Abstract
Purpose: The present study aims to investigate the antitumor effect and possible mechanisms of chlorin e6 (Ce6)-mediated sono-photodynamic therapy (Ce6-SPDT) on murine 4T1 mammary cancer cells in vitro.
Materials: Cellular uptake and intracellular distribution of Ce6 in 4T1 cells were detected by flow cytometry and confocal microscope. Cells after loading with 1 μg/mL Ce6 were exposed to ultrasound at 1.0 MHz for up to 1 minute with an intensity of 0.36 W/cm2 and laser light with total radiation dose of 1.2 J/cm2. Cell viability and clonogenicity were determined by MTT assay and colony formation assay. Apoptosis was analyzed by DAPI staining, Western blots were used to detect the activity of Caspase-3. DNA damage, mitochondrial membrane potential (MMP), and intracellular reactive oxygen species (ROS) of 4T1 cells were also evaluated by flow cytometry. FD500 was employed to detect changes of membrane permeability after ultrasound.
Results: Ce6 rapidly entered 4T1 cells within 4 hours after it has been added and displayed a mitochondria-localization pattern. Compared with sonodynamic therapy (SDT) and photodynamic therapy (PDT) alone, the combined SPDT treatment further enhanced cell viability loss, DNA damage, and clonogenicity inhibition. DAPI staining and western blots analysis reflected that cells with apoptotic morphological characteristics and the activity of Caspase-3 were apparently increased in the combined group. Besides, SPDT caused obvious MMP loss and intracellular ROS generation at early 1 hour post treatment. Interestingly, the SPDT induced cell viability loss and cell apoptosis was greatly inhibited by pre-treatment with ROS scavenger N-acetylcysteine and Caspase inhibitor z-VAD-fmk. FD500 detection showed that ultrasound enhanced cell membrane permeability, implying much higher uptake of Ce6 might be involved in PDT therapy by pre-ultrasound treatment.
Conclusions: The findings demonstrated that Ce6-mediated SPDT enhanced the antitumor efficacy on 4T1 cells compared with SDT and PDT alone, a Caspase-dependent apoptosis and loss of MMP, generation of ROS may be involved.
Key words: : 4T1 cells, apoptosis, Chlorin e6, photodynamic therapy, sonodynamic therapy
Introduction
Photodynamic therapy (PDT) is a clinical treatment of various cancers, with cytotoxicity resulting from the production of reactive oxygen species (ROS), which involves the administration of a photosensitizer, followed by light irradiation in the target tissue.1–3 First proposed by Umemura et al. in 1989, sonodynamic therapy (SDT) is a new approach for cancer therapy derived from PDT,4 and is still in the experimental study with distinct advantage of focusing ultrasound energy to target deep tissues sites and activate the sonosensitizing compound locally.5
With the continuous development and improvement of both PDT and SDT, there comes Sono-Photodynamic Therapy (SPDT), which combines light and ultrasound to activate photosensitizer to produce both photochemical and sonochemical activities. Although there are very few reports about it and little information concerning the responses of cancer to SPDT is known, the combined therapy has shown a more remarkable anticancer effect than any monotherapy.6–8
Undoubtedly, the sensitizers are the key factors for PDT and SDT. Chlorin e6 (Ce6) is a hydrophilic sensitizer derived from porphyrin.9 Ce6 has been shown to accumulate more effectively in tumors, absorb more strongly at longer wavelengths (670 nm), clear faster from the organism, and could be activated by both light and ultrasound.10–12 It has been reported that apoptosis induction is arguably the most potent defense against cancer.13 Recently, Yumita et al. reported that the combination of ultrasound and chlorin-e6 can sonochemically induce apoptosis and necrosis in HL-60 cells.14 Nevertheless, the effectiveness of cell damage induced by Ce6 mediate combination of SDT and PDT has barely been reported.
Breast cancer has been one of the most fatal cancers threatening the health of women for decades. Traditional therapies such as radiotherapy, chemotherapy, and surgery have some limitations because of poor prognosis and serious side effects. The growth and metastasis of 4T1 cancer cell in Balb/c mice are similar to that of breast cancer in human body. In the present study, we select murine 4T1 mammary cancer cell line as experimental model to evaluate the antitumor effect and the underlying mechanisms of Ce6-mediated SPDT with the presence of 1 μg/mL Ce6, exposure to ultrasound with the frequency of 1.0 MHz, intensity of 0.36 W/cm2 for up to 1 minute, and laser light with total radiation dose of 1.2 J/cm2. As evidenced by this article, the antitumor efficacy of combined therapy is remarkably enhanced compared with SDT and PDT alone accompanied by an apoptotic response and proposed some possible mechanism such as loss of MMP; generation of ROS may be associated with the action. We hope this study should provide some new findings to SPDT therapy.
Materials and Methods
Chemicals
Ce6 was purchased from Sigma Chemical Company (St Louis) and was dissolved in phosphate buffered saline (PBS) at 2.5 mg/mL, sterilized, and stored at −20°C in dark. Rhodamine-123 (RHO123), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide etrazolium (MTT), FITC dextran (FD500), z-VAD-fmk (z-VAD), propidium iodide (PI), 4′-6-diamidino-2-phenylindole (DAPI), N-acetylcysteine (NAC), and Ho33342 (Ho) were obtained from the Sigma Chemical Company. Mito Tracker Green (MT-G) and 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) were supplied by Molecular Probes, Inc.(Invitrogen). All other reagents were commercial products of analytical grade.
Cell culture
Murine 4T1 mammary cancer cell line was obtained from the department of basic medicine, Union Medical College, Beijing, China. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco, Life Technologies, Inc.) supplemented with 10% fetal bovine serum (FBS; Hyclone), 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 mM L-glutamine, in a incubator with 5% CO2 and 100% humidity at 37°C. 4T1 cells cultured for 12 hours to 70% of confluence and exponential phase of growth were used in our study.
Ultrasound and laser light treatment
A 35 mm diameter planar transducer (Institution of Applied Acoustics, Shaanxi Normal University) was submerged in an acrylic container filled with cold degassed water (4°C), temperature increase was monitored with a digital thermometer, and no significant variation of temperature was detected (≤2°C) to avoid thermal effect. Continuous-wave ultrasound was generated by the generator (T&C Power Conversion, Inc.). Cells in 35 mm culture dish were placed in the water bath and 10 mm above the top of the transducer in a horizontal position for sonication. The frequency of 1.0 MHz, intensity of 0.36 W/cm2, and duration of 1 minute was used for ultrasound treatment.
The diode laser (excitation wavelength: 650 nm; power intensity: 10.4 mW/cm2) was used as a source for evocation of the photodynamic effect. Irradiance was measured by the radiometer (Institute of Photonics & Photon-technology, Department of physics, Northwest University). The process lasted 2 minutes and total dose of radiation was 1.2 J/cm2.
Sonodynamic therapy and/or photodynamic therapy
4T1 cells cultured on 35 mm culture dishes (Corning Company) to 70% of confluence were used in the photodynamic and ultrasound treatment protocol and were randomly divided into four groups: (1) control (no treatment), (2) SDT (Ce6 plus ultrasound), (3) PDT (Ce6 plus laser light), and (4) SPDT (Ce6 plus ultrasound plus laser light). The cells were incubated with 1 μg/mL Ce6 in serum-free DMEM for 4 hours in dark, allowing sufficient time for cell uptake of Ce6 to a maximum level, control cells were incubated with serum-free DMEM without Ce6 for an equivalent time. Cells in SDT group were exposed to ultrasound with frequency of 1.0 MHz and intensity of 0.36 W/cm2 for 1 minute. Cells in PDT group were irradiated with total laser light dose of 1.2 J/cm2. For SPDT group, cells were exposed to ultrasound then immediately irradiated with laser light under the same condition. After treatment, cells were cultured for an additional time as specified in the text and subjected to different analysis. All procedures were carried out in low-level light to minimize any influence of photo-activation.
Special ROS scavenger NAC and broad-spectrum Caspase inhibitor z-VAD were added to culture medium 1 hour prior to loading Ce6 for inhibitory studies. The inhibitors at the used concentrations did not yield any significant cell damage to the cultured cells.
Evaluation of cellular uptake and intracellular distribution of Ce6
Cells cultured in 24-well culture plates for 12 hours to 70% of confluence were used to evaluate the cellular uptake of Ce6, which is natively fluorescent. 4T1 cells were incubated with various concentrations of Ce6 (0, 0.5, 1, 2, and 5 μg/mL) in serum-free DMEM at different times (from 0 to 5 hours). At different time points, cells were trypsinized after being washed by PBS, then re-suspended by PBS, and the specific fluorescence intensity of Ce6 was immediately measured by flow cytometry (Guava technologies, Inc., Millipore) under laser emitting excitation light at 488 nm with an Argon laser, which indirectly reflected the cellular uptake of Ce6 in 4T1 cells with different incubation time.
For subcellular localization, 4T1 cells were incubated with 1 μg/mL Ce6 for 4 hours at 37°C in a CO2 incubator. Cells were co-loaded with 3 nM Mito Tracker Green (MTG; mitochondria probe) at 37°C for 20 minutes. Then, they were co-loaded with 1 μg/mL Hoechst 33342 (Ho, nucleus dye) at room temperature for 10 minutes. Cells were washed with PBS after each loading, and imaged with an inverted confocal microscope (TCS SP5 Leica). In multichannel imaging, photo-multiplier sensitivities and offsets were set to a level at which bleed-through effects from one channel to another were negligible.
Cellular viability assay
The cytotoxic effect and the protective effect of NAC and z-VAD on 4T1 cells were determined by MTT assay. After different treatments, cells were washed by PBS then harvested by trypsinization and resuspended by DMEM with 10% FBS, and added to 96-well culture plates (100 μL/well), followed by adding 10 μL MTT solution (5 mg/mL, dissolved in PBS), and the mixture was incubated for 4 hours at 37°C in a CO2 incubator. The MTT solution was carefully removed after centrifugation and 150 μL dimethyl sulfoxide (DMSO) was added to solubilize the violet formazan crystals. The absorbance of the resulting solution was measured in a 96-well microplate reader (BIO-TEK ELX800) at 570 nm against the reference value at 630 nm. The results were determined as percentage of control.
Analysis of DNA fragmentation
Krysko et al. described an easy and quantitative method to analyze DNA fragmentation based on flow cytometry detection of DNA hypoploidy after the procedure of staining with PI and permeabilizing them by freeze-thawing.15 PI intercalates in the DNA and the size of DNA fragmentations appears as a hypoploid DNA histogram. At 16 hours post treatment, 4T1 cells were harvested and washed with PBS then re-suspended in 200 μL serum-free DMEM containing 5 μg/mL PI in 1.5 mL eppendorf tube. The tubes were immediately placed in liquid nitrogen for 30 seconds then thawed in 37°C for 5 minutes. Samples were analyzed for DNA content under laser emitting excitation light at 488 nm using a flow cytometry (Millipore).
Colony formation assay
Colony formation assay was performed to evaluate the long-term proliferative potential of 4T1 cells following SPDT therapy. Cells were harvested by trypsinization and diluted gradient ratio by DMEM with 10% FBS, and seeded into six-well plates at a density of 100 cells/well and cultured for 14 days, the medium was changed every 3 days until visible colonies formed. The experiment was carried out in triplicate and colonies were fixed with formaldehyde at 4°C for 15 minutes after washed by PBS, and then stained with Giemsa for 30 minutes. Colonies containing 50 cells were manually counted, and cloning efficiency was calculated using the following equation: relative clone formation rate (%)=number of clones formed in treatment group/number of clones formed in control group ×100%.
Fluorescence microscopy for morphological detection
Fluorescence probe DAPI can bind to natural double-stranded DNAs and represent the change of the nuclei morphology. At 4 hours after treatment, 4T1 cells were washed by PBS and fixed with 3.5% paraformaldehyde at 4°C for 10 minutes and stained with 4 μg/mL DAPI in PBS at 37°C for 15 minutes. Moreover, we co-administrated NAC (5 mM) and z-VAD (5 μM) in SPDT group for this detection. Both fluorescent image and the corresponding phase-contrast image were acquired with the same exposure settings using a fluorescence microscopy (Nikon E-600).
Western blot analysis of Caspase-3 activity
Sodium dodecyl sulfate-poly-acrylamide gel electrophoresis gel (SDS-PAGE) and immunoblotting were performed according to standard procedures to detect the activity of apoptosis-related protein Caspase-3. Briefly, at 4 hours after different treatment, cells were harvested and lysed with RIPA buffer (containing 50 mM Tris-HCl (pH7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, 1 μM leupeptin, and 0.01 μM aprotinin) on ice. The protein content of the lysate was measured using the BCA protein assay reagent. Similar amount of protein was analyzed on SDS-PAGE and transferred onto polyvinylidene nitrocellulose membranes (Millipore). Membranes were then incubated at room temperature for 1 hour in blocking buffer (5% low-fat milk powder in tris-buffered saline-tween 20) and incubated with Caspase-3 primary antibodies (Cell Signaling Technology) overnight at 4°C. The bound primary antibodies were then tagged with IR Dye 680-labeled secondary antibodies (Li-cor, Biosciences) at room temperature for 1 hour. The infrared fluorescence was detected with the Odyssey infrared imaging system (Li-Cor Bioscience). Anti-β-actin (Santa Cruz) was used to ensure equal loading.
Measurement of MMP and ROS production
Changes of mitochondrial membrane potential (MMP) were measured by uptake of fluorescent cationic Rhodamine 123 (RHO123) in mitochondria with an intact membrane potential.16 Once the MMP is lost, it cannot provide enough potential gradient for RHO123 to be absorbed and retained in the mitochondria, and the fluorescence intensity decreases. At 1 hour post different treatment, 4T1 cells were washed with PBS, incubated with 1 μg/mL RHO123 in serum-free DMEM for 30 minutes at 37°C in dark, then harvested by re-suspended with PBS and approximate 2×103 cells of each treatment were detected immediately detected by flow cytometry (Millipore) with the excitation wavelength at 488 nm. Data analysis was performed using FCS ExpressV3 software.
Intracellular ROS production was assessed by measuring the fluorescence intensity of dichlorofluorescein (DCF) as described by other investigators.17 A non-fluorescent cell-permeant compound, 2,7-DCF-diacetate (DCFH-DA) is cleaved by endogenous esterases to DCFH when taken up by cell. DCFH reacts with intracellular ROS to generate a new highly fluorescent compound (DCF), which can be analyzed with flow cytometry to reflect the ROS generation. At 1 hour after different treatment, cells were incubated with 1 μM DCFH-DA for 30 minutes in dark with gentle shaking. Then, cells were washed and resuspended by PBS and immediately detected by flow cytometry (Millipore); histograms were analyzed using FCS Express V3.
Detection of the permeability of cell membrane
FD500 was used to detect the changes of cell membrane permeability after ultrasound. FD500 is the conjugate of fluorescein FITC and dextran with a molecular weight of 500,000, which makes it difficult to penetrate the cellular membrane under normal conditions. FD500 can enter cells when membrane permeability is enhanced during sonoporation. 4T1 cells cultured in 35 mm dishes for 12 hours to 70% confluence were washed with PBS and 2 mL FD500 (1 mg/mL) was added, and then immediately exposed to ultrasound at a frequency of 1.0 MHz and intensity of 0.36 W/cm2 for 1 minute. Cells were washed thrice with PBS and observed using a fluorescence microscope.
Statistical analysis
All data in figures were presented as mean±standard deviation of three independent experiments. The statistical significance was determined using one-way analysis of variance; differences between the groups and control were considered statistically significant when p-values <0.05.
Results
Cellular uptake and intracellular distribution of Ce6
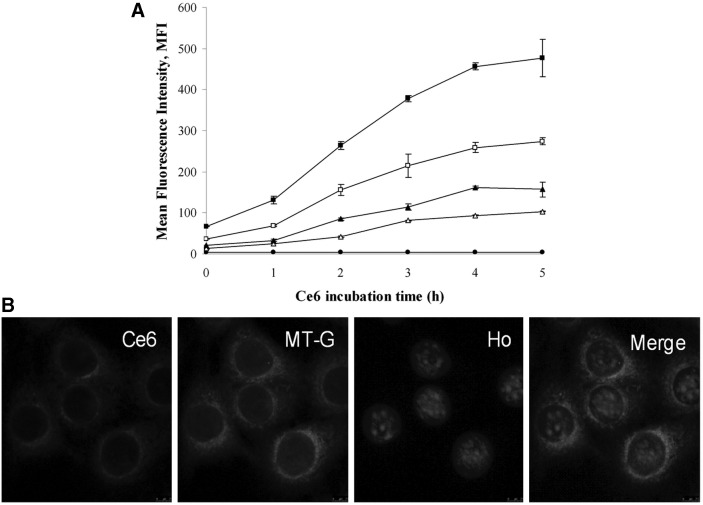
Cellular uptake of Ce6 following various concentration and different incubation times is shown in Figure 1A, indicating Ce6 in intracellular metabolic process was a dynamic change trend. Intracellular Ce6 content rapidly increased at the first few hours when 4T1 cells were co-incubated with various initial concentration of Ce6 (0.5, 1, 2, 5 μg/mL) and reached a relatively high level at 4 hours after incubation. The result suggested that 4 hours may be the optimal incubation time of Ce6 with 4T1 cells for our follow-up ultrasound and laser light irradiation experiments.
FIG. 1.
Uptake and intracellular distribution of Ce6 in 4T1 cells. (A) Alterations of Ce6 fluorescence intensity in 4T1 cell at various Ce6 concentrations with different incubation time was measured by flow cytometry. Data are presented as mean±SD from three independent experiments. Ce6 concentration: ■, 5 μg/mL; □, 2 μg/mL; ▲, 1 μg/mL; △, 0.5 μg/mL; ●, 0 μg/mL. (B) After incubation with 1 μg/mL Ce6 for 4 hours, cells were stained with 3 nM MT-G (mitochondria probe) and 1 μg/mL Ho (nucleus dye). After co-loading, cells were visualized by confocal microscopy to evaluate intracellular distribution of Ce6 in 4T1 cells.
The intracellular distribution of photosensitizer is important for its activity; therefore, the distribution of Ce6 in 4T1 cells was examined. After 4 hours incubation of Ce6, 4T1 cells were co-loaded with mitochondria probe MT-G and nuclear probe Ho. The result displayed that Ce6 labeling pattern corresponded well with the mitochondria probe MT-G, but it did not overlap with Ho (Fig. 1B). The co-staining images of Ce6 and MT-G showed a good overlapping of both fluorescent signals indicating a higher concentration of Ce6 in mitochondria in 4T1 cells, where Ce6 mainly accumulated.
Antitumor effect on 4T1 cells after Ce6-SPDT
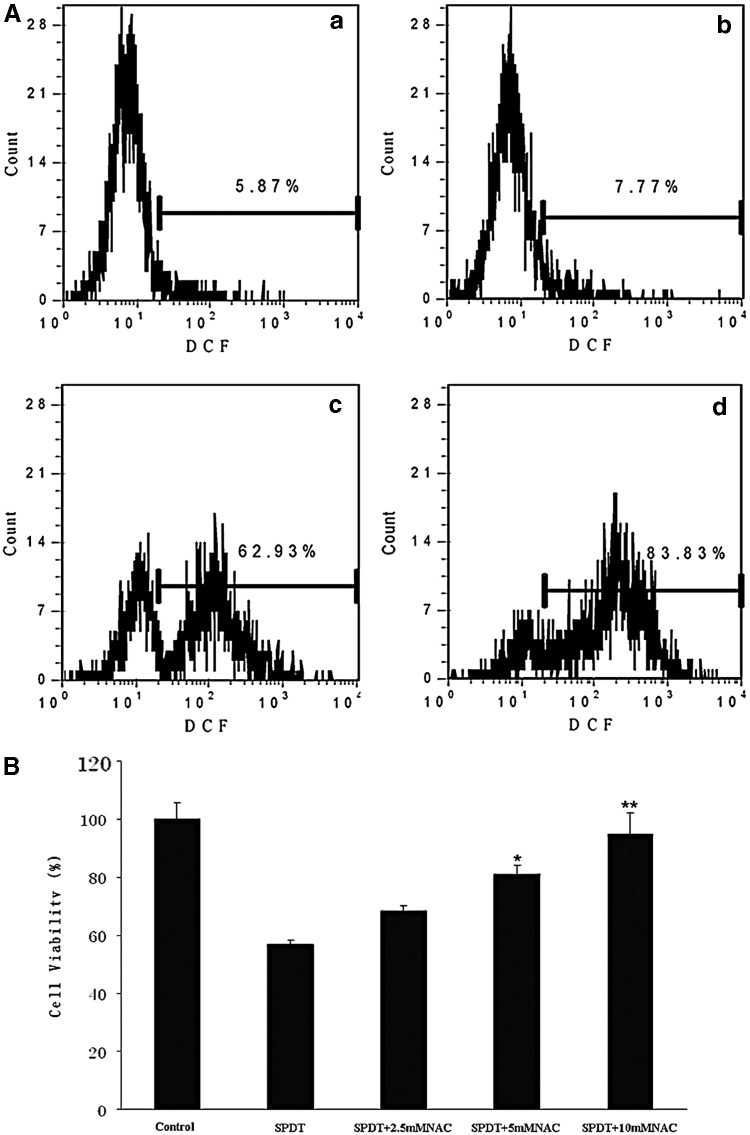
Cytotoxicity of 4T1 cells was assessed by MTT assay 4 hours after treatment. 1 μg/mL Ce6 alone has no cytotoxicity with the viability of 101.52%, ultrasound alone, light alone, and ultrasound plus light, all showed no inhibitory effect on 4T1 cells and their viability were 99.41%, 101.84%, and 100.77%, respectively (Fig. 2A). As shown in Figure 2B, the cell viability of SDT alone was 85.06%, showing no significant cytotoxic effect on 4T1 cells compared with control (p>0.05). The cell viability declined to 69.11% after PDT (p<0.05). Cell viability apparently declined to 47.8% (p<0.01) following SPDT, showing significant difference with both SDT group and PDT group (p<0.01 and p<0.05, respectively), displaying a synergistic enhancement of cytotoxicity induced by combined therapy of SDT with PDT.
FIG. 2.
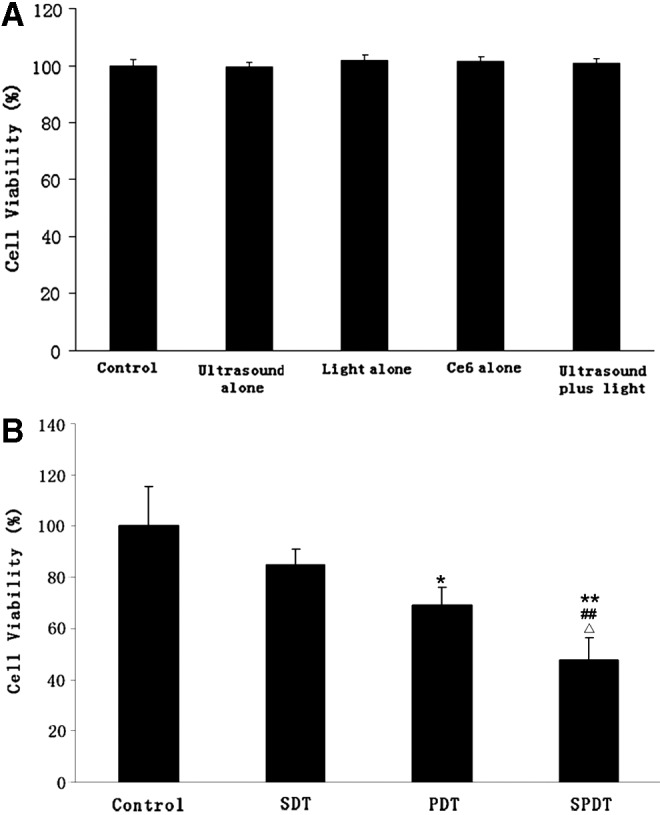
Viability of 4T1 cells at 4 hours after different treatment. (A) Control, no treatment; ultrasound alone, cells exposed to ultrasound alone for 1 minute (without Ce6); Light alone, cells irradiated by laser light alone (without Ce6); Ce6 alone, cell administrated with Ce6 alone (without light or ultrasound); ultrasound plus light, ultrasound irradiated for 1 minute plus laser light. (B) Control, no treatment; SDT, ultrasound exposed for 1 minute in presence of Ce6; PDT, laser light irradiated in presence of Ce6; SPDT, sono-photodynamic therapy, ultrasound irradiated for 1 minute in presence of Ce6 followed by laser light immediately. The concentration of Ce6 was 1 μg/mL. Ultrasound with frequency of 1.0 MHz, intensity of 0.36 W/cm2, laser light with total radiation dose of 1.2 J/cm2 were applied in the treatment. All data are expressed as percentage of control; error bars represent SD of the mean from three independent experiments. *p<0.05 and **p<0.01 versus untreated control, ##p<0.01 versus SDT group and ▵p<0.05 versus PDT group. SDT, sonodynamic therapy; PDT, photodynamic therapy.
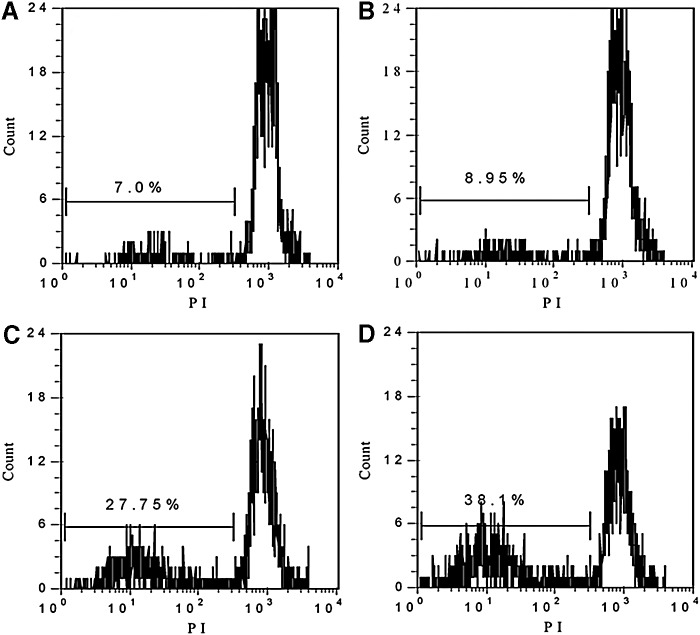
As described in the Materials and Methods section, the DNA fragmentation was detected by flow cytometry. Figure 3 showed that DNA fragmentation of control is 7.0% after 16 hours of incubation post treatment, those of SDT alone and PDT alone were 8.95% and 27.75%, respectively, the DNA fragmentation increased to 38.1% after SPDT treatment. The results implied that Ce6-mediated SPDT markedly induced DNA damage of 4T1 cells compared with SDT and PDT alone.
FIG. 3.
DNA fragmentation of 4T1 cells at 16 hours after different treatment. The experiment was carried out in triplicate as described in the Materials and Methods section. (A, Control; B, SDT; C, PDT; D, SPDT.) SPDT, Sono-Photodynamic therapy.
Decreased cell viability and increased DNA damage showed an overall decline in the proliferative potential of population cells. Colony formation assay was performed to evaluate the ability of proliferation and clonogenicity of single 4T1 cell following SPDT therapy. Relative colony formation rate of SDT alone, PDT alone, and SPDT were 84.93%, 59.59%, and 26.02%, respectively (Fig. 4), displaying Ce6-SPDT resulted in more significant inhibition of clonogenicity and long-term proliferation upon single 4T1 cell compared with SDT (p<0.01) and PDT alone (p<0.05).
FIG. 4.
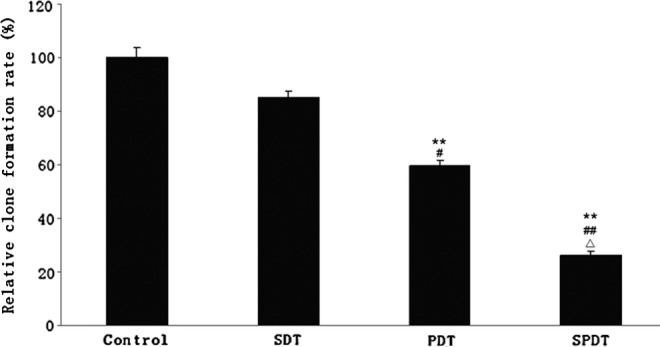
Colony formation assay of 4T1 cells after different treatment. The relative clone formation rate expressed as percentage of clones formed in experimental group as control group. **p<0.01 versus untreated control, #p<0.05, ##p<0.01 versus SDT group and ▵p<0.05 versus PDT group.
Apoptosis induction in 4T1 cells after Ce6-SPDT
Increased DNA fragmentation maybe a typical biochemical feature of apoptosis. To determine whether the anti-proliferative effect of Ce6-SPDT on 4T1 cells was related to apoptotic cell death, we performed DAPI staining to examine the morphological changes induced by Ce6-SPDT. As shown in Figure 5, the nuclei of control cells appeared to be round with undamaged chromatin and homogenously stained with faint fluorescence, normal growth state was shown with intact cell plasma membrane in phase contrast (Fig. 5A, a). Cells in SDT alone had no significant morphological changes compared with control, displaying a weak well-distributed fluorescence with integrated morphology and had no visible nuclei changes (Fig. 5B, b). Cells in PDT group showed slight enhancing DAPI staining, few cells indicated damaged nuclei with decreased size, certain degree of cellular retraction was also observed in phase contrast (Fig. 5C, c). Decreased size nucleus, enhanced DAPI staining observed in SPDT group seemed to reflect the typical apoptosis characteristics of altered nuclei morphology such as nuclear agglutination and fragment, phase contrast showed more serious cellular shrinkage with abnormal round type, too (Fig. 5D, d).
FIG. 5.
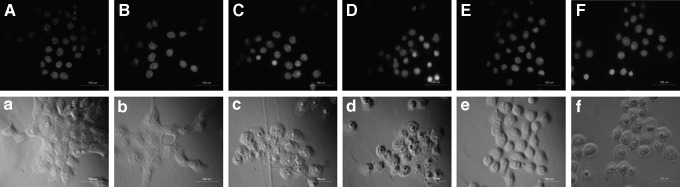
Apoptosis observed by DAPI staining for morphology detection. Fluorescence and phase contrast images were captured at 4 hours post treatment with the same exposure settings under fluorescent microscope. (A) Control; (B) SDT; (C) PDT; (D) SPDT; (E) SPDT in presence with 5 mM NAC; (F) SPDT in presence with 5 μM z-VAD. The corresponding phase contrast images were shown (a–f ). (Scale bar: 100 μm.) NAC, N-acetylcysteine.
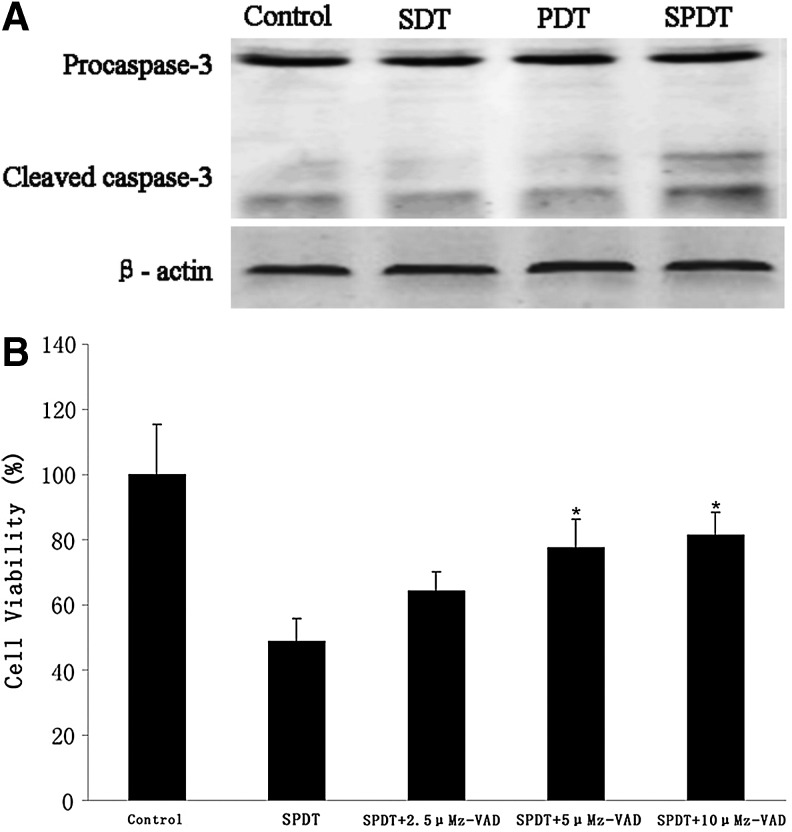
Further, we attempted to verify the activity of key apoptosis related protein Caspase-3 by western blot analysis to evaluate whether SPDT induced apoptosis was Caspase dependent. The result in Figure 6A showed that SPDT enhanced the expression level of cleaved Caspase-3, indicating SPDT resulted in more obvious Caspase-3 activation compared with SDT and PDT. Broad-spectrum Caspase inhibitor z-VAD rescued the cytotoxicity of 4T1 cells induced by SPDT in a concentration dependent manner (Fig. 6B). Additionally, typical apoptosis characteristics of nucleus were partially inhibited when pretreated with z-VAD, even though serious cell shrinkage was observed (Fig. 5F, f). These suggested Ce6-SPDT could induce apoptotic cell death of 4T1 cells, which might be Caspase dependent.
FIG. 6.
Activity of caspase-3 and effect of z-VAD on cell viability of 4T1 cells. (A) Western blotting analysis of Caspase-3 cleavage. (B) MTT assay of cell viability in 4T1 cells 4 hours post SPDT treatment with or without Caspase inhibitor z-VAD pretreatment. *p<0.05 versus SPDT treatment.
MMP changes induced by Ce6-SPDT
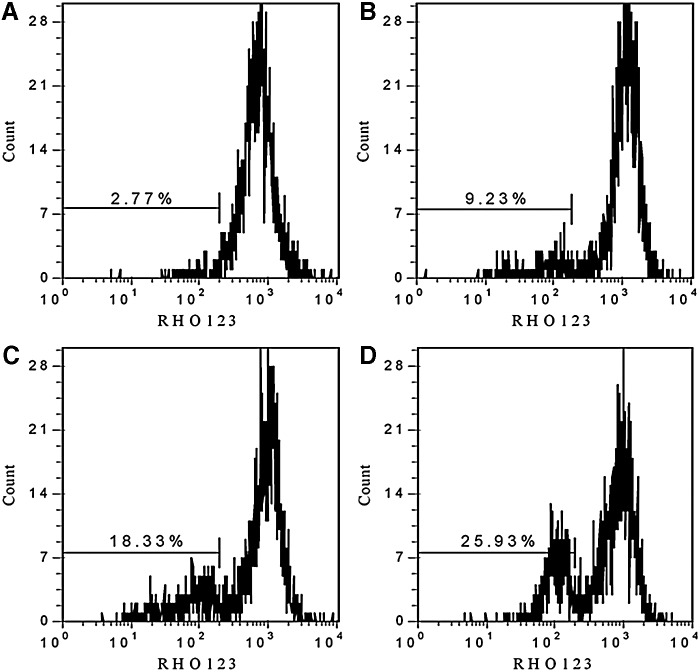
Figure 1B showed that Ce6 co-localized well with mitochondria, suggesting that mitochondria may be one of the dominating target of PDT and SDT. To detect the mitochondrial damage, we adopted RHO123 staining with flow cytometry to evaluate MMP changes induced by Ce6-SPDT. The mean fluorescence intensity of RHO123 directly proportional to MMP was used as an indicator. Results in Figure 7 showed that compared with control, SDT could not cause obvious MMP change, PDT alone caused certain degree of MMP loss in which about 18.33% of cells showed low RHO fluorescence, while in SPDT, the MMP loss was further enhanced, and the percentage of cells with low RHO123 fluorescence increased to 25.93%.
FIG. 7.
Changes of mitochondrial membrane potential detected by flow cytometry. Histograms show number of cell channel (vertical axis) versus RHO123 fluorescence (horizontal axis) 1 h-post treatment. (A) Control; (B) SDT; (C) PDT; (D) SPDT.
ROS involved in SPDT-induced apoptosis
We examined the possible mechanisms involved after determining the induction of apoptosis by SPDT. Presently, the proposed mechanisms of SDT-induced apoptosis mainly focus on the generation of intracellular ROS.18 Therefore, we monitored the intracellular ROS generation 1 hour after different treatment by measuring the conversion of non-fluorescent DCFH-DA to fluorescent DCF using flow cytometry. Statistic analysis of Figure 8A showed that there were no obvious cells in SDT group displaying higher DCF fluorescence compared with control, but 62.93% of cells in PDT group showed higher DCF fluorescence, and that increased to 83.83% in SPDT group, indicating Ce6-SPDT significantly enhanced the production of ROS. We co-administrated a special ROS scavenger NAC to further investigate the role of ROS in Ce6-SPDT-induced cell damage in 4T1 cells. MTT assay revealed that decreased cell viability caused by SPDT was obviously rescued by NAC in a concentration-dependent manner (Fig. 8B). Moreover, addition of NAC (5 mM) in SPDT group attenuated DAPI fluorescence and nuclei changes, its corresponding phase-contrast image indicated that cell shrinkage and cell morphological changes was rescued, too (Fig. 5E, e).
FIG. 8.
Reactive oxygen species (ROS) generation and effect of NAC on cell viability of 4T1 cells. (A) Measurement of intracellular ROS generation in 4T1 cells detected by flow cytometry 1 hour post treatment. (a) Control; (b) SDT; (c) PDT; (d) SPDT. (B) MTT assay of cell viability in 4T1 cells 48 hours post SPDT treatment with or without ROS scavenger NAC. *p<0.05 and **p<0.01 versus SPDT treatment.
Permeability of cell membrane enhanced by ultrasound
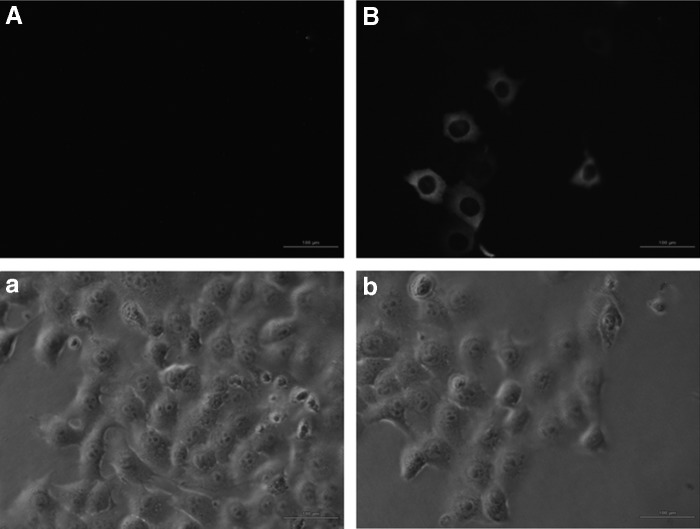
Changes of cell membrane permeability by ultrasound treatment were investigated via FD500. Images in Figure 9 showed obvious FD500 fluorescence in 4T1 cells immediately after ultrasound treatment, while there was no FD500 fluorescence in control cells, suggesting ultrasound enhanced cell membrane permeability and much higher uptake of Ce6 may be involved in PDT therapy by pre-ultrasound treatment. The corresponding phase-contrast image showed integrated cell morphology after ultrasound treatment, this correlate with the MTT assay that ultrasound alone caused no cellular toxicity. The result implied ultrasound merely enhanced cell membrane permeability, but neither altered cell morphology, nor decreased cell viability, so that more Ce6 might be uptaken by 4T1 cells postultrasound treatment, which may explain the enhanced PDT cytotoxicity by pre-ultrasound treatment.
FIG. 9.
Detection of cell membrane permeability. The fluorescence images and the corresponding phase-contrast image of 4T1 cells before (A, a) and immediately after (B, b) exposure to ultrasound with the macromolecule FD500 were acquired using a fluorescence microscopy with the same exposure settings. (Scale bar: 100 μm.)
Discussion
SPDT is a late-model and promising anticancer therapy using the combination of SDT and PDT. Preliminary studies have suggested the enhanced therapeutic effect compared with each monotherapy,6–8 but the potential mechanisms have not been fully explored. Therefore, this study was to investigate the antitumor effect of Ce6-mediated SPDT therapy on murine 4T1 mammary cancer cells and the potential mechanism of it.
Previous studies were focused on the factors that influence the efficiency of SDT or PDT, such as cell types, sensitizers, and the ultrasound or light exposure parameters. Studies about the potential of photosensitizer have suggested that PDT and SDT are effective to inhibit cancer growth by the induction of apoptosis.19–21 As an essential biologic process, apoptosis is characterized by the activation of Caspase and other features, and it is a complex mechanism that could be induced and regulated by many signal stimulus pathways. Recent investigations have revealed that combination of low-energy ultrasound with sensitizers could induce apoptosis in many cancer cells, and assumed that apoptosis induction is one of the potent defense against cancer.22
Sensitizer is a key component in PDT/SDT, and the site of sensitizer distribution and its uptake in cells is potentially critical for the therapeutic effect, because of the very short lifetime and very short diffusion distance of some radical products derived from the sensitizer produced during the process.23,24 In the present study, Figure 1A illustrated Ce6 reached a relatively high level within 4 hours post its adding, suggesting that Ce6 co-incubated with 4T1 cells for 4 hours, which may be the optimal time for ultrasonic and light irradiation. Next, decreased cell viability and clonogenic cell survival were detected on 4T1 cells following Ce6-SPDT compared with SDT alone or PDT alone group. Apoptosis involves specific morphological and biochemical changes such as chromatin condensation, cell shrinkage, DNA fragmentation, activation of Caspase-3 and others. We conducted further experiments to determine whether the inhibition of cell viability observed after Ce6-SPDT was the result of induction of cell apoptosis. DAPI staining showed morphological change of apoptosis characteristics such as decreased size nuclear and enhanced DAPI fluorescence with serious cellular shrinkage in Ce6-SPDT compared with SDT alone or PDT alone group. Consistent with it, flow cytometric analysis displayed a significant increase of DNA fragmentation after SPDT compared with SDT alone or PDT alone group. These data suggested that an enhanced cytotoxicity and apoptotic response occurred in 4T1 cells following Ce6-SPDT.
Confocal microscopy showed that Ce6 primarily accumulated in the mitochondria of 4T1 cells, indicating mitochondria was the main target for photochemical or sonochemical damage and mitochondria damage might be the major cause for Ce6-SPDT-enhanced cytotoxicity of 4T1 cells. Increasing evidences show that changes in MMP is linked to apoptosis.25 Subsequently, we monitored an initial MMP drop in 4T1 cells after Ce6-SPDT, indicating disaggregation of MMP and functional impairment of mitochondrial after SPDT treatment. As the major energy generators, mitochondria-mediated apoptosis occurs in response to a wide range of stimuli. This result was consistent with our previous study of SDT,26 suggesting the mitochondria where Ce6 enriches tend to be the most effective damaging sites in SPDT treatment. And the decrease of MMP as a result of mitochondria depolarization in association with apoptosis appears to be a common event.
The most critical events that occur in the mitochondria during apoptosis are the structural and functional remodeling of this organelle and the subsequent apoptotic events initiated in the cytosol and nucleus. Many factors such as cytochrome C release from damaged mitochondria activate Caspase-9 proenzyme, and the active Caspase-9 in turn activates Caspase-3 that subsequently executes cell apoptosis.27 In our study, western blot showed visible enhancement of Caspase-3 cleavage in the combined group, and the caspase inhibitor z-VAD protected SPDT-induced cytotoxicity in 4T1 cells, indicating Caspase-dependent apoptosis might be involved in Ce6-SPDT.
In addition, investigations also show that damaged mitochondria stimulate increased ROS production, which subsequently activates the signaling pathways that regulate cell apoptosis.28,29 In our study, we found a rapid generation of intracellular ROS in PDT alone, SPDT group demonstrated even significant increase in ROS levels, whereas there was no occurrence in the control and SDT alone groups. The rapid generation of ROS found in treated cells would induce damage to mitochondria and lead to MMP changes, thereby triggering the activation of the intrinsic apoptotic caspase cascade.30 Moreover, co-administration with NAC, a special scavenger of ROS, cell cytotoxicity effect of 4T1 cells induced by SPDT was inhibited, and this inhibition was more obvious as the concentration of NAC increased. These results suggest an oxidative stress mechanism may be involved in response to Ce6-SPDT in 4T1 cells.
Moreover, SPDT effect was further evaluated by ultrasound-enhanced cell membrane permeability detection in the present study, which showed the big molecular FD-500 entered 4T1 cells after ultrasound treatment, implying much higher uptake of Ce6 might be involved in PDT therapy by pre-ultrasound treatment. Ultrasound significantly enhanced subsequent PDT effect, although the applied ultrasound would not cause serious cytotoxicity, which might be due to more Ce6 accumulation in 4T1 cells by ultrasound exposure. These results partially agree with previous reports, suggesting ultrasound promotes the aggregation content of a sensitizer and changed its distribution characteristics in cells.31,32
Conclusions
In summary, this study evaluated the therapeutic potential of Ce6-mediated SPDT on murine 4T1 mammary cancer cells, suggesting the combined SDT and PDT therapy enhanced antitumor effect and induced more evident cell apoptosis than either monotherapy. Although further investigations concerning combination effect of SPDT are needed, it appears that the principal mechanism of Ce6-SPDT stems from the loss of MMP and generation of ROS that subsequently results in Caspase-dependent apoptosis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81000999 and No. 10904087), the Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20100202110006), the Natural Science Foundation of Shaanxi Province, China (Grant No. 2011JQ4012), and the Fundamental Research Funds for the Central Universities (No. GK201102020).
Disclosure Statement
No financial conflict of interests exist.
References
- 1.Schuitmaker JJ, Baas P, van Leengoed HL, et al. Photodynamic therapy: A promising new modality for treatment of cancer. J Photochem Photobiol B 1996;34:3. [DOI] [PubMed] [Google Scholar]
- 2.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: An update. CA Cancer J Clin 2011;61:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastle M, Grimm S, Nagel R, et al. Combination of PDT and inhibitor treatment affects melanoma cells and spares keratinocytes. Free Radic Biol Med 2011;50:305. [DOI] [PubMed] [Google Scholar]
- 4.Umemura S, Yumita N, Nishigaki R, et al. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn J Cancer Res 1990;81:962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang W, Liu QH, Wang XB, et al. Potential mechanism in sonodynamic therapy and focused ultrasound induced apoptosis in sarcoma 180 cells in vitro. Ultrasonics 2009;49:786. [DOI] [PubMed] [Google Scholar]
- 6.Jin ZH, Miyoshi N, Ishiguro K, et al. Combination effect of photodynamic and sonodynamic therapy on experimental skin squamous cell carcinoma in C3H/HeN mice. J Dermatol 2000;27:294. [DOI] [PubMed] [Google Scholar]
- 7.Kessel D, Lo J, Jeffers R, et al. Modes of photodynamic vs. sonodynamic cytotoxicity. J Bio chem 1995;28:219. [DOI] [PubMed] [Google Scholar]
- 8.Kolarova H, Tomankova K, Bajgar R, et al. Photodynamic and sonodynamic treatment by phthalocyanine on cancer cell lines. Ultrasound Med Biol 2009;35:1397. [DOI] [PubMed] [Google Scholar]
- 9.Mojzisova H, Bonneau S, Vever-Bizet C, et al. Cellular uptake and subcellular distribution of chlorin e6 as functions of pH and interactions with membranes and lipoproteins. Biochim Biophys Acta 2007;1768:2748. [DOI] [PubMed] [Google Scholar]
- 10.Gijsens A, Missiaen L, Merlevede W, et al. Epidermal growth factor-mediated targeting of chlorin-e6 selectively potentiates its photodynamic activity. Cancer Res 2000;60:2197. [PubMed] [Google Scholar]
- 11.Sheleg SV, Zhavrid EA, Khodina TV, et al. Photodynamic therapy with chlorin-e(6) for skin metastases of melanoma. Photodermatol Photoimmunol Photomed 2004;20:21. [DOI] [PubMed] [Google Scholar]
- 12.Shi HT, Liu QH, Qin XF, et al. Pharmacokinetic study of a novel sonosensitizer chlorin-e6 and its sonodynamic anti-cancer activity in hepatoma-22 tumor-bearing mice. Biopharm Drug Dispos 2011;32:319. [DOI] [PubMed] [Google Scholar]
- 13.Trentham-Dietz A. Epidemiologic breast cancer research at the UW-Madison: A summary of past accomplishments and future directions. Wis Med J 2009;108:284. [PubMed] [Google Scholar]
- 14.Yumita N, Han QS, Kitazumi I, et al. Sonodynamically-induced apoptosis, necrosis, and active oxygen generation by mono-l-aspartyl chlorin e6. Cancer Sci 2008;99:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krysko DV, Vanden Berghe T, D'Herde K, et al. Apoptosis and necrosis: Detection, discrimination and phagocytosis. Methods 2008;44:205. [DOI] [PubMed] [Google Scholar]
- 16.Wang ZB, Liu YQ, Zhang Y, et al. Reactive oxygen species, but not mitochondrial membrane potential, is associated with radiation-induced apoptosis of AHH-1 human lymphoblastoid cells. Cell Biol Int 2007;31:1353. [DOI] [PubMed] [Google Scholar]
- 17.Duranteau J, Chandel NS, Kulisz A, et al. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 1998;273:11619. [DOI] [PubMed] [Google Scholar]
- 18.El-Sikhry HE, Miller GG, Madiyalakan MR, et al. Sonodynamic and photodynamic mechanisms of action of the novel hypocrellin sonosensitizer, SL017: Mitochondrial cell death is attenuated by 11, 12-epoxyeicosatrienoic acid. Invest New Drugs 2011;29:1328. [DOI] [PubMed] [Google Scholar]
- 19.Bednarz N, Zawacka-Pankau J, Kowalska A. Protoporphyrin IX induces apoptosis in HeLa cells prior to photodynamic treatment. Pharmacol Rep 2007;59:474. [PubMed] [Google Scholar]
- 20.Du HY, Olivo M, Tan BK. Hypericin-mediated photodynamic therapy induces lipid peroxidation and necrosis in nasopharyngeal cancer. Int J Oncol 2003;23:1401. [PubMed] [Google Scholar]
- 21.Jin H, Zhong X, Wang Z. Sonodynamic effects of hematoporphyrin monomethyl ether on CNE-2 cells detected by atomic force microscopy. J Cell Biochem 2011;112:169. [DOI] [PubMed] [Google Scholar]
- 22.P Lipponen. Apoptosis in breast cancer: Relationship with other pathological parameters. Endocr Relat Cancer 1999;6:13. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita M, Hynynen K. Mechanism of porphyrin-induced sonodynamic effect: Possible role of hyperthermia. Radiat Res 2006;165:299. [DOI] [PubMed] [Google Scholar]
- 24.Yumita N, Umemura S, Magario N, et al. Membrane lipid peroxidation as a mechanism of sonodynamically induced erythrocyte lysis. Int J Radiat Biol 1996;69:397. [DOI] [PubMed] [Google Scholar]
- 25.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria as targets for chemotherapy. Apoptosis 2009;14:624. [DOI] [PubMed] [Google Scholar]
- 26.Mi N, Liu QH, Wang XB, et al. Induction of sonodynamic effect with protoporphyrin IX on isolate hepatoma-22 cells. Ultrasound Med Biol 2009;35:680. [DOI] [PubMed] [Google Scholar]
- 27.Sheridan C, Martin SJ. Mitochondrial fission/fusion dynamics and apoptosis. Mitochondrion 2010;10:640. [DOI] [PubMed] [Google Scholar]
- 28.Choi H, Chun YS, Shin YJ, et al. Curcumin attenuates cytochrome P450 induction in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin by ROS-dependently degrading AhR and ARNT. Cancer Sci 2008;99:2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozben T. Oxidative stress and apoptosis: Impact on cancer therapy. J Pharm Sci 2007;96:2181. [DOI] [PubMed] [Google Scholar]
- 30.Li WT, Tsao HW, Chen YY, et al. A study on the photodynamic properties of chlorophyII derivatives using human hepatocellular carcinoma cells. Photochem Photobiol Sci 2007;6:1341. [DOI] [PubMed] [Google Scholar]
- 31.El Maalouf J, Bera JC, Alberti L, et al. In vitro sonodynamic cytotoxicity in regulated cavitation conditions. Ultrasonics 2009;49:238. [DOI] [PubMed] [Google Scholar]
- 32.Hiraoka W, Honda H, Feril LB Jr, et al. Comparison between sonodynamic effect and photodynamic effect with photosensitizers on free radical formation and cell killing. Ultrason Sonochem 2006;13:535. [DOI] [PubMed] [Google Scholar]