Abstract
Purpose: Study distribution, pharmacokinetics, and safety of intraperitoneal (IP) 212Pb-TCMC-trastuzumab in patients with HER-2-expressing malignancy.
Experimental Design: IP 212Pb-TCMC-trastuzumab was delivered, after 4 mg/kg intravenous (IV) trastuzumab, to 3 patients with HER-2-expressing cancer who had failed standard therapies. Patients were monitored for toxicity and pharmacokinetics/dosimetry parameters.
Results: Imaging studies after 0.2 mCi/m2 (7.4 MBq/m2) show little redistribution out of the peritoneal cavity and no significant uptake in major organs. Peak blood level of the radiolabeled antibody, determined by decay corrected counts, was <23% injected dose at 63 hours; maximum blood radioactivity concentration was 6.3nCi/mL at 18 hours. Cumulative urinary excretion was ≤6% in 2.3 half-lives. The maximum external exposure rate immediately post-infusion at skin contact over the abdomen averaged 7.67 mR/h and dropped to 0.67 mR/h by 24 hours. The exposure rates at the other positions monitored (axilla, chest, and femur) decreased as a function of distance from the abdomen. The data points correlate closely with 212Pb physical decay (T1/2=10.6 hours). Follow-up >6 months showed no evidence of agent-related toxicity.
Conclusions: Pharmacokinetics and imaging after 0.2 mCi/m2 IP 212Pb-TCMC-trastuzumab in patients with HER-2-expressing malignancy showed minimal distribution outside the peritoneal cavity, ≤6% urinary excretion, and good tolerance.
Key words: : antibody immunotherapy, cancer, intraperitoneal, ovarian, 212Pb-TCMC-trastuzumab
Introduction
The majority of patients with ovarian cancer are diagnosed after the disease has spread through the abdominal cavity. Although complete responses are common with optimal surgery and standard adjuvant chemotherapy, about half will experience recurrence that is usually confined to the abdominal cavity. Intensification of treatment by intraperitoneal (IP) chemotherapy and/or radionuclides has shown therapeutic efficacy, and some Phase III studies have documented improved survival and/or decreased abdominal failure rate.1,2 Much work remains to optimize the agents and their integration with other modalities to achieve improved outcome. Prior experience with IP radionuclide conjugate therapy suggests that a radionuclide with shorter half-life than the 2.7 days of 90Y and less γ emissions than 131I or 177Lu would allow dose escalation without excess toxicity.3
The potential for therapeutic targeted delivery of α-emitters has been recognized for many years as their cytotoxicity efficacy is ∼1000× that of β particles.4–10 Although the intense radiation and considerably shorter path length features of α-particles make them attractive, and may be optimal for radioimmunotherapy, their development/implementation has been challenging due to lack of availability, and poor stability of radiolabeled conjugates using chelators that were developed for β-emitters.11,12 The use of 212Pb (10.6 hours half-life) provides clinical feasibility. 212Pb itself is not an α-emitter, but its physical decay results in the emission of two short-lived α-particles with potent therapeutic efficacy to cellular nuclei. 212Pb decays to 212Bi via β emission. 212Bi, with a 60-minute half-life, has a split decay chain and emits an α particle at 36% frequency at an average of 6.1 MeV; 212Bi decays to 212Po the remaining 64% via β emission. The 212Po decays to stable 208Pb in microseconds by emission of an 8.8 MeV α particle. Betas are of low energy and/or frequency such that they are not expected to contribute significantly to toxicity or efficacy. Cumulative energy from the γ emissions is <12% of those from the αs, but the 238.6 keV γ ray with a 43% yield can be exploited for imaging.
The synthesis of the 2-(4-isothiocyanotobenzyl)-1, 4, 7, 10-tetraaza-1, 4, 7, 10-tetra-(2-carbamonyl methyl)-cyclododecane, or TCMC, chelator has overcome the problem that 212Pb was not stable with prior β-conjugate chelators. This has allowed extensive in vitro and animal model testing of 212Pb-TCMC-trastuzumab prior to this human trial. IP administration of 212Pb-TCMC-trastuzumab in a preclinical setting has demonstrated therapeutic activity against a variety of human tumor xenografts, and has allowed assessment of redistribution from the peritoneal cavity.13–17 Those studies provided the information required to progress to this initial clinical trial of IP 212Pb-TCMC-trastuzumab.
Materials and Methods
Patient population
Three patients with ovarian cancer who had progressed after multiple therapies were treated in the initial imaging/pharmacokinetics cohort of a Phase I trial. At the time of trial entry they had no evidence of significant compromise of normal organ function or other major illnesses. All had ascites but none had required paracentesis.
Trial design
The trial design included delivery of the investigational agent, 212Pb-TCMC-trastuzumab, as a single IP injection in patients with HER-2-expressing malignancies mainly confined to the peritoneal cavity who had failed standard therapy. The study was approved by the Western Institutional Review Board and was authorized by the food and drug administration (FDA). HER-2 expression of at least 1+ by immunohistochemistry in >10% of the cells was acceptable for gastric cancer; 30% was required for other diseases. Alternatively, HER-2 serum levels >15 ng/mL by enzyme-linked immunosorbent assay (ELISA) were allowed. Patients had to have free flow of fluid in the peritoneal cavity and were excluded for serious cardiac dysfunction, left ventricular ejection fraction <50%, poor organ function (defined as any of the following: elevated creatinine, total bilirubin >1.5× normal, aspartate transaminase (AST) and alanine aminotransferase (ALT) >2.5× normal, absolute neutrophil counts <1.5×103/μL, or platelets <100×103/μL), or other conditions that might compromise safety. Other exclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status >2, pregnancy or breast feeding, evidence of bowel obstruction or transmural involvement, prior radiation to the whole abdomen, prior IP radionuclide therapy, stem cell transplant, history of human immunodeficiency virus (HIV) or Hepatitis A antibody positivity, or detectable antibody to trastuzumab.
Eligible, consenting, adult patients were housed in a Clinical Research Unit where they received a single IP injection of 0.2 mCi/m2 (7.4 MBq/m2) 212Pb-TCMC-trastuzumab in 50 mL <4 hours following 4 mg/kg IV trastuzumab. Additional saline was instilled into the peritoneal cavity before and after 212Pb-TCMC-trastuzumab, for a total volume of ≤1000 mL. Post-treatment blood pharmacokinetics, urinary excretion, and biodistribution studies were performed. Blood samples were obtained immediately post-infusion and at 2, 8, 12, 18, 24, and 63 hours; urine was collected for 24 hours. Each void was collected, the volume was determined, and then 1 mL was counted. Blood samples were allowed to clot and spun, and a 1 mL aliquot of serum was counted. The well counter was operated with the window open to include 238.6 keV γ detection. Counts were corrected for decay between the time of collection and measurement. Simultaneous whole-body anterior and posterior γ images were obtained post-treatment and were repeated at 18–24 hours using a dual-headed Phillips Skylight Camera. These used a peak-energy window of 238.6 keV, which corresponds to a 212Pb γ emission. Images were obtained with a medium-energy general-purpose collimator at 12 cm/min, and high-resolution matrix settings. Dosimetry data were obtained with radiation detector counts immediately post-treatment and at 3 additional times over 24 hours. Probe measurements were taken at the axilla, the mid femur, the umbilicus, and over the sternum using the Inspector 1000 portable radiation detector (Canberra). The patients were followed for toxicity as defined in the Common Terminology Criteria for Adverse Events (NCI CTCAE v.4.03). As a precautionary measure (based on prior studies of other α-emitter conjugates) adjuvant medications were used. A saturated solution of potassium iodide (SSKI) was initiated the evening before treatment and it was continued for 3 days. Furosemide (40 mg) was also started the day before 212Pb-TCMC-trastuzumab and it was used for 10 days, and then followed by 100 mg spironolactone daily for 6 months as renal protective agents.
Investigational agent 212Pb-TCMC-trastuzumab
Trastuzumab is an FDA-approved humanized monoclonal antibody (Genentech) which has therapeutic efficacy by immunologic mechanisms in tumors that overexpress the HER-2 receptor.18 TCMC-trastuzumab was provided in a form for further manufacturing of an investigational drug product for human use, having passed required quality control tests. It was stored in 200-μL vials at a concentration of 5 mg/mL. Labeling was performed at 1 mg of TCMC-trastuzumab per mCi of purified 212Pb eluate. 212Pb generators were provided by AREVA Med and were shipped from Bessines-sur-Gartempes, France. Manufacturing of the final product was at the University of Alabama at Birmingham under good manufacturing practices.19 Quality control testing included instant thin layer chromatography (ITLC), endotoxin, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS PAGE), visual inspection, and quantification. The 212Pb-TCMC-trastuzumab was administered within 5 hours after passing all quality control measures. Sterility testing and radioimmunoassay were also performed post-infusion. Radioimmunoassay was used to test the affinity of the 212Pb-TCMC-trastuzumab product to HER-2.
Results
Three patients with ovarian cancer entered this Phase I trial after they had failed multiple prior therapies. Initially, the 3 patients had CA125 values of 748–115,000 at the time of diagnosis of Stage Ic–IIIc disease. Their CA125 values normalized after debulking surgery and initial six cycles of chemotherapy. All received at least six cycles of carboplatin plus another agent. Two received the standard of a taxane but patient No. 1 had difficulty with the first cycle of taxane and received gemcitabine as the second agent for the remaining five cycles. Patient No. 2 received bevacizumab in addition to the standard chemotherapy doublet and continued to receive bevacizumab until relapse at 15 months. Patients No. 1 and No. 3 did not receive additional therapy until they relapsed at 28 and 52 months, respectively. None had additional surgery for recurrence but all had computed tomography (CT) evidence of widespread disease in the peritoneal cavity. Table 1 provides a summary of information on these patients pre-212Pb-TCMC-trastuzumab.
Table 1.
Patient Demographics
| |
Patient |
||
|---|---|---|---|
| No. 1 | No. 2 | No. 3 | |
| Age at 212Pb-TCMC-trastuzumab infusion (years) |
46 |
67 |
83 |
| Months after surgery to first relapse |
28 |
15 |
52 |
| Months after surgery to 212Pb-TCMC-trastuzumab |
40 |
27 |
71 |
| No. of prior chemotherapy courses |
16 |
>23 |
>20 |
| No. of prior chemotherapy regimens | 2 | 4 | 3 |
The patients had placement of an IP catheter 1–2 days prior to therapy. All demonstrated free flow of fluid in the peritoneal cavity by serial 99mTc scans that were performed on the day of catheter placement. None had evidence of significant leakage from the cavity with the second scan 2 hours later. The patients tolerated therapy at the 0.2 mCi/m2 level with no more than mild acute discomfort associated with phlebotomy and catheter removal. Later adverse events were mild, and attributed to adjuvant medications rather than the experimental agent. Patient No. 3 had Grade 1 nausea and dizziness associated with SSKI. Subsequently, she developed an allergic reaction to spironolactone that resolved with discontinuation. Patient No. 1 had Grade 1 fatigue and increased muscle cramps. These were felt to be related to mild dehydration associated with the renal protective agents. There was no evidence of marrow suppression, new laboratory abnormalities, electrocardiogram (ECG), or echocardiography changes within 6 weeks post-treatment. No late toxicity attributed to the investigational agent has been noted at 6 months.
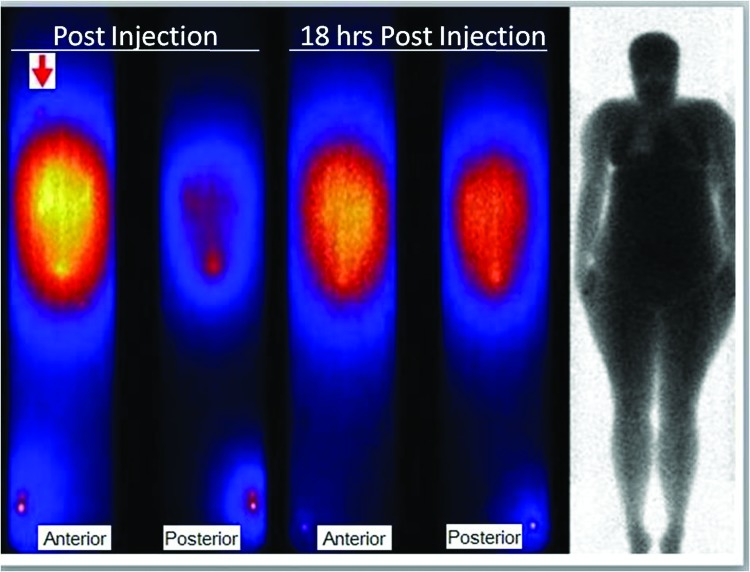
Gamma camera imaging studies after IP 212Pb-TCMC-trastuzumab showed no distribution of radioactivity out of the peritoneal cavity or normal organ uptake. As shown in Figure 1 even the delayed, repeat scan the day after therapy did not show activity outside the peritoneal cavity such that a shoulder marker plus a standard at the ankle were needed as anatomic indicators of body regions. The day-2 images illustrate the shift to more uniform anterior/posterior abdominal distribution over 18 hours and the loss of intensity due to radioactive decay compared with the early post-treatment images. The camera-measured drop in radioactivity from the abdomen in day 2 was similar to that of the other body regions that showed no evidence of accumulation and all were consistent with the physical decay half-life of 212Pb.
FIG. 1.
The immediate anterior and posterior whole-body scan images after IP 212Pb-TCMC-trastuzumab (left) are compared with the repeat scan the next day. 99mTc marker at the right shoulder (arrow) plus the 212Pb standard adjacent to the right ankle provide anatomic locations outside the abdominal area as does the transmission scan (right) that displays body anatomy.
Despite the lack of visualization of activity outside the peritoneal cavity, slow absorption/distribution occurred based on detectable radioactivity in the blood and urinary loss.
Peak blood conjugate level activity was <23% injected dose at the last time point of 63 hours as determined with decay correction (Table 2). The maximum blood concentration of 6 nCi/mL occurred at 18 hours (patient No. 2). Although this patient had a higher percent injected dose at 63 hours, the concentration was less then due to ensuing radioactivity decay. As shown in Table 2, the range of peak blood concentration was <1%–22.9%, since patient No. 3 had only 0.1 nCi/mL at the time of peak activity. Cumulative urinary loss ranged from 0.3% to 6% in 24 hours among the 3 patients. Patient No. 3, who had the lowest blood concentration, also had the lowest urinary loss.
Table 2.
% Injected Dose in Blood Determined by Decay Correction
| |
Hours post-infusion |
||
|---|---|---|---|
| 2 hours | 24 hours | 63 hours | |
| Patient No. 1 |
4.9 |
7.2 |
18.8 |
| Patient No. 2 |
4.4 |
21.0 |
22.9 |
| Patient No. 3 | 0.1 | 0.1 | 0.7 |
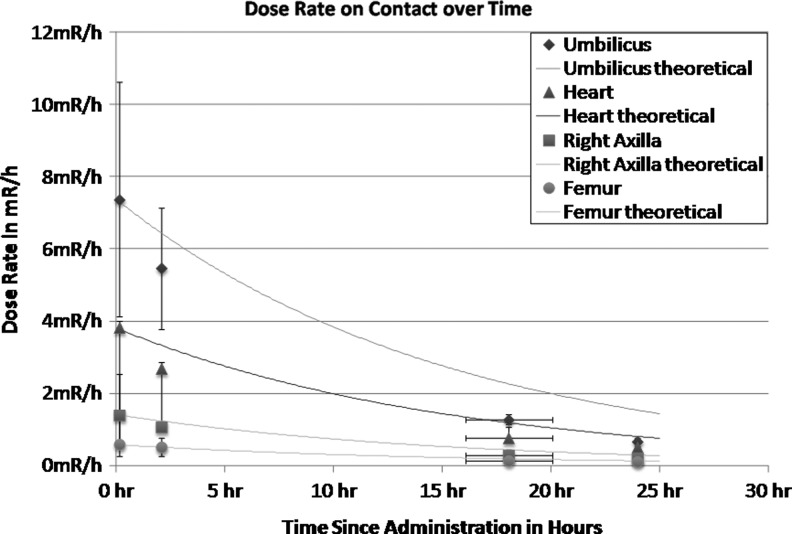
Detector-measured exposure rates at four body sites were monitored over 24 hours. The maximum dose rate immediately post-infusion over the abdomen averaged 7.67 mR/h and dropped to 0.67 (range 0.55–0.8) by 24 hours. The rate at the other sites monitored (axilla, chest, and femur) decreased as a function of their distance from the abdomen. The ratio of the average exposure rates at the abdomen over other sites was 2–12.8 post-infusion and changed to 1.3–5.9 by 24 hours. These changes over time were consistent with a very small amount of redistributed activity from the abdomen. The data points correlate closely with theoretic curves (Fig. 2), even without adjustment for urinary excretion of radioactivity.
FIG. 2.
Data points represent mean exposure rate of radioactivity at 4 time points in the initial 24 hours after IP 212Pb-TCMC-trastuzumab. Measurements over the abdomen are compared with those at the chest, axilla, and femur. The actual data points (mean±one standard deviation) are shown along with theoretical curves of physical radioactive decay. There was no adjustment for urinary excretion of radioactivity.
Discussion
Targeted IP therapy using a radionuclide with 10.6 hours of half-life and predominance of α emissions has potential for improved efficacy and decreased toxicity compared to β emitters used in treatment of malignancy that has spread throughout the peritoneal cavity. This first-in-human experience with IP 212Pb-TCMC-trastuzumab confirms its medical potential and feasibility.
The data obtained are consistent with those of prior non-human studies in showing prolonged retention of the 212Pb-TCMC-trastuzumab within the peritoneal cavity and no evidence for localization to normal organs on planar images.13–17 The starting dose level for this first cohort in a Phase I trial was low compared to theoretical tolerance and experience in other species but was consistent with the starting dose for another α particle radioimmunotherapy trial using 211At.20 This dose allowed monitoring of blood, urine, and imaging with minimal exposure to laboratory personnel. Subsequent dose groups will also be followed for toxicity but will have less quantitative pharmacokinetics/dosimetry data collection.
Some low-frequency and mainly low-energy γ-emissions from the decay path, plus bremsstrahlung radiations, allowed detection of gross biodistribution by γ-camera imaging. This was helpful to rule out targeted localization to normal organs such as the heart, thyroid, or kidneys. Given the relatively low activity administered, whole-body imaging of 212Pb was quite sensitive. After administration of 0.4 mCi, the anterior or posterior counts were 4.7 and 4.5 million counts, respectively, and 10 μCi of 212Pb in the counting standard at the patient's ankle was very clear in the image. The inability to distinguish anatomy outside the abdomen despite evidence of limited redistribution is influenced by scatter from 212Pb in the abdomen that affects contrast of body areas with background. Serial quantitative monitoring of spectral distribution at four body sites also confirmed the modest level of redistribution from the peritoneal cavity. The amount of radioactivity detected in the blood and urinary loss is consistent with that of other radiolabeled antibody conjugates.9,10,20–22 As a precaution to possible localization to organs/tissues outside the peritoneal cavity that might have HER-2 expression, a standard loading dose of 4 mg/kg trastuzumab was given intravenously within 4 hours prior to the IP injection of 212Pb-TCMC-trastuzumab.
212Pb-TCMC-trastuzumab is being developed to improve upon results with other radionuclides that have been proposed or evaluated for IP therapy such as 90Y and 177Lu by providing more potent radiation to targeted malignant cells while limiting radiation exposure to normal tissues.3 212Pb has a shorter half-life and path length (range of α radiation) compared with radionuclides that predominantly emit β particles.6–10,22 IP therapy using another α-emitting conjugate, 211At-MX35F(ab′)2, has shown promise as an adjuvant therapy for ovarian cancer in patients with no evidence of gross disease.20 Based on clinical experience and preclinical data, it is reasonable to expect that treatment at the time when disease deposits are only microscopic should provide the maximum therapeutic benefit.7,8,10,20212Pb-TCMC-trastuzumab may have beneficial activity against a number of malignancies that have HER-2 expression. Additional study of 212Pb-TCMC-trastuzumab and other α-emitting agents is warranted.10,22–32
Conclusions
Pharmacokinetics and imaging after IP 212Pb-TCMC-trastuzumab in patients with HER-2-expressing malignancy showed minimal distribution outside the peritoneal cavity, consistent with preclinical studies. This α-emitter radioimmunotherapy has potential for improved therapeutic ratio over β-emitter-targeted conjugate therapy and could add to the overall armamentarium of treatments for patients with HER-2-expressing malignancies. Additional study of this α-emitting radioimmunotherapy is warranted.
Acknowledgments
The authors express their appreciation to Tracey Cotton-Young, Charles Landen, Andres Forero, Max Austin, Mike Pfaff, Alma Del Grosso, and Martin Brechbiel for their contributions. This study was sponsored by AREVA Med and the National Institutes of Health (NIH) and the Center for Clinical and Translational Science (CCTS) grant 1UL1RR025777.
Author Disclosure Statement
The author has no significant financial interests that are related to or would reasonably appear to be affected by the proposed article. All authors involved in the article have been informed of their obligations under federal regulations governing disclosure of significant financial interests and have no conflicts of interest or potential conflicts of interest that have not been disclosed.
References
- 1.Verheijen RH, et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol 2006;24:571. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DK, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34. [DOI] [PubMed] [Google Scholar]
- 3.Macey DJ, Meredith RF. A strategy to reduce red marrow dose for intraperitoneal radioimmunotherapy. Clin Cancer Res 1999;5:3044s. [PubMed] [Google Scholar]
- 4.Sgouros G, et al. MIRD Pamphlet No. 22 (abridged): Radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med 2010;51:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young RC. Initial therapy for early ovarian carcinoma. Cancer 1987;60:2042. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs AJ, et al. A phase I trial of a rhenium 186-labeled monoclonal antibody administered intraperitoneally in ovarian carcinoma: Toxicity and clinical response. Obstet Gynecol 1993;82:586. [PubMed] [Google Scholar]
- 7.Hird V, et al. Adjuvant therapy of ovarian cancer with radioactive monoclonal antibody. Br J Cancer 1993;68:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epenetos AA, et al. Long term survival of patients with advanced ovarian cancer treated with intraperitoneal radioimmunotherapy. Int J Gynecol Cancer 2000;10:44. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblum MG, et al. Phase I study of 90Y-labeled B72.3 intraperitoneal administration in patients with ovarian cancer: Effect of dose and EDTA coadministration on pharmacokinetics and toxicity. Clin Cancer Res 1999;5:953. [PubMed] [Google Scholar]
- 10.Meredith R, et al. Predictors of long-term outcome from intraperitoneal radioimmunotherapy for ovarian cancer. Cancer Biother Radiopharm 2012;27:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macklis RM, et al. Alpha particle radio-immunotherapy: Animal models and clinical prospects. Int J Radiat Oncol Biol Phys 1989;16:1377. [DOI] [PubMed] [Google Scholar]
- 12.Jurcic J. Phase I trial of the targeted alpha-particle nano-generator actinium-225 (225Ac)-lintuzumab (anti-CD33; HuM195) in acute myeloid leukemia (AML). Blood 2011;118:768 [Google Scholar]
- 13.Milenic DE, et al. Potentiation of high-LET radiation by gemcitabine: Targeting HER2 with trastuzumab to treat disseminated peritoneal disease. Clin Cancer Res 2007;13:1926. [DOI] [PubMed] [Google Scholar]
- 14.Milenic DE, et al. Multimodality therapy: Potentiation of high linear energy transfer radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin Cancer Res 2008;14:5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milenic DE, et al. Improved efficacy of alpha-particle-targeted radiation therapy: Dual targeting of human epidermal growth factor receptor-2 and tumor-associated glycoprotein 72. Cancer 2010;116:1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azure M. Radiolabeling and imaging of 212Pb-TCMC-trastuzumab. World Molecular Imaging Congress, Kyoto, Japan, 2010 [Google Scholar]
- 17.Yong KJ, et al. Sensitization of tumor to (212)Pb radioimmunotherapy by gemcitabine involves initial abrogation of G2 arrest and blocked DNA damage repair by interference with Rad51. Int J Radiat Oncol Biol Phys 2013;85:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010;376:687. [DOI] [PubMed] [Google Scholar]
- 19.Tan Z, et al. Significant systemic therapeutic effects of high-LET immunoradiation by 212Pb-trastuzumab against prostatic tumors of androgen-independent human prostate cancer in mice. Int J Oncol 2012;40:1881. [DOI] [PubMed] [Google Scholar]
- 20.Andersson H, et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: Pharmacokinetics and dosimetry of (211)At-MX35 F(ab′)2—a phase I study. J Nucl Med 2009;50:1153. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez RD, et al. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: A phase I/II study. Gynecol Oncol 1997;65:94. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez RD, et al. A phase I study of combined modality (90)Yttrium-CC49 intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res 2002;8:2806. [PubMed] [Google Scholar]
- 23.Elgqvist J. Targeted alpha therapy: Part I. Curr Radiopharm 2011;4:176. [DOI] [PubMed] [Google Scholar]
- 24.Baidoo KE, et al. Molecular pathways: Targeted alpha-particle radiation therapy. Clin Cancer Res 2013;19:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horak E, et al. Radioimmunotherapy targeting of HER2/neu oncoprotein on ovarian tumor using lead-212-DOTA-AE1. J Nucl Med 1997;38:1944. [PubMed] [Google Scholar]
- 26.Song EY, et al. Bismuth-213 radioimmunotherapy with C595 anti-MUC1 monoclonal antibody in an ovarian cancer ascites model. Cancer Biol Ther 2008;7:76. [DOI] [PubMed] [Google Scholar]
- 27.Palm S, et al. Therapeutic efficacy of astatine-211-labeled trastuzumab on radioresistant SKOV-3 tumors in nude mice. Int J Radiat Oncol Biol Phys 2007;69:572. [DOI] [PubMed] [Google Scholar]
- 28.Elgqvist J, et al. Alpha-radioimmunotherapy of intraperitoneally growing OVCAR-3 tumors of variable dimensions: Outcome related to measured tumor size and mean absorbed dose. J Nucl Med 2006;47:1342. [PubMed] [Google Scholar]
- 29.Elgqvist J, et al. Intraperitoneal alpha-radioimmunotherapy in mice using different specific activities. Cancer Biother Radiopharm 2009;24:509. [DOI] [PubMed] [Google Scholar]
- 30.Elgqvist J, et al. Repeated intraperitoneal alpha-radioimmunotherapy of ovarian cancer in mice. J Oncol 2010;2010:394913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borchardt PE, et al. Targeted actinium-225 in vivo generators for therapy of ovarian cancer. Cancer Res 2003;63:5084. [PubMed] [Google Scholar]
- 32.Gustafsson AM, et al. Comparison of therapeutic efficacy and biodistribution of 213Bi- and 211At-labeled monoclonal antibody MX35 in an ovarian cancer model. Nucl Med Biol 2012;39:15. [DOI] [PubMed] [Google Scholar]