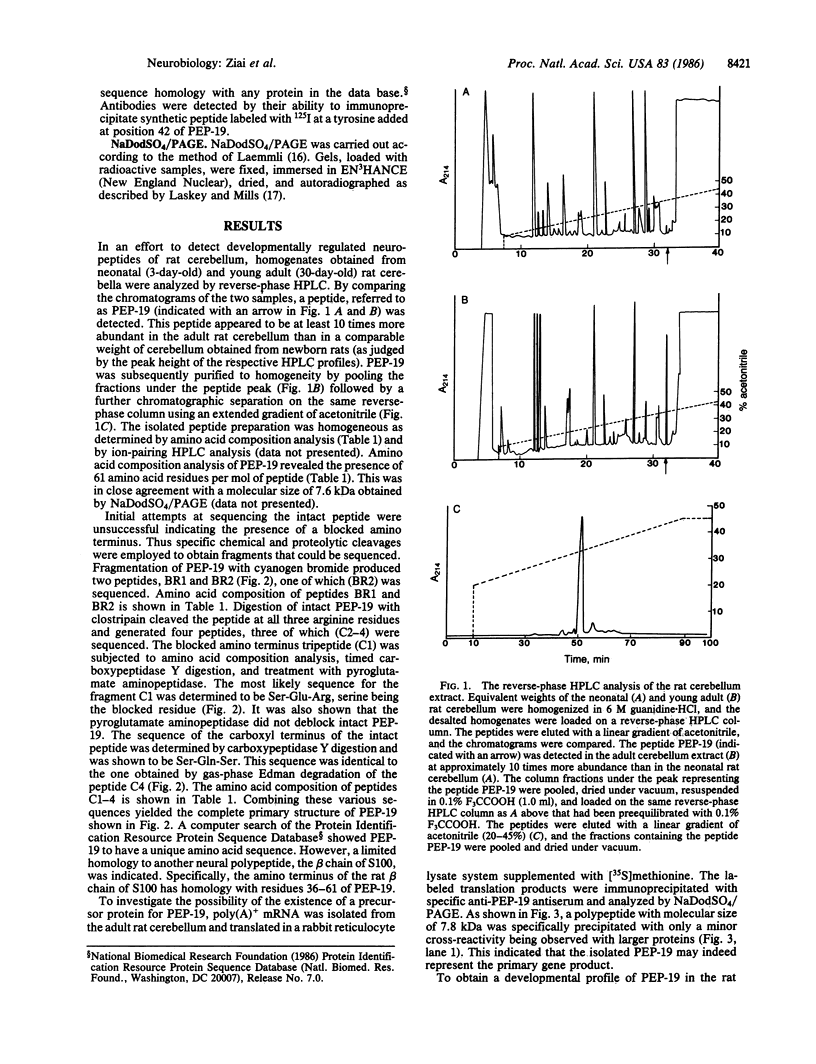
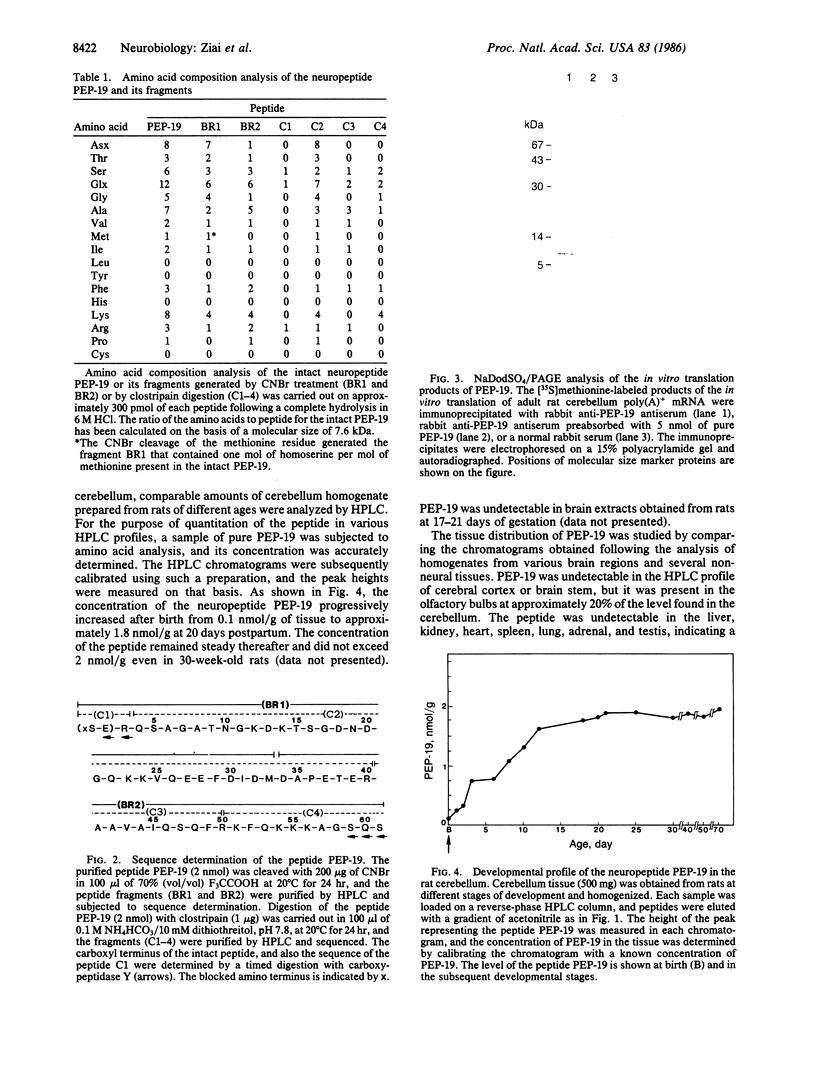
By comparing the HPLC profiles of cerebellar extracts from adult and neonatal rats, a developmentally regulated polypeptide, termed PEP-19, was identified. The concentration of PEP-19 rose from 0.1 nmol/g of cerebellum at birth to 2 nmol/g at 20 days postpartum. The polypeptide could also be detected at lower levels in olfactory bulbs of adult rats but was absent in cerebral cortex, brain stem, and all non-neural tissues examined. HPLC-purified PEP-19 contained 61 amino acids and had a molecular size of 7.6 kDa. The native polypeptide is blocked at its amino terminus but was sequenced following proteolytic and chemical fragmentation. The primary amino acid sequence was determined to be: X (S-E) R Q S A G A T N G K D K T S G D N D G Q K K V Q E E F D I D M D A P E T E R A A V A I Q S Q F R K F Q K K K A G S Q S. PEP-19 has a unique sequence, but shares some homology with several calcium binding proteins including the beta chain of S100 and intestinal calcium binding protein. This polypeptide is the primary translation product of cerebellar poly(A)+ mRNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Postnatal development of the cerebellar cortex in the rat. 3. Maturation of the components of the granular layer. J Comp Neurol. 1972 Aug;145(4):465–513. doi: 10.1002/cne.901450403. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972 Jul;145(3):353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972 Aug;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock E., Jorgensen O. S. Rat brain synaptic vesicles and synaptic plasma membranes compared by crossed immunoelectrophoresis. FEBS Lett. 1975 Mar 15;52(1):37–39. doi: 10.1016/0014-5793(75)80632-6. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Ebert R. F., Bell W. R. Localization of a fibrinogen calcium binding site between gamma-subunit positions 311 and 336 by terbium fluorescence. J Biol Chem. 1985 Aug 15;260(17):9713–9719. [PubMed] [Google Scholar]
- Desplan C., Heidmann O., Lillie J. W., Auffray C., Thomasset M. Sequence of rat intestinal vitamin D-dependent calcium-binding protein derived from a cDNA clone. Evolutionary implications. J Biol Chem. 1983 Nov 25;258(22):13502–13505. [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Herschman H. R., Levine L., De Vellis J. Appearance of a brain-specific antigen (S-100 protein) in the developing rat brain. J Neurochem. 1971 Apr;18(4):629–633. doi: 10.1111/j.1471-4159.1971.tb11993.x. [DOI] [PubMed] [Google Scholar]
- Hexham J. M., Totty N. F., Waterfield M. D., Crumpton M. J. Homology between the subunits of S100 and a 10kDa polypeptide associated with p36 of pig lymphocytes. Biochem Biophys Res Commun. 1986 Jan 14;134(1):248–254. doi: 10.1016/0006-291x(86)90554-1. [DOI] [PubMed] [Google Scholar]
- Hofmann T., Kawakami M., Hitchman A. J., Harrison J. E., Dorrington K. J. The amino acid sequence of porcine intestinal calcium-binding protein. Can J Biochem. 1979 Jun;57(6):737–748. doi: 10.1139/o79-092. [DOI] [PubMed] [Google Scholar]
- Isobe T., Okuyama T. The amino-acid sequence of the alpha subunit in bovine brain S-100a protein. Eur J Biochem. 1981 May;116(1):79–86. doi: 10.1111/j.1432-1033.1981.tb05303.x. [DOI] [PubMed] [Google Scholar]
- Jacque C. M., Jorgensen O. S., Baumann N. A., Bock E. Brain-specific antigens in the Quaking mouse during ontogeny. J Neurochem. 1976 Oct;27(4):905–909. doi: 10.1111/j.1471-4159.1976.tb05153.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen O. S., Bock E. Brain specific synaptosomal membrane proteins demonstrated by crossed immunoelectrophoresis. J Neurochem. 1974 Oct;23(4):879–880. doi: 10.1111/j.1471-4159.1974.tb04419.x. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Ratner L., Clarke M. F., Westin E. H., Reitz M. S., Wong-Staal F. Transforming potential of human c-sis nucleotide sequences encoding platelet-derived growth factor. Science. 1984 Aug 10;225(4662):636–639. doi: 10.1126/science.6740330. [DOI] [PubMed] [Google Scholar]
- Kligman D., Marshak D. R. Purification and characterization of a neurite extension factor from bovine brain. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7136–7139. doi: 10.1073/pnas.82.20.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lau S. Y., Sanders C., Smillie L. B. Amino acid sequence of chicken gizzard gamma-tropomyosin. J Biol Chem. 1985 Jun 25;260(12):7257–7263. [PubMed] [Google Scholar]
- Rönnbäck L., Rubin M., Parnes H. S-100 protein in guinea pig brain during prenatal development. Dev Neurosci. 1978;1(3-4):186–189. doi: 10.1159/000112572. [DOI] [PubMed] [Google Scholar]
- Shimono T., Nosaka S., Sasaki K. Electrophysiological study on the postnatal development of neuronal mechanisms in the rat cerebellar cortex. Brain Res. 1976 May 28;108(2):279–294. doi: 10.1016/0006-8993(76)90186-4. [DOI] [PubMed] [Google Scholar]
- Slemmon J. R., Blacher R., Danho W., Hempstead J. L., Morgan J. I. Isolation and sequencing of two cerebellum-specific peptides. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6866–6870. doi: 10.1073/pnas.81.21.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmon J. R., Danho W., Hempstead J. L., Morgan J. I. Cerebellin: a quantifiable marker for Purkinje cell maturation. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7145–7148. doi: 10.1073/pnas.82.20.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg J., Pansini A. R., Christakos S. Vitamin D-dependent rat renal calcium-binding protein: development of a radioimmunoassay, tissue distribution, and immunologic identification. Endocrinology. 1984 Aug;115(2):640–648. doi: 10.1210/endo-115-2-640. [DOI] [PubMed] [Google Scholar]
- Stein S., Brink L. Amino acid analysis of proteins and peptides at the picomole level: the fluorescamine amino acid analyzer. Methods Enzymol. 1981;79(Pt B):20–25. doi: 10.1016/s0076-6879(81)79008-6. [DOI] [PubMed] [Google Scholar]
- Szebenyi D. M., Obendorf S. K., Moffat K. Structure of vitamin D-dependent calcium-binding protein from bovine intestine. Nature. 1981 Nov 26;294(5839):327–332. doi: 10.1038/294327a0. [DOI] [PubMed] [Google Scholar]
- Weir M. D., Patel A. J., Hunt A., Thomas D. G. Developmental changes in the amount of glial fibrillary acidic protein in three regions of the rat brain. Brain Res. 1984 Aug;317(2):147–154. doi: 10.1016/0165-3806(84)90092-0. [DOI] [PubMed] [Google Scholar]
- Wilson P. W., Harding M., Lawson D. E. Putative amino acid sequence of chick calcium-binding protein deduced from a complementary DNA sequence. Nucleic Acids Res. 1985 Dec 20;13(24):8867–8881. doi: 10.1093/nar/13.24.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller N. K., Hunkeler M. J., Campagnoni A. T., Sprague J., Lazzarini R. A. Characterization of mouse myelin basic protein messenger RNAs with a myelin basic protein cDNA clone. Proc Natl Acad Sci U S A. 1984 Jan;81(1):18–22. doi: 10.1073/pnas.81.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman J. E., Herschman H. R., Levine L. Appearance of a brain specific antigen (th S-100 protein) during human foetal development. J Neurochem. 1970 Feb;17(2):247–251. doi: 10.1111/j.1471-4159.1970.tb02207.x. [DOI] [PubMed] [Google Scholar]