Abstract
Aging of the extracellular matrix (ECM), the protein matrix that surrounds and penetrates the tissues and binds the body together, contributes significantly to functional aging of tissues. ECM proteins become increasingly cross-linked with age, and this cross-linking is probably important in the decline of the ECM's function. This article reviews the role of ε-(γ-glutamyl)-lysine (EGGL), a cross-link formed by transglutaminase enzymes, and particularly the widely expressed isozyme transglutaminase 2 (TG2), in the aging ECM. There is little direct data on EGGL accumulation with age, and no direct evidence of a role of EGGL in the aging of the ECM with pathology. However, several lines of circumstantial evidence suggest that EGGL accumulates with age, and its association with pathology suggests that this might reflect degradation of ECM function. TG activity increases with age in many circumstances. ECM protein turnover is such that some EGGL made by TG is likely to remain in place for years, if not decades, in healthy tissue, and both EGGL and TG levels are enhanced by age-related diseases. If further research shows EGGL does accumulate with age, removing it could be of therapeutic benefit. Also reviewed is the blockade of TG and active removal of EGGL as therapeutic strategies, with the conclusion that both have promise. EGGL removal may have benefit for acute fibrotic diseases, such as tendinopathy, and for treating generalized decline in ECM function with old age. Extracellular TG2 and EGGL are therefore therapeutic targets both for specific and more generalized diseases of aging.
Tissue Cross-Links and Aging: Transglutaminase's Role?
It is a truism that we become less flexible as we age. This is literally true of many tissues, which become more mechanically rigid and less amenable to tissue remodeling with age. Loss of flexibility is due at least in part to increased cross-linking of the extracellular protein in the tissue, converting a flexible network of molecules into a rigid mesh. Stiffness increases have been recorded in lung,1 major arteries,2–4 bone,5–7 muscle,8,9 tendon,10 and lens of the eye11,12 among other tissues. In a range of diseases, changes in the mechanical properties of the extracellular matrix (ECM) have been linked to cellular processes as well as mechanical changes in tissue (for review, see ref. 13). In many of these studies, degradation of mechanical properties has been linked directly to chemical cross-linking of extracellular protein.
Extracellular proteins are chemically cross-linked by several mechanisms in vivo, any of which could contribute to age-related matrix stiffening. Lysyl oxidase (LO) converts the side-chains of lysines in collagen and elastin to aldehyde-containing groups, which can then react with lysines, arginines, and histidines in other protein chains to form complex cross-links. The pattern of cross-links changes with age, but also with fibrosis and tissue scarring.14 Increase in LO cross-links with age could therefore be due to development of chronic fibrotic and inflammatory disease rather than endogenous trends in protein chemistry.15,16
Protein glycation is known to cross-link proteins in vivo, and causes degradation of tissue function.3,17 Reducing sugars react non-enzymatically with lysine and arginine side-chains in protein, forming complex products that can then undergo oxidation and react with other basic side-chains on other proteins, forming cross-links. The process does not involve any enzymes, and hence is not under biological control (except to the extent that sugar levels and temperature are controlled). The end products of glycation, called advanced glycation end products (AGEs), are stable under physiological conditions, and so accumulate in long-lived proteins.
Transglutaminases (TGs) are a group of enzymes that catalyze the formation of a peptide bond between the side-chains of glutamine and lysine residues. They are widely expressed, known to be related to disease, and could, in principle, contribute to the loss of mechanical properties of tissues with age. TGs cross-link proteins inside and outside cells, and so could be a significant contributor to ECM cross-linking and stiffness in health, disease, and old age. There have been many reviews of the role of TGs in specific disease states, and these are not repeated here, nor is the extensive literature on the complex mechanisms of TG action. Reviews of these studies are cited below. This review asks a more specific question. Are extracellular TG cross-links a potential target for blocking or reversing the increased cross-linking of the ECM that occurs with age, and which is likely to be associated with the general increase in morbidity with age? Specifically, do TG cross-links increase with age, and, if they do, is this because TG cross-links:
1. inherently increase with the aging of tissue, independent of specific disease,
2. increase with aging as a result of low levels of pathology that generally increase with age, without overt or florid disease,
3. increase as a consequence of specific episodes of diseases (acute or chronic) that emplace cross-links in long-lived proteins.
If TG cross-links do increase with age, we are also interested in whether this results in significant changes in ECM function.
This article starts by reviewing the TGs, and specifically tissue transglutaminase, transglutaminase 2 (TG2), which is the most ubiquitously expressed of the TG enzyme family. Then the meager evidence is reviewed that TG cross-links increase with age, before discussing the much larger body of evidence linking TGs with disease. This article then discusses whether TG enzyme activity or TG cross-links could be good therapeutic targets for the age-related increase in general incapacity and morbidity. I conclude that there is some evidence that an ε-(γ-glutamyl)-lysine (EGGL)-breaker could be an attractive new therapeutic option for tendinopathies, in future may be valuable for treating chronic cardiovascular and kidney disease, and may also provide a route to reversing some of the general ECM cross-linking that accumulates with aging. However the basic biology should be made more robust before substantial investment is made.
Transglutaminases
The transglutaminases are a family of enzymes that catalyze the formation of isopeptide bonds between γ-carboxamide groups of glutamine residues and either the ε-amino group of lysine residues (of other protein molecules) or primary amines (for review, see refs. 18–21). The transglutaminase family in humans has eight catalytically active members, including the blood clotting enzyme Factor XIIIa, and one homologous protein without transglutaminase activity.18,20
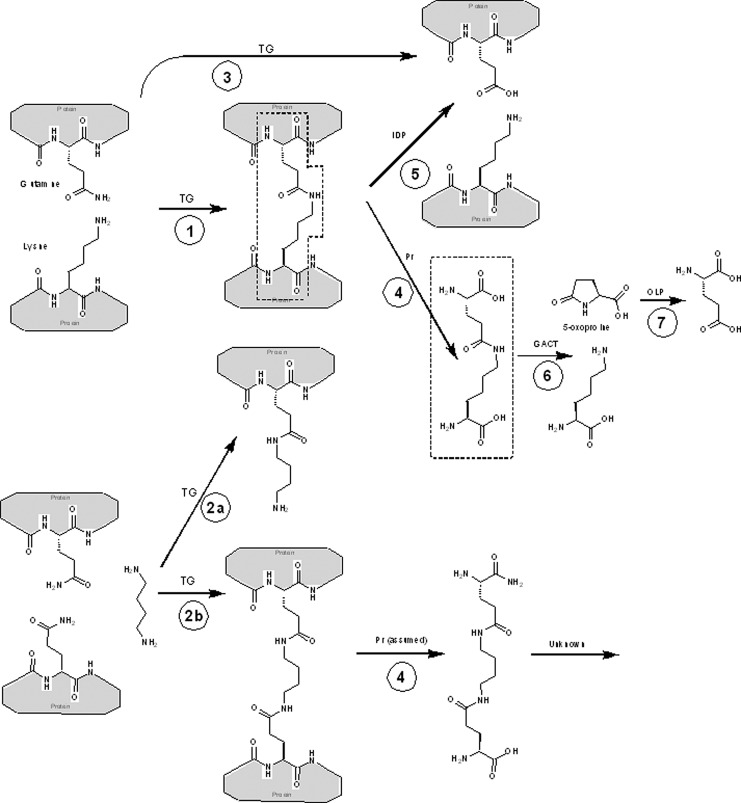
Transglutaminases can catalyze the direct cross-linking of proteins via a lysine–glutamine isopeptide bond, or indirectly via a polyamine (Fig. 1). Transglutaminases only cross-link specific proteins, and vary in their ability to link glutamines to primary amines or to hydrolyze glutamine (reactions 2 and 3 in Fig. 1). Table 1 summarizes the relevant properties of the known, catalytically active human transglutaminases. This review focuses on TG2, which is widely expressed and present in the ECM, and so could cause some of the ECM cross-linking that accumulates with age.
FIG. 1.
Transglutaminase cross-links. Key chemical structures discussed in this review and their interconversion. ε-(γ-glutamyl)-lysine (EGGL) moieties are outlined in dotted lines. Key: Gray boxes represent proteins, whose sequence contains the indicated amino acids. Specific catalyzed reactions are: (1) cross-linking of two proteins with transglutaminase (TG); (2a) linking of a protein with an amine (here putrescine used as an example) with TG, and (2b) cross-linking of two proteins via an amine; (3) hydrolysis of glutamine to glutamate, catalyzed by TG; (4) proteolysis of proteins with any protease (PR) releases free cross-link; (5) isodipeptidase (IDP) cleavage of cross-link regenerates original, unlinked proteins; (6) γ-glutamylamine cyclotransferase (GACT) cleaves isolated EGGL to lysine and 5-oxoproline; (7) 5-oxo-l-prolinase (OLP) cleaves 5-oxoproline to form l-glutamate.
Table 1.
Summary of Known Human Transglutaminases
| Protein | Synonyms | Gene locus/ chromosomal location | Structure/species (if not human) | Tissue distribution | Cellular location of active enzyme | Likely physiological substrates for cross-linking |
|---|---|---|---|---|---|---|
| TG1 |
Epidermal transglutaminase |
TGM1/14q11.2 |
|
Skin (keratinocytes) |
Plasma membrane, extracellular |
Keratin, stratum corneum proteins |
| TG2 |
Tissue transglutaminase (tTG), transglutaminase C (TG-C), and Gh |
TGM2/20q11-12 |
1kv3 (inactive), 2q3z (active) |
All tissues (See text) |
Intracellular, plasma membrane, extracellular |
Collagen, elastin, fibronectin, fibrinogen, laminin, latent transforming growth factor-β-binding protein-1, nidogen, osteocalcin, osteonectin, osteopontin and vitronectin, wide variety of intracellular proteins |
| TG3 |
Epidermal transglutaminase |
TGM3/20q11-12 |
|
Skin |
Extracellular |
Trichohyalin (hair) |
| TG4 |
Prostate transglutaminase, TGP |
TGM5/3p22-p21.33 |
|
Prostate |
Extracellular |
Unknown |
| TG5 |
|
TGM5/15q15.2 |
|
Skina |
Extracellular |
Vimetinb |
| TG6 |
|
TGM6/20p13 |
|
Central nervous system |
Unknown |
Unknown |
| TG7 |
Transglutaminase Z |
TGM7/15q15.2 |
|
Unknown |
Unknown |
Unknown |
| Factor XIIIa (A=activated form of zymogen) | Factor XIII, Fibrin Stabilizing Factor | F13A1/6p24–25 | Produced in liver, secreted into blood. Also in a range of extracellular fluids | Extracellular | Fibrin (can also substitute for TG2 in broad extracellular matrix cross-linking). |
Tissue distribution not well characterized.
Substrates only partly defined.
Data from ref. 241 and http://www.ncbi.nlm.nih.gov
Transglutaminase 2
TG2 is a multifunctional protein involved in processes inside and outside the cell. TG2 (also sometimes abbreviated as TGM2) is also known as tissue transglutaminase (tTG), transglutaminase C (TG-C), and Gh. TG2 acts as a transglutaminase, a protein kinase,22 a disulfide isomerase,23 and a G-protein GTPase in selected G-protein–coupled receptors,24 and interacts directly with fibronectin and integrin, thus mediating cell:ECM interactions (for review, see refs. 19, 25–27). The TG2 protein is expressed ubiquitously and abundantly, although, as discussed below, transglutaminase enzyme activity is much more restricted. TG2 is found in the cell nucleus and in the cytosol, can be embedded in the membrane, and is found in the ECM. TG2's known molecular roles include a pivotal role in the control of apoptosis, control of necrosis and repair of cell damage, and roles in both up- and down-regulating a variety of fibrotic and inflammatory processes, in wound healing, and in formation and remodeling of a variety of mesenchymal tissues. It is expressed in several splice variants that have significantly different cellular and disease state distributions.28–30 The extent to which different splice variants have different extracellular roles is not known. Because of this multiplicity of function, correlating TG2 levels with any physiological outcome is only the start of understanding what the protein is actually doing.
Intracellular and extracellular TG2 levels are strongly linked in several cell types.31 The mechanism for exporting TG2 to the ECM was until very recently largely unknown because TG2 has no classical signal peptide to mark it for export. Studies in muscle cells suggest an excretion pathway involving TG2 cross-linking itself into microparticles, which are then released32 through a common but poorly characterized pathway.33 Microparticle release has been most studied in the cardiovascular system, where microparticles are released that contain high levels of cytokines and microRNAs, and are associated with inflammatory disease, autoimmune disease, and atherosclerosis.34 TG2 is also associated with inflammatory, autoimmune, and atherosclerotic disease, which is probably not a coincidence.
The cross-linking enzymatic activity of TG2 creates cross-links between many proteins, although it is not indiscriminate. Extensive studies of the sequence specificity of TG2 show that it has some restriction on the sequence around glutamine, although the amino acids immediately flanking the Gln residue have little effect on the TG2 reaction rate.35–38 TG2 has almost no sequence preference for the sequence around lysine. (I note, however, that surface lysines in ECM proteins are twice as likely to be flanked by hydrophobic amino acids as expected by chance from the distribution of all exposed surface amino acids, and so we might expect some limited selectivity for a flanking hydrophobic environment in TG2. [W.B., unpublished observation.]) It is not known if the hydroxylysine residues in collagen can also be substrates for TG2. The chance that a specific glutamine and lysine pair will be cross-linked therefore probably depends more on steric effects than on sequence, i.e., whether the proteins are near enough to cross-link.39 TG2 has been shown to favor linkages between glutamines and lysines in regions of the protein showing high disorder in crystal structures,40 which supports the idea that the enzyme cross-links side-chains with sufficient mobility to approach within linking distance.
TG2 has been demonstrated to link polyamines to some proteins under physiological conditions,41,42 and gamma-glutamyl–linked polyamines have been detected in a variety of tissues (for review, see ref. 43). These can form polyamine-bridged cross-links in vitro (reaction 2b in Fig. 1),44 but it is not known the extent to which cross-links formed in vivo are direct lysine-glutamine (EGGL) cross-links or involve polyamines. The amounts of various polyamines, such as putrescine, spermidine, and spermine, in the ECM normally are very low,45 so EGGL is believed to be the major TG2-catalyzed cross-link in the ECM.19
Regulation of TG2
Discussion of the regulation of TG2 is important for our purposes because much of the research on TG2 and its role measures protein levels or enzyme activity, not cross-link levels. TG2 is expressed abundantly in many tissues, but most of the intracellular and extracellular protein is not an active transglutaminase,46 indicating tight control of enzyme activity. (Note that TG2 is multifunctional, so lack of transglutaminase activity does not mean that the protein is biologically inert; it could be carrying out its other functions while its transglutaminase activity is inhibited.)
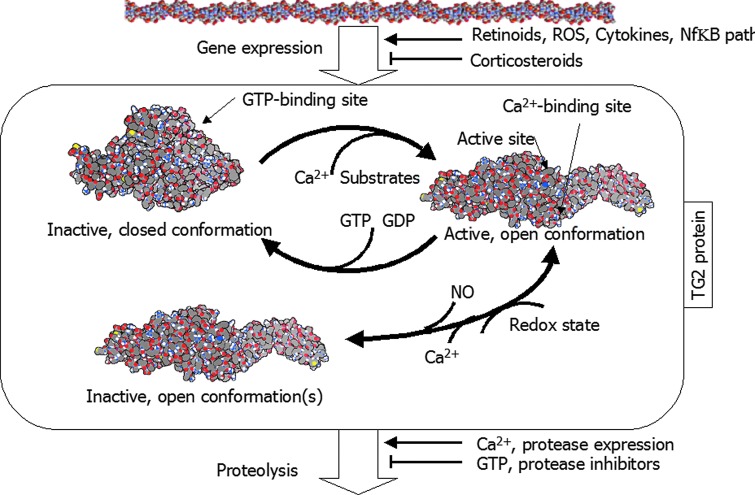
Purified TG2 requires about 1 mM Ca2+ for activity,26,47 although this requirement can be reduced to as low as 5 μM in presence of substrate and other proteins, depending on the substrate.48 Guanosine-5′-triphosphate (GTP) and guanosine diphosphate (GDP) are allosteric inhibitors of TG2,49 stabilizing the compact, inactive form,50–53 and GTP is hydrolyzed by TG2, although this is not part of the cross-linking chemistry. The binding of GTP or GDP reduces TG2 affinity for Ca2+. Thus, inside the cell, with very low Ca2+ and significant GTP levels, TG2 is expected to be inactive. However, binding to other proteins, proteolytic processing, or alternative splicing may all reduce sensitivity to GTP (for review, see ref. 48). TG2 transamidation activity is also reduced by S-nitrosylation by nitric oxide (NO)54 in a Ca2+ concentration–dependent manner,55 and high levels of Ca2+ caused release of NO from TG2, providing another path in the mechanism of TG2 activation.
In the ECM, Ca2+ levels are typically higher than inside the cell, with normal concentrations ranging from 1.0 to 2.5 mM, depending on tissue and physiological circumstance.56–60 Whether this represents the free Ca2+ levels that cells “see” is debatable. Many cell types respond to Ca2+ levels in the 0.1–1 mM range, suggesting that these are physiologically relevant concentrations for cells to detect (e.g., keratinocytes,61–63 osteoblasts,64 macrophages,65 fibroblasts,66 and sperm67,68). Thus, Ca2+ may be limiting TG2 activity in some circumstances in the ECM. GTP levels are typically low outside the cell (∼0.6 μM69), so GTP is not a relevant extracellular inhibitor of TG2 activity.
Recently, it has been found that TG2 is modulated by redox, through rearrangement of a disulfide bond between three cysteines outside the catalytic site. Oxidation can lock the enzyme into an open but inactive form.48,70 The redox reaction is reduced by the presence of Ca2+. It seems likely that TG2 is controlled through several pathways in the ECM, making predictions of its activity impractical.
The half-life of TG2 is relatively short, with an average of 11 hr.26 TG2 gene expression is increased by retinoids,71 reactive oxygen species (ROS) through proinflammatory cytokines,72 and steroid hormones such as vitamin D and progesterone.73 Other steroid hormones such as glucocorticoids seem to reduce its expression. This is consistent with a major role of TG2 in chronic inflammation. Additionally, Ca2+ seems to promote TG2 proteolysis, whereas GTP appears to inhibit it.53,74 Therefore, Ca2+ and GTP have contrasting effects on protein activity and protein stability. Splice variants of TG2 containing the GTP-binding domain have a longer half-life than those lacking it.29 Ca2+ is a general stimulator of proteolysis via activation of calpains.75 It is not known whether this is the mechanism of calcium's effect on TG2, or whether the more open form of the enzyme, which Ca2+ stabilizes, is more proteolytically labile. In addition, any physiological change that increases protease levels or decreases protease inhibitor levels may change TG2 turnover.
The regulatory paths around TG2 catalytic activity are summarized in Fig. 2. An important conclusion from this wealth of regulatory data is that the presence of TG2 protein in the ECM (and even more so the presence of TG2 protein or mRNA in cells) does not imply that cross-links are being formed in the ECM. The only effective way to detect TG2 activity is with fluorescent irreversible inhibitors that directly test for transglutaminase catalytic activity (e.g., dansyl cadaverine [DC]). Active TG2 cross-linking enzyme activity detected in this way outside cells may, with caution, be taken as an indication that EGGL cross-links are being formed in the ECM. Additionally, absence of TG2 protein or mRNA does not necessarily mean the absence of extracellular EGGL cross-links, nor that cross-links are not being formed, because other transglutaminase enzymes may form extracellular cross-links, especially Factor XIIIa, as noted below.
FIG. 2.
Regulation of transglutaminase 2 (TG2) catalytic activity. Summary of regulatory influences on TG2. See text for details. TG2 structures are derived from Protein Data Bank (PDB) entries 1kv3 and 2q3z, after ref. 51. The structure of the inactive open form of the enzyme is unknown: it is represented here as the same as the same as the active open form for illustrative purposes only. Color images available online at www.liebertpub.com/rej
TG2 and the ECM
TG2 is able to cross-link a variety of proteins, both cytosolic and extracellular. Those that are important for the ECM of various tissues include collagen, elastin, fibronectin, fibrinogen, laminin, latent-transforming growth factor-β–binding protein 1 (LTBP-1), nidogen, osteocalcin, osteonectin, osteopontin, and vitronectin.26 TG2 cross-links help define structure of elastic fibers76 by aligning the proteins. TG2 has a high binding affinity for fibronectin,77 which is crucial for cell spreading, adhesion, and wound healing,78 although this role may not involve TG2 catalytic activity.79 Osteonectin cross-linking by TG2 also seems to be important for cartilage development.80
Several types of collagen can be cross-linked by TG2: Cross-links are formed between the globular portions of the collagen molecule, not the triple-helical segments.19,81 TG2 cross-linked collagen is less ordered and more protease-resistant than collagen cross-linked by LO, consistent with its role in pathology rather than normal development of the ECM, discussed below. Type III collagen can be bound to the diamine putrescine by TG2,82 and it is possible that this conjugate might go on to form N,N-bis(γ-glutamyl)-diamine cross-links. Some research suggests that some other collagens, such as types I and IV, appear to be poor TG2 substrates.81
“Healthy” Functions of Transglutaminases
Because our goal is to consider TG2 as a target for therapeutic intervention, we must consider the positive, healthy roles of TG2, and of the transglutaminases in general, to gain some understanding of potential adverse effects of blocking TG2 action or undoing its effects by cleaving EGGL cross-links. This section only summarizes a large body of work on TG2 in health and disease; the reader is directed to the references for more detail on the many roles of this multifunctional protein.
Role of transglutaminases
Transglutaminases are essential enzymes for healthy growth and development in mammals. Factor XIIIa was the first transglutaminase discovered and is central to the blood clotting cascade. Genetic deficiency in Factor XIIIa causes severe clotting disorders in humans.83 As noted below, Factor XIIIa also probably has a role complementing TG2.
TG-1 and TG-384 have a central role in assembling cornified cell envelope in stratum corneum, the outermost layer of the epidermis.85 Epidermal TG is mainly in the granular layer of the epidermis, where terminal differentiation of the keratinocytes leads to development of the stratum corneum. One of the cross-linked proteins is filaggrin, whose mutation compromises skin barrier function and predisposes carriers to atopic dermatitis.86 Homozygous TG1 knockout (KO) mice suffer severe dehydration from a few hours after birth,87 and often die. Those that survive show massive keratosis (thickening of the upper layers of the skin), which partially compensates for loss of barrier function in the skin.88 Human mutants of the gene suffer the life-threatening lamellar ichthyosis, a disease of profound disordering of the skin.89 Transglutaminases are also important in providing mechanical strength to epithelial surface of lung alveoli.90
TG2 is known to be expressed abundantly in muscle development, especially cardiac muscle, during development in humans.91 However, as noted below (the section “Effect of genetic ablation of TG2”), it is not clear whether it is formally essential in this role.
Wound healing
TG2's transglutaminase activity has an important role in would healing, and the enzyme is elevated in healing wounds in cell culture46 and in vivo.92,93 Initially, TG2 activity is a result of activation of previously inactive enzyme, not synthesis of new protein, but smooth muscle cells, fibroblasts, and myofibroblasts all synthesize new TG2 in the ECM of the healing wound.94,95 TG2 stabilizes the matrix forming in the wound as cells move in and replace the original clot matrix (itself stabilized by Factor XIIIa cross-linking). Collagen fibrils laid down in the wound by myofibroblasts are cross-linked by TG2.26,93
Abnormal wound healing responses, notably hypertrophic scar formation, are associated with elevated TG2.96 Putrescine, a known inhibitor of TG2, has been tested as a treatment to prevent hypertrophic scar formation, but did not work (Procyon Biopharma press release, January 18, 2005).
Transglutaminase activity may also important in healing wounds in other tissue. In nerve axon injury, the axolemma (the cell membrane surrounding the axon) fails to seal after injury in low extracellular calcium.97 Calcium triggers a number of enzymes,98 and this may include TG2. The role of TG2 in normal wound healing fits with its pathological role in fibrotic diseases, discussed below.
Intracellular roles and apoptosis
TG2 has a major role in the induction and control of apoptosis. A common signal for apoptosis is the influx of calcium into the cell, which will inherently activate the catalytic activity of TG2.48 Different isoforms of the enzyme have different effects, and TG2 in the cytoplasm has different effects from that in the nucleus (for review, see ref. 99). Activity of TG2 in apoptotic cells appears to cause massive cross-linking of intracellular proteins,25,52,100 probably to keep the dying cell intact and to prevent leakage of cell contents into the extracellular milieu.
TG2 has been implicated in many aspects of cancer biology, including cell migration and the evasion of apoptosis that cancer cells achieve. The focus of this review is effects in the extracellular space, thus, these important aspects of the role of TG2 in cancer biology will not be discussed further here. The interested reader is directed toward recent reviews in the field.27,99–105
A number of studies show that inhibiting transglutaminases with irreversible inhibitors such as DC blocks internalization of extracellular ligands or their receptors in cultured cells (e.g., see refs. 106–108). DC blocks clustering of receptors in coated pits, which is therefore likely to be mediated by the catalytic activity of TG2.106 TG2 is also involved in phagocytosis in macrophages.109
Effect of genetic ablation of TG2
Given this diversity of role, it would be expected that mice in which both copies of any of the transglutaminase genes have been knocked out would exhibit multiple severe morbidities. As noted above, homozygous knock-out (KO) mice lacking TG1, TG3, or Factor XIIIa87 do suffer severe defects of the appropriate tissues or organs.
However, two independently derived TG2 KO mice strains both show no overt pathology.110,111 The homozygotes are born at expected Mendelian frequencies, suggesting little prenatal mortality. Despite the widespread expression of TG2 in musculoskeletal development,91 no obvious musculoskeletal abnormalities are seen in TG2 KO mice, apparently because Factor XIIIa can replace TG2 in musculoskeletal development.112 Despite the role of TG2 in wound healing, TG2 KO mice are reported to show similar post-surgical wound healing to normal mice,113 although others report defects in wound healing models.114 Despite the widespread importance of apoptosis in many aspects of development and the proven role of TG2 in apoptosis, no abnormalities are seen in TG2 KO mice that can be traced to poor or excessive apoptosis in development. Fibroblasts from the mice showed reduced adherence in culture, and thymocytes from the KO mice induced to apoptosis died off faster. There are some abnormalities of macrophage function clearing apoptotic cells in some models,109 but not others.111 TG2 KO mice show a variety of changes in disease models, noted below, but in general are astonishingly healthy for animals that have lost a ubiquitously expressed, multifunctional protein. De Laurenzi et al.111 and Johnson et al.115 attribute this largely to the ability of other transglutaminases, notable TG1 and Factor XIIIa, to “step in” and take over TG2's role in a variety of normal physiological processes.
Extracellular TG2 Cross-Links and Age
Levels of TG2 protein and mRNA have been measured in a wide variety of animals, diseases, and ages, but relatively little of this work has addressed protein cross-linking. This section focuses on the ECM and touches on other aspects of TG2 only when needed to dissect the overall pattern of TG2 presence of activity from its role in the mechanical properties of tissue.
Evidence for EGGL accumulation with age
There is very little information on whether EGGL cross-link densities change with age, other than in direct association with a disease or pathology. What evidence there is suggests that EGGL may increase with age in the absence of overt pathology, but methodological issues dog these studies. A range of antibody tests have been suggested,90,94,116,117 but a recent survey suggests that some of the antibodies to EGGL cross-react with other antigens (Ustok et al., in preparation), and those that are specific may not detect cross-links efficiently when they are embedded in a dense protein matrix.118 Measuring TG2 catalytic activity is a common substitute for measuring EGGL, but TG2 catalytic activity will only correlate with relative EGGL levels if protein turnover is the same between the compared states and if TG2 is the only transglutaminase forming EGGL in that tissue. In most non-diseased tissue, the majority of the transglutaminase activity is generally believed to be TG2 (although Factor XIIIa is seen to substitute for TG2 functionally in many circumstances, as noted in the section “Effect of genetic ablation of TG2,” above, so TG2 cannot be assumed to be the only extracellular transglutaminase). Because protein turnover is different in many of the diseases discussed below, transglutaminase activity can only be regarded as a semiquantitative indicator of likely EGGL levels.
As noted above, much of the TG2 in the ECM is catalytically inactive, so measuring TG2 protein levels is only very circumstantial evidence of cross-link densities. Direct chemical measurement of EGGL is complex and time-consuming, and thus rarely performed, but it is possible to simultaneously measure lysine and EGGL levels, and thereby obtain cross-linked lysine residues as a percentage of total lysine.119 Even chemical measurements of EGGL do not provide data that can be compared between studies. Studies report EGGL as a fraction of total lysine, of collagen, of protein, or of tissue mass without providing the data on the ratios of lysine to collagen to protein to tissue mass that would allow interstudy comparisons. The data set is, therefore, poor.
One study has shown clearly that EGGL levels increase with aging in healthy humans in arterial walls, as evidenced by immunohistochemical staining showing an approximate 2.5-fold increase in staining in aged human subjects compared to young ones.54 This correlated with an increase in TG2 activity of approximately 75% from young to aged subjects. Similar increases were observed in rat aorta. Other work in the same report shows that at least some of the increase in stiffness in mouse artery with age is attributable to increase in TG2 activity, caused both by an increase in protein expression and decrease in inhibition by NO. The mechanism for this increase was determined to be decreased S-nitrosylation of TG2, likely because of decreased NO bioavailability in the ECM with aging.120 TG2 protein levels did not change significantly in this study, consistent with an activation of pre-existing enzyme.
Nemes et al.121 also show that EGGL–cross-linked proteins are present in significantly higher levels in old, healthy human brains compared to young brain tissue. However, we should note that “healthy” old brains often show signs of neuronal loss, β-amyloid accumulation, and other molecular markers of neurodegenerative disease, even if overt cognitive or behavioral symptoms are not seen. Thus, it is unclear what a “healthy” brain actually looks like in this context. It is also not clear how much (if any) of this EGGL was extracellular.
Suzuki et al.122 show data suggesting that EGGL cross-links in stratum corneum decline with age, which may be related to the changes in barrier function of skin with old age (for review, see ref. 123). Because this is a specifically differentiated tissue, it is not representative of the ECM elsewhere, although aging of skin is itself a significant issue. Skin EGGL is primarily laid down by TG1 and TG3, not TG2, in normal skin growth.
Several studies examine the EGGL cross-link as an isolated entity in serum or urine. These are interesting (especially in the diagnosis of neurodegenerative disease, discussed below) but are not helpful to our quest. Serum and urine EGGL derives both from turnover of ECM and from intracellular cross-linking and subsequent breakdown of proteins associated with apoptosis. Therefore, it is a marker of tissue turnover, not stable EGGL levels, and is only tenuously related to ECM EGGL. For example, collagen cross-link excretion is elevated 1.5- to 2-fold in young, fit, healthy men during space flight due to loss of bone and muscle loading and consequent increase in tissue turnover. Excretion returns to normal on landing.124
Evidence for increase in TG2 activity with age
TG2 activity is easier to measure than EGGL cross-links, and as a consequence a range of studies have looked at protein levels or enzyme activity rather than cross-link accumulation. As noted above, this is indicative of cross-link densities only.
Some studies in human tissue show increase in TG2 or transglutaminase activity with age in absence of pathology. Normal (non-arthritic) knee meniscus tissue and chondrycytes show an increase of TG2 activity with age125 (as well as an additional increase with disease, as noted below). A range of animal studies have looked at TG2 levels in different species and tissues. In general, they find an increase of TG2 catalytic activity with age. Total rat liver TG2 activity increases about 1.5-fold (with substantial variation) between young and old animals, while levels in the brain increase seven-fold in a much more systematic pattern.126 TG2 mRNA, however, does not increase in rat brain with age: If anything there is a slight decrease.127 Total TG2 levels are found to be elevated in old versus young pig articular chondrocytes.128 There is an increase in TG2 in the macrophages of old versus young mice,129 which, however, may be related to a higher background level of immune activation in old mice. TG2 levels in murine macrophages are also found to vary between strains, so the generality of age-related effects is unknown. As noted above, rat aorta shows an increase in TG2 activity with age.54 In rat kidney, TG2 mRNA increases with age in male but not female rats, which is probably related to testosterone induction.130
Changes in substrate availability
The availability of substrates for transglutaminases may change with age. In general, these changes suggest that the potential for ECM proteins to be cross-linked by transglutaminases may decline with age rather than increase.
Glutamine spontaneously deamidates over time, especially when in a Gln-Ser or Gln-Gly sequence.131,132 Aspartate and asparagine racemization is a feature of the same chemistry: D-aspartate can be expected to make up 10% of aspartate in elastin in the tendon and aortic walls of a 50-year-old human,133 so removal of accessible glutamines may significantly reduce the substrate availability in old tissue. Some studies show that polyamines decline with age,134 others suggest that they do not.135 Polyamine-supplemented diets have been suggested to reduce age-related kidney and liver function decline,136 although the rationale behind this, while passionately defended by some, is not generally supported.
ECM proteins probably change with age, but quantitative specifics are limited. Collagen levels increase with age in mouse137 and rat138,139 models of muscle aging, but are not significantly changed in human muscle140 and decrease in human skin with age.141 Collagen packing may become less dense in some tissues with age, reflecting different organization.142 This may affect all forms of collagen cross-linking, because proteins have to be physically near to each other in the ECM to be cross-linked. The organization of other proteins has not been systematically measured.
EGGL and long-lived proteins
Accumulation of cross-links is the result of a balance between their synthesis and their removal. EGGL could accumulate because its rate of synthesis increased with age, as implied by the sections above. Or it could increase because its rate of removal declined, or (equivalently) was so slow that EGGL, once made, stayed with you forever. Either could lead us to expect EGGL levels to rise with age.
Enzymes that cleave the isopeptidase bond in EGGL would be classed as isopeptidases. While such enzymes are known in non-mammalian species (discussed in the section “EGGL removal agents,” below), it is generally believed that there is no human isopeptidase that cleaves the EGGL link in the ECM. The body removes extracellular EGGL by proteolysis of the cross-linked protein as part of ECM turnover and remodeling, and then cleavage of the isolated EGGL. γ-glutamylamine cyclotransferase (GACT) can cleave isolated EGGL143,144 converting EGGL to free lysine and 5-oxoproline (reaction 6 in Fig. 1). It is quite non-specific to the lysine moiety, acting on d- or l-lysine and on other, non–amino acid side chains with more than three methylene groups. The enzyme can also accommodate quite bulky substituents on the lysine analog.145 However, GACT only acts on the free isopeptide, not on cross-links in proteins. Another enzyme, γ-glutamyltransferase (GT), a membrane protein with an extracellular active site,146 has been found to liberate free lysine and glutamic acid from the EGGL cross-link during gastrointestinal digestion of cross-linked protein.147 Overexpression of GT has been linked to increased risk of tumor growth and metastasis,148 as well as osteoporosis.149 5-Oxo-L-prolinase, an enzyme widely distributed in mammalian tissues, catalyzes the conversion of 5-oxo-l-proline back to l-glutamate (reaction 7 in Fig. 1).144
Loewy150 used a thin-layer chromatography (TLC)-based assay to report the identification of isopeptidases in a variety of organisms from Bacillus cereus to chicken brains and claimed widespread enzyme activity in mammalian tissues including brain.151 However, this work was never followed up, so it is not possible to confirm that this activity is due to a general EGGL-cleaving enzyme. Their assay may have been detecting the intracellular isopeptidases that cleave the isopeptide bond between ubiquitin and proteins targeted for proteosomal destruction (isopeptidase T), which is known in many cells and all species.152 This enzyme is quite specific, and (as far as is known) will not cleave EGGL links between matrix proteins. Thus, EGGL itself is not turned over with age. If EGGL accumulates with age, it is most likely because the proteins in which it is placed do not get turned over.
Some proteins are extremely long-lived, being turned over very slowly. Radiotracer experiments tracking the isotopes incorporated into children's eye lens crystallins in the late 1940s and early 1950s (when atmospheric testing of nuclear weapons performed a “pulse-chase” radiolabeling of every human then alive) suggest that some of the crystallins of the lens of the human eye are not turned over at all.153 Aspartate racemization measurements suggest that the collagen in articular cartilage has a half-life of at least 70 years,154,155 and that of human skin collagen as approximately14 years. Aspartate racemization studies suggest that collagen in bone and teeth also contains long-lived proteins. Other Asp-racemization determined half-lives include Aggrecan in intervertebral discs (∼11 years),156 elastin in skin elastic fibers (at least a year, varying substantially with age),157 and bone osteocalcin (at least 50 years).158 Total protein in bone, skin, brain, teeth, and eye all show systematic increase in d-aspartate levels with age.
Aspartate racemization data should be viewed with care. The rate of aspartate racemization is sequence and structure dependent. Different proteins (including different collagens) will generate d-Asp at significantly different rates, and proteins that have limited peptide backbone mobility—due to native folding, amyloid formation, or cross-linking—will have substantially lower rates of aspartate racemization than ones that are naturally flexible, or are denatured.131,132,159,160 We should also note that the d-Asp levels are often measured in “acid-insoluble” fractions of tissue, and some cross-links are acid-unstable, so proteins will be selectively lost through this preparation method. However, aspartate racemization studies do suggest that some ECM components have half-lives of years, possibly decades, and so EGGL cross-linking them may last for years or decades as well.
The ECM is turned over slowly as a whole (apart from large-scale replacement after wounding or severe pathology). Replacement ECM is often less well organized than the original (for review, see ref. 161), and so may be more susceptible to TG2 cross-linking. In general, protein turnover is observed to decline with age (for review, see ref. 162), along with the general metabolic slowdown of the elderly. ECM turnover declines significantly with age in intervertebral discs163,164 and articular cartilage,165 although apparently not in muscle, where exercise and diet dominate collagen synthesis rates.166 Disease states (such as the tendinopathies discussed below) can however substantially accelerate ECM turnover, as can loading changes and other factors.124,167,168
It is plausible, therefore, to suggest that TG2 action to create EGGL cross-links in tissue will increase the level of EGGL for some time afterwards, and possibly for a substantial proportion of the individual's life. In the next section, information on TG2 activity is considered in the context of age-related disease, which could provide the episodes of EGGL formation necessary to create cross-links in these long-lived proteins.
TG2 in age-related diseases
In contrast to the paucity of data on EGGL cross-links in aging in the absence of specific pathology, there is a wealth of data on EGGL, TG2 activity, and TG2 protein in specific disease states. These are summarized briefly, both because specific diseases contribute to the disabilities of old age and because they suggest mechanisms that will drive cross-link formation and accumulation in “healthy” aging.
TG2 is likely to have at least three roles in cancer: (1) A modulator of apoptosis and cell autophagy, (2) changing the ECM to enable cell transformation, and (3) as a modeler of the ECM through which cancer cells must migrate. However, this is not a potential role of TG2 in “healthy” aging or in the chronic diseases of old age, and so I will not discuss TG2 and cancer in this article. The role of TG2 in cancer, and the potential of TG2 as an anticancer therapeutic target, has been discussed extensively elsewhere (see refs. 101 and 169–174 and references therein)
TG2 in inflammatory and autoimmune disease
It is often stated that TG2 activity is elevated in inflammatory and immune diseases (see, e.g., refs. 174–176). The actual picture is more complex, and TG2 activity and EGGL accumulation is better thought of as a feature of fibrotic diseases that result from chronic inflammation or other insult. TG2 and EGGL levels have either not been examined, or have been examined and been found to be unchanged, in a range of inflammatory diseases. In particular, citations in reviews to papers on diabetes177,178 and rheumatoid arthritis179 are actually referring to studies showing elevation of anti-TG2 auto-antibodies, not TG2 itself or its cross-links. Therefore, it is important to identify which disease, etiology, and underlying mechanisms are relevant to our quest.
Some chronic inflammatory diseases show clear elevation of TG2 expression, protein, catalytic activity, or EGGL cross-links. TG2 expression is elevated in a range of inflammatory myopathies,180,181 where it is seen specifically in damaged and regenerating muscle fibers.182 TG2 is a marker of osteoarthritis disease severity in guinea pig,183 and significant levels of TG2 activity and cross-links are reported in human osteoarthritis,125,184 although without comparable control levels in normal knees. In asthma, TG2 protein and protein cross-linking are all elevated.185,186 (I note that chronic asthma has many of the features of a chronic fibrotic disease. For review, see ref. 187). High levels of TG activity and cross-linking in psoriasis are probably related to skin-specific transglutaminases, not TG2.188 It is worth noting that wound healing in adults has a substantial inflammatory component, and hydration of skin (i.e., support of the barrier function) reduces this189 (for review, see ref. 190).
We should note that intracellular TG2 is activated post-translationally by ROS in a TGF-β–related mechanism,191,192 so the role of TG2 in inflammatory disease is not solely extracellular, and rise in EGGL in a tissue may represent intracellular activity, not ECM cross-linking.
Sohn et al.193 used a TG2 inhibitory peptide that could not enter cells to block inflammatory uveitis. Kim et al.175 used the same peptide to reduce the effect of a murine model of asthma, demonstrating that at least some of the role of TG2 in this model was outside the cell (although the peptide also has phospholipase A2 (PLA2)-inhibitory properties,194 so its action cannot be unambiguously attributed to TG2 inhibition). Generalized immune activation in mice by lipopolysaccharide (LPS) injection in mice can cause increase in TG2 activity and EGGL in a variety of tissues on different timescales.195 However, different inflammatory inducers can produce septic shock models that are enhanced or reduced by TG2 KO, depending on the inducer,196 illustrating that this is a complex and highly non-linear system. Extracellular TG2 is inactive in the small intestinal mucosa of mice until stimulated by poly(I:C) (mimicking viral infection).46 Morbidity in poly(I:C)-treated mice was substantially greater in TG2 KO mice than in controls,197 suggesting a protective role for TG2 in acute inflammation.
TG2 has a unique role in celiac disease, where it is the auto-antigen against which the immune system reacts.198,199 Anti-TG2 antibodies are highly diagnostic of the disease.200–202 Gliadin peptides from wheat gluten are an excellent substrate for TG2, which may result in TG2 cross-linking itself to gliadin,203 thereby sensitizing the immune system to the enzyme, which is then removed, stopping the formation of effective tight junctions along the intestinal mucosa.204
Auto-antibodies against TG2 are reported to be common in some other inflammatory diseases, although not at the level found in Crohn disease (for review, see ref. 176). However Sánchez et al.205 report looking for auto anti-TG2 IgA antibodies in lupus, type I diabetes, multiple sclerosis, autoimmune thyroiditis, and non-Crohn inflammatory bowel disease, and seeing no difference from controls. There is a gradual increase in TG2 autoantibodies with age in healthy people (although to much lower levels than in Crohn disease).206 This might suggest that TG2 activity levels are reduced with age in the absence of disease, as the enzyme is cleared by the immune system.
TG2, AGEs, and immune changes
As well as overt immune or inflammatory disease, EGGL synthesis might be enhanced with age as a result of the increase in “background” inflammation with age, arising from two causes—AGEs and inherent immune decline.
High glucose, high insulin, and AGEs can increase TG2 activity.207,208 ROS, (molecules such as superoxide and hydrogen peroxide), can increase the expression of TG2. Because AGEs can act as pro-inflammatory mediators by binding to the receptor for AGEs (RAGE)209 and thereby increase the production of ROS,210 AGEs could have an indirect effect on transglutamination. The accumulation of AGEs with age is well known,3,17,211,212 and so it is expected that this mechanism would increase TG2, and hence EGGL, with age. The link between TG2 and glucose metabolism may be a major factor driving the link between TG2 and diabetic nephropathy (see the section “TG2 and fibrosis,” below)
The immune system itself declines with age (for review, see ref. 213), and because a major function of the immune system is to keep itself in check, we might expect inflammatory activation to rise with age. However, the evidence for increases in inflammatory damage resulting from immune dysregulation is more mixed: Polymorphonuclear neutrophil (PMN) cells and natural killer (NK) cells appear to be as functional in the elderly as in the young,214,215 and if anything to have lower levels of activation to generate ROS. If there is an immune-related increase in TG2 activity, and hence EGGL synthesis, with age, it is likely to be a result of other chronic damage such as AGE accumulation rather than decline in immune function.
TG2 and fibrosis
Much of the reported role of TG2 in inflammatory disease can be attributed to its role in fibrosis, the excessive or aberrant accumulation of extracellular material in reaction to inflammation. TG2's role in fibrosis fits with its role in wound healing and scarring, because fibrosis is considered an abberant or over-exuberant wound healing response, often to inflammatory injury (for review, see refs. 216, 217). A major role of TG2 in inflammation appears to be to control the effects of cell death by cross-linking cellular components to stabilize them against release and induction of inflammation and bystander cell killing. TG2 KO mice have reduced clearance, enhanced tissue toxicity and enhanced inflammatory reaction in liver cirrhosis models.109,218 TG2 KO mice have fewer Mallory bodies (intracellular cross-linked protein bodies accumulated in some liver diseases) than normal mice, and higher levels of gallstones, jaundice, and ductal abnormalities in a model of liver damage, suggesting enhanced cellular damage.219 Transforming growth factor-β (TGF-β) both stimulates macrophages to clear apoptotic cells and induces TG2. In this regard, it is interesting that TG2 activity tends to rise in early stages of animal models of hepatitis and to decline later when LO cross-links are more abundant in the fibrosis,220 implying TG2 has a transient stabilizing influence. Hepatitis studies are complicated by TG2's role in the apoptosis of hepatocytes in many disease models.99,221
Fibrotic diseases commonly are caused by chronic tissue damage and associated inflammatory processes that over-induce these protective mechanisms. TG2 over-expression is a feature of the renal scarring that is associated with chronic kidney disease in humans31 and in streptozotocin-induced diabetic rats,222 where EGGL levels rose eight-fold in 120 days. Inhibitors that specifically target extracellular TG2 reduce fibrosis and scarring in an animal model of diabetic nephropathy223 and kidney fibrosis.224 TG2 KO mice have reduced fibrosis in a model of idiopathic lung fibrosis225 due in part to reduction of the nuclear factor-κB (NF-κB) pathway,226 and there is strong correlation between TG2 levels, EGGL, and nephrosis in humans.226
As well as diseases where fibrosis builds up in normally non-fibrous tissue, TG2 is implicated in diseases where tensile connective tissue such as tendon or cartilage is injured. Tendinopathy is a particularly important case for aging, because the collagen in tendons has a very long half-life in humans, as noted above, and so could be a candidate for mechanism (3) in the first section of this article. TG2 is elevated in rotator cuff tendinopathy,227 although TG2 gene expression may paradoxically be reduced in ruptured tendons, with elevated TG1 and Factor XIII compensating228 (again, speaking to TG2 activity as a sequela of injury rather than a cause or immediate response to it). We can note also that cataract formation is probably accelerated by transglutaminase cross-linking of lens crystallins,229 probably as a result of inflammatory damage to proteins and/or induction of transglutaminase230 (for review, see ref. 20).
Cardiovascular and metabolic disease
TG2 is associated with a wide range of cardiovascular systems and processes, including modulating platelet activity, contributing to the development of hypertension, influencing the progression of atherosclerosis, regulating vascular permeability and angiogenesis, and contributing to myocardial signaling, contractile activity, and ischemia/reperfusion injury (for review, see ref. 231). TG2 activity is associated with arterial stiffening in humans232,233 and rats.234 Remodeling of small arteries, which is dependent on TG2,235 is defective in TG2 KO mice.113
EGGL concentrations are elevated in atherosclerotic plaque in humans,236 although the enzyme forming cross-links is Factor XIIIa, not TG2.237 Apolipoprotein E (ApoE)/TG2 double KO mice did not show any effect of TG2 on plaque chemistry or stability,238 suggesting TG2 elevation is an effect and not a cause of atherosclerotic pathology. (ApoE KO mice can be driven to develop atherosclerosis rapidly on high-fat diets.) However pharmaceutical blockade of TG2 and Factor XIIIa reduces macrophage infiltration in atheroscleoric lesions in mice,239 suggesting the reverse. Mice engineered to express high levels of TG2 show enhanced cardiac hypertrophy and fibrosis and apoptosis under stress, an effect in which elevated TG2 interacts with COX-2 and is modulated by COX-2 inhibitors.240 However, it is not known if this is dependent on the transglutaminase activity or G-protein activity of the TG2 protein.241
TG2-catalyzed protein cross-linking is also elevated intracellularly in the heart during the damage that occurs after after ischemia and reperfusion (called reperfusion injury),242,243 although this effect is related at least in part of changes in mitochondrial energy metabolism and changes in the mitochondrial arm of the apoptotic response,109,244,245 and may also be related to TG2's protein disulfide isomerase activity.23,246
The majority of these effects can be seen as an extension of TG2's role in inflammatory disease. Atherosclerosis and reperfusion injury have strong immune components, and TG2's role in them may therefore represent a response to, or perhaps over-compensation for, inflammatory disease.
Neurodegenerative disease
The association of TG2 cross-links and EGGL with neurodegenerative disease, especially Alzheimer disease (AD), is well known.103,247–249 EGGL247,250 and TG2251 are found in the cerebrospinal fluid (CSF) of AD patients at levels significantly higher than those in normal patients, although not in the CSF of patients with vascular dementia.252 EGGL and TG2 are elevated in a mouse model of Huntington disease,253 but the course of the disease is the same in TG2+ and TG2− mice.254 TG2 is elevated in human HD patients' brains,255,256 and in serum and CSF (but not spinal cord) of early stage amyotrophic lateral sclerosis (ALS) patients.257
This association of TG2 activity is likely to be related to neuronal cell death. As mentioned, TG2 is strongly implicated in apoptosis,258 and neuronal cell death is a major feature of AD. Thus, much of the EGGL and TG2 in neurodegenerative disease is likely to be a marker for cell death, not extracellular effects. (The same caveat applies to the studies above noting increased TG2 levels in old, “healthy” animal brains.)
TG2 does induce rapid aggregation of amyloid-beta (Aβ) protein in vitro at physiological Aβ concentrations, whereas Aβ aggregation without TG2 required much higher Aβ concentrations.259 TG2 can cross-link tau protein into aggregates,260,261 and EGGL cross-links are found between tau tangles and Aβ.151
There may nevertheless be a relationship between extracellular transglutaminase or EGGL and neurodegenerative disease. I note a preliminary report of an intriguing link between progression of ALS and reduced skin elasticity.262,263 This has not been formally reported, and the informal report suggests that there are a range of biochemical changes in skin causing the mechanical changes: Cross-linking was not reported explicitly. However, it would be worth exploring whether extracellular EGGL metabolism was abnormal in this neurodegenerative disease in skin and spinal tissue.
Conclusions: EGGL, TG2, and aging
The picture is, to say the least, not clear. Inflammatory, autoimmune, and fibrotic diseases all have an immune component, as does chronic arterial disease. The role of TG2 in these diseases is consistent with its role in modulating tissue damage following immune activation, and hence its pathological role is related to over-activity in this mode.
There is some limited evidence that EGGL increases with age, independent of its (short-term) increase in specific, overt disease. However discriminating as to why this happens is hard. In terms of the three options listed in the first section of this article, does EGGL:
1. inherently increase with the aging of tissue, independent of specific disease? There is little evidence for this, but this is mainly because it has not been studied. Changes in anti-TG2 antibodies and substrate availability suggest that TG2 activity (and hence EGGL accumulation, although not necessarily absolute levels) decreases with age.
2. increase with aging as a result of low levels of pathology that generally increase with age, without overt or florid disease? There is substantial evidence that EGGL is deposited in tissues by disease mechanisms that increase in incidence with age, such as chronic inflammatory diseases.
3. increase as a consequence of specific episodes of diseases (acute or chronic) that emplace cross-links in long-lived proteins? It is highly likely that long-lived proteins are cross-linked by TG2, and therefore that any extracellular TG2 activity will cross-link those proteins with EGGL that will accumulate with age. It is particularly likely that rising burden of inflammatory disease with age would deposit EGGL in a variety of tissues.
The rise of EGGL cross-links with age, and their effect on tissue elasticity, could be relatively easily checked by looking at the tissue physiology and biomechanics of TG2 KO mice in the absence of induced disease. As noted above, TG2 KO mice show no obvious (i.e., young-onset) abnormalities and do show differences in models of age-related disease. Do they show differences in “normal” aging (i.e., aging in the absence of an acute, induce pathology)? This would be an issue worth exploring.
What is not known is whether the rise of EGGL with age is quantitatively significant, or whether the other cross-links, such as AGEs or LO-formed cross-links mentioned in the first section of this article dominate ECM physiology. The evidence for the quantitative role of different cross-links will be reviewed elsewhere (Bains and Grainger, in preparation), but the data are not strong. There is no study of which I am aware that quantifies the three classes of cross-links mentioned in the first section in the same tissue, so the relative levels of EGGL and hence their relative contribution to ECM stiffening are unknown. This is a major gap in the literature on ECM biology.
Therapeutic approaches
The analysis in the section “Conclusions: EGGL, TG2, and aging” is not classification for the sake of classification. Excessive cross-linking of the ECM is probably a bad thing, which we might wish to avoid, block, or remove. TG2 may contribute to this. But what is the best route to blocking or removing EGGL?
If EGGL accumulates in tissue because of continued, chronic increase in TG2 activity (option 2 above), then blocking this activity will reduce TG2 and hence EGGL levels, reversing the effect. Equally, blocking immune activation would also be effective. Evidence of the KO mice suggests that whole-scale blockade of TG2 may not have serious adverse effects. However, if EGGL inherently accumulates with age, or accumulates in long-lived proteins as a result of past TG2 activity, then it will have to be actively cleared. Blocking TG2 today will not reverse its effects yesterday. At the moment, we do not know which of these strategies might have value. The pragmatic approach is to try them all (an approach the pharmaceutical industry takes by default in many disease areas, even if each company believes that their strategy is “the best”). But are all three equally practical?
Discussion of anti-inflammatory drugs is beyond the remit of this review. An entire literature exists on the therapeutic modulation of the immune system. Here I will focus on approaches to block TG2 activity and to remove EGGL cross-links.
TG2 inhibitors
TG2 inhibitors have been a research target in academia and industry for several decades, primarily because of TG2's role in cancer,264,265 but also targeted at neurodegenerative disease.266 Approaches to developing TG2 inhibitors are to screen known compounds for (fortuitous) ability to block TG2, designing mechanism-based irreversible inhibitors, and designing conventional, reversible inhibitors for the enzyme's many binding sites.
The catalytic mechanism of the transglutaminases is well known. The active site of TG2 and other TGs contains a so-called catalytic triad of amino acids, consisting of Cys-314, His-373, and Asp-396.267 This catalytic triad bears high similarity to the catalytic triad found in cysteine proteases.268 On the basis of the mechanistic and structural understanding of the enzyme, substantial effort has been expended developing transglutaminase inhibitors (for review, see refs. 21 and 102). Indiscriminate blockade of all transglutaminase activity is likely to be very harmful. So therapeutic development has focused on trying to produce isotype-specific inhibitors.21 Many inhibitors that show strong blockade of enzyme activity have been demonstrated in the laboratory, but so far none have progressed to clinical development.
As noted above, gluten peptides are extremely good substrates for TG2. A range of inhibitors have been designed by modifying these with chemical groups that hijack TG2's mechanism and chemically link the inhibitor to the enzyme.269 Typically, there are peptides or peptide mimetics with a reactive group on a side-chain instead of an epsilon amine group. Reactive groups have included epoxide, maleimide, and diazo groups. Other irreversible inhibitors that are not based on peptides have also been designed.270,271
Of the other binding sites on TG2, only the GTP binding site has been targeted by an inhibitor, Tyrphostin 47, an epidermal growth factor receptor (EGFR) kinase inhibitor that binds to TG2 competitively with GTP.272 Some kinase-related inhibitors of TG2 have been found, but are competitive inhibitors of enzyme activity,272 not (as far as is known) allosteric inhibitors acting through the GTP site. For anti-cancer applications, inhibitors that are competitive with GTP may be particularly effective. Mutants of TG2 that cannot bind GTP strongly promote apoptosis,273 suggesting that an antagonist of GTP binding might also be pro-apoptotic. As far as I know, the very extensive libraries of kinase inhibitors (for review, see refs. 274 and 275) and GTPase inhibitors (e.g., refs. 276 and 277) have not been screened for activity as TG2 allosteric inhibitors. However, some small libraries of compounds developed for other targets have been screened, and several found that inhibit TG2 at low nM potency, suitable for development as drugs.272
Inhibitors that target the enzyme's active site only act on active protein and so will not affect enzyme that is currently inactive. Because much of TG2's activity is the activation of pre-existing enzyme, it is not clear whether this is a useful therapeutic strategy.
Inhibitors tested in animal models have proven effective in animal models of kidney fibrosis and scarring,208,223,224,278 and models of Crohn disease.279–282 However none of these agents have come close to clinical development. This is probably because they have all been developed for specific, overt diseases for which other therapeutic approaches are precedented, and so preferred
EGGL removal agents
A more ambitious goal is to remove EGGL cross-links from the body. There are two approaches to removing EGGL cross-links from the ECM. The cross-links can be cleaved, releasing the cross-linked proteins, or the proteins as a whole can be degraded. As wholesale degradation of proteins in the ECM is clearly undesirable, this latter approach means that the natural turnover of proteins in the ECM must be stimulated. Enhancing turnover has been reviewed elsewhere in the context of removing glycation cross-links,283 and will not be discussed further here.
Removal of EGGL itself is a more specific target. If TG2 cross-links are found at high enough levels to play a major role in the age-related diseases of the ECM and other tissues in humans, it would be desirable to find a way to reverse their formation. This requires a chemical cleavage agent or an enzyme. Chemical cleavage of the isopeptide bond seems an unlikely possibility. Any reagent that could cleave the isopeptide bond would also cleave at least a small fraction of all of the peptide bonds in the body, rendering it extremely toxic. Targeting a peptide bond-cleavage agent to the isopeptide bond would require multiple interactions, effectively an artificial enzyme, which is beyond conventional drug chemistry.
Enzymatic cleavage is plausible and precedented. Isopeptidases are known to be produced by organisms that eat highly cross-linked proteins, such as the Brown House Moth, which can live off the highly cross-linked keratin in wool.284 Other organisms that live on materials that are highly cross-linked with EGGL (or indeed other cross-links mentioned in the first section of this article) may be fertile sources for specific isopeptidases.
Mammalian isopeptidases are known to exist, although none are suitable as ECM-opening agents without substantial modification. I have mentioned the intracellular isopeptidases that cleave the isopeptide bond between ubiquitin and proteins targeted for proteosomal destruction (isopeptidase T).152 Isopeptidase T is specific to polyubiquitin and so could not be used as a general EGGL cleavage agent in vivo, but engineering the protein for broader specificity might be practical. Thyroglobulin is cross-linked extensively in thyroid gland lumen; 20%–70% of cross-links are EGGL, depending on species.285 Thyroglobulin has to be endocytosed to be broken down and thyroid hormones released, so large multimers must be broken down from “globules” of protein before endocytosis. A plasminogen-like protein286 and possibly cathepsin-like proteases287 are involved in breaking up the very large cross-linked masses, and may be able to cleave the EGGL links within them. As mentioned above, GACT cleaves free EGGL cross-links,143,144 converting them to free lysine and 5-oxoproline. However, GACT has a deeply buried active site,288 which is only suited to cleave small molecule substrates and not peptides linked by EGGL cross-links. Thus, while we might think that a mammalian enzyme would be an easier starting point for developing a therapeutic, because it is less likely to be immunogenic, it is likely that the EGGL-cleaving enzyme from the House Moth will be a more practical target for protein engineering.
Therapeutic targets for EGGL removal
Developing any treatment for the disability of aging is an ambitious target. Apart from academic discussions of whether aging is a disease, a precursor to disease, or something that is part of our “normal” biology (like plague and starvation),289,290 there is the more practical issue of what efficacy and toxicity end points would be acceptable for a clinical trial of a new drug aimed at reducing disability in older people who were not diagnosed with overt, acute pathology. It would ease the regulatory route for a treatment if the drug could be applied to a recognized disease or disorder for development, and then have its use extended once launched. This is a well-trodden strategy for drugs from aspirin to erythropoietin (EPO). What would the initial therapeutic target be?
The evidence above suggests that TG2 has an effect cross-linking the ECM in a range of fibrotic disease. Reducing cross-linking in the walls of major arteries or the capillaries of the kidneys is liable to have toxicity risks, making it a high-risk strategy over other, more conventional treatment for cardiovascular disease and nephropathy. Targeting a non–life-threatening but disabling condition might provide a better risk–reward ratio for a developer. Two possible areas are cataracts and tendinopathy.
TG2 is plausible as a mechanism for formation of anterior polar cataracts. Although TG2 is not the principal cause of cataracts (they are triggered by TGF-β–mediated inflammatory processes), once TG2 has cross-linked lens proteins, their extremely long lifetimes153 mean that the only plausible treatment is cleavage of the cross-links or removal of the lens. Treatment with an EGGL cleavage agent topically limited to the lens capsule by injection is plausible, and an interesting potential alternative to surgical replacement of the lens with a synthetic lens, because the restored lens would have some flexibility, also (partially) reversing age-related presbyopia.
Tendinopathies may also be an attractive therapeutic target. Over-use or inflammatory injuries to tendons, especially the rotator cuff tendons of the shoulder, result in substantial limitations of movement that can be partly relieved by long-term physiotherapy. Relaxation of the over-rigid tendon could benefit these patients, providing an overshoot did not render them liable to dislocation of the shoulder, which is extraordinarily painful and results in further inflammatory damage.
There is precedent for replacing surgical procedures with enzyme treatment. Dupuytren contracture is a disabling but not life-threatening pathology of the tendons of the palmar fascies of the hand in which formation of fibrotic scar tissue around the tendons of the palm and fingers results in increasingly restricted movement of the affected fingers. It can be treated surgically, under general or (more rarely) local anesthetic, as an outpatient procedure. However, in 2010 collagenase from Clostridium histolyticum was approved as an alternative treatment. Collagenase is injected into the fibrotic bands around the tendon, weakening them and allowing the surgeon to straighten the affected fingers.291,292 The procedure is faster and less invasive than surgery—whether it is as effective remains to be seen. But the precedent for replacing day surgery with relatively non-specific enzyme treatment has been established and offers hope for an early path to an EGGL cleavage agent being developed as a therapeutic.
Conclusion
There is limited but encouraging precedence for extracellular TG2 activity and extracellular EGGL cross-links as therapeutic targets for the diseases and disabilities of aging. However, substantially more work needs to be done to quantitate the level of cross-links, as opposed to relative TG2 enzyme, protein, or gene levels, both in disease conditions and in aging in the absence of acute pathology. A significant and major lack is whether EGGL cross-links are quantitatively important in the aging ECM.
If further work supports the concept of preventing or removing EGGL cross-links as an approach to reversing age-related changes in the ECM, then enzyme-based therapeutic strategies for cleaving EGGL cross-links in situ appear plausible. I suggest two “conventional” disease states—rotator cuff tendonitis and anterior polar cataracts—for which development of such a therapy may be valuable under conventional drug development processes and economics and which would open the route to the therapy's more ambitious use in combating a wider range of disabilities of old age.
Acknowledgments
I am very grateful for funding from the SENS Research Foundation (Mountain View, CA) to support this work. I am grateful to Johan Sjöberg for early input into the study of TG2, Sven Bulterijs for comments, Melanie Leitner and Harvey Arbesman for bringing the observations of ALS to my notice, two anonymous referees for helpful and constructive comments, and to Rhian Grainger for unflagging cheerfulness.
Author Disclosure Statement
The author admits to having had surgery for Dupuytren contracture and physiotherapy for “frozen shoulder” after a dislocation, and to be personally interested in a better way of treating both. However, he has no financial interest in the subject of this article.
References
- 1.Lai-Fook SJ, Hyatt RE. Effects of age on elastic moduli of human lungs. J Appl Physiol 2000;89:163–168 [DOI] [PubMed] [Google Scholar]
- 2.Haskett D, Johnson G, Zhou A, Utzinger U, Vande Geest J. Microstructural and biomechanical alterations of the human aorta as a function of age and location. Biomechan Modeling Mechanobiol 2010;9:725–736 [DOI] [PubMed] [Google Scholar]
- 3.Sell DR, Monnier VM. Molecular basis of arterial stiffening: Role of glycation—a mini-review. Gerontology 2012;58:227–237 [DOI] [PubMed] [Google Scholar]
- 4.Soldatos G, Cooper ME. Advanced glycation end products and vascular structure and function. Curr Hypertens Rep 2006;8:472–478 [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann EA, Schaible E, Bale H, Barth HD, Tang SY, Reichert P, Busse B, Alliston T, Ager JW, Ritchie RO. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci USA 2011;108:14416–14421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang SY, Vashishth D. Non-enzymatic glycation alters microdamage formation in human cancellous bone. Bone 2010;46:148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouxsein ML. Overview of bone structure and strength. In: Genetics of Bone Biology and Skeletal Disease. Academic Press, San Diego, 2013, pp. 25–34 [Google Scholar]
- 8.Zimmerman SD, McCormick RJ, Vadlamudi RK, Thomas DP. Age and training alter collagen characteristics in fast- and slow-twitch rat limb muscle. J Appl Physiol 1993;75: 1670–1674 [DOI] [PubMed] [Google Scholar]
- 9.Gosselin LE, Adams C, Cotter TA, McCormick RJ,. Thomas DP. Effect of exercise training on passive stiffness in locomotor skeletal muscle: Role of extracellular matrix. J Appl Physiol 1998;85:1011–1016 [DOI] [PubMed] [Google Scholar]
- 10.Couppé C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjær M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 2009;107:880–886 [DOI] [PubMed] [Google Scholar]
- 11.van Boekel MA, Hoenders HJ. Glycation-induced crosslinking of calf lens crystallins. Exp Eye Res 1991;53:89–94 [DOI] [PubMed] [Google Scholar]
- 12.Heys KR, Cram SL, Truscott RJW. Massive increase in the stiffness of the human lens nucleus with age: The basis for presbyopia? Mol Vision 2004;10:956–963 [PubMed] [Google Scholar]
- 13.Karsdal MA, Nielsen MJ, Sand JM, Henriksen K, Genovese F, Bay-Jensen A-C, Smith V, Adamkewicz JI, Christiansen C, Leeming DJ. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol 2013;11:70–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev 1998;106:1–56 [DOI] [PubMed] [Google Scholar]
- 15.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol 2011;300:R655–R662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritze O, Romero B, Schleicher M, Jacob MP, Oh DY, Starcher B, Schenke-Layland K, Bujan J, Stock UA. Age-related changes in the elastic tissue of the human aorta. J Vasc Res 2012;49:77–86 [DOI] [PubMed] [Google Scholar]
- 17.Sjoberg JS, Bulterijs S. Characteristics, formation, and pathophysiology of glucosepane: A major protein cross-link. Rejuvenation Res 2009;12:137–148 [DOI] [PubMed] [Google Scholar]
- 18.Esposito C, Caputo I. Mammalian transglutaminases. FEBS J 2005;272:615–631 [DOI] [PubMed] [Google Scholar]
- 19.Lorand L, Graham RM. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat Reviews Med Cell Biol 2003;4:140–156 [DOI] [PubMed] [Google Scholar]
- 20.Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: Lessons from genetically engineered mouse models and inherited disorders. Physiol Rev 2009;89:991–1023 [DOI] [PubMed] [Google Scholar]
- 21.Badarau E, Collighan RJ, Griffin M. Recent advances in the development of tissue transglutaminase (TG2) inhibitors. Amino Acids 2013;44:119–127 [DOI] [PubMed] [Google Scholar]
- 22.Mishra S, Murphy LJ. Tissue transglutaminase has intrinsic kinase activity: identification of transglutaminase 2 as an insulin-like growth factor-binding protein 3 kinase. J Biol Chem 2004;279:23863–23868 [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa G, Suwa M, Ichikawa Y, Ohtsuka T, Kumagai S, Kikuchi M, Sato Y, Saito Y. A novel function of tissue-type transglutaminase: Protein disulphide isomerase. Biochem J 2003;373:793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakaoka H, Perez DM, Baek KJD, Das T, Husain A, Misono K, Im MJ, Graham RM. Gh: A GTP-binding protein with transglutaminase activity and receptor signaling function. Science 1994;264:1593–1596 [DOI] [PubMed] [Google Scholar]
- 25.Park D, Choi SS, Ha KS. Transglutaminase 2: A multi-functional protein in multiple subcellular compartments. Amino Acids 2010;39:619–631 [DOI] [PubMed] [Google Scholar]
- 26.Griffin M, Casadio R, Bergamini CM. Transglutaminases: Nature's biological glues. Biochem J 2002;368:377–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fesus L, Piacentini M. Transglutaminase 2: An enigmatic enzyme with diverse functions. Trends Biochem Sci 2002;27:534–539 [DOI] [PubMed] [Google Scholar]
- 28.Monsonego A, Shani Y, Friedmann I, Paas Y, Eizenberg O, Schwartz M. Expression of GTP-dependent and GTP-independent tissue-type transglutaminase in cytokine-treated rat brain astrocytes. J Biol Chem 1997;272:3724–3732 [DOI] [PubMed] [Google Scholar]
- 29.Lai T-S, Liu Y, Li W, Greenberg CS. Identification of two GTP-independent alternatively spliced forms of tissue transglutaminase in human leukocytes, vascular smooth muscle, and endothelial cells. FASEB J 2007;21:4131–4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phatak VM, Croft SM, Rameshaiah Setty SG, Scarpellini A, Hughes DC, Rees R, McArdle S, Verderio EAM. Expression of transglutaminase-2 isoforms in normal human tissues and cancer cell lines: Dysregulation of alternative splicing in cancer. Amino Acids 2013;44:33–44 [DOI] [PubMed] [Google Scholar]
- 31.Fisher M, Jones RA, Huang L, Haylor JI, Nahas M, Griffin M, Johnson TS. Modulation of tissue transglutaminase in tubular epithelial cells alters extracellular matrix levels: A potential mechanism of tissue scarring. Matrix Biol 2009;28:20–31 [DOI] [PubMed] [Google Scholar]
- 32.van den Akker J, van Weert A, Afink G, Bakker ENTP, van der Pol E, Boing AN, VanBavel E. Transglutaminase 2 is secreted from smooth muscle cells by transamidation-dependent microparticle formation. Amino Acids 2012;42:961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res 2003;59:277–287 [DOI] [PubMed] [Google Scholar]
- 34.Mause SF, Weber C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ Res 2010;107:1047–1057 [DOI] [PubMed] [Google Scholar]
- 35.Keresztessy Z, Csősz É, Hársfalvi J, Csomós K, Gray J, Lightowlers RN, Lakey JH, Balajthy Z, Fésüs L. Phage display selection of efficient glutamine-donor substrate peptides for transglutaminase 2. Protein Sci 2006;15:2466–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hitomi K, Kitamura M, Sugimura Y. Preferred substrate sequences for transglutaminase 2: Screening using a phage-displayed peptide library. Amino Acids 2009;36:619–624 [DOI] [PubMed] [Google Scholar]
- 37.Lee J-H, Song C, Kim D-H, Park I-H, Lee S-G, Lee Y-S, Kim B-G. Glutamine (Q)-peptide screening for transglutaminase reaction using mRNA display. Biotechnol Bioeng 2012;110:353–362 [DOI] [PubMed] [Google Scholar]
- 38.Sugimura Y, Yamashita H, Hitomi K. Screening of substrate peptide sequences for tissue-type transglutaminase (TGase 2) using T7 phage cDNA library. Cytotechnology 2011;63:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groenen PJTA, Smulders RHPH, Peters RFR, Grootjans JJ, Van Den Ijssel PRLA, Bloemendal H, De Jong WW. The amine-donor substrate specificity of tissue-type transglutaminase. Eur J Biochem 1994;220:795–799 [DOI] [PubMed] [Google Scholar]
- 40.Csosz E, Bagossi P, Nagy Z, Dosztanyi Z, Simon I, Fesus L. Substrate preference of transglutaminase 2 revealed by logistic regression analysis and intrinsic disorder examination. J Mol Biol 2008;383:390–402 [DOI] [PubMed] [Google Scholar]
- 41.Williams-Ashman HG, Canellakis ZN. Transglutaminase-mediated covalent attachment of polyamines to proteins: Mechanisms and potential physiological significance. Physiol Chem Phys 1980;12:457–472 [PubMed] [Google Scholar]
- 42.Folk JE, Park MH, Chung SI, Schrode J, Lester EP, Cooper HL. Polyamines as physiological substrates for transglutaminases. J Biol Chem 1980;255:3695–3700 [PubMed] [Google Scholar]
- 43.Tabolacci C, Lentini A, Provenzano B, Beninati S. Evidences for a role of protein cross-links in transglutaminase-related disease. Amino Acids 2012;42:975–986 [DOI] [PubMed] [Google Scholar]
- 44.Beninat S, Senger DR, Cordella-Miele E, Mukherjee AB, Chackalaparampil I, Shanmugam V, Singh KM, Muhkerjee BB. Osteopontin: Its transglutaminase-catalyzed posttranslational modifications and cross-linking to fibronectin. J Biochem 1994;115:255–258 [DOI] [PubMed] [Google Scholar]
- 45.Gilad GM, Casero RAJ. Busto R, Globus MY. Polyamines in rat brain extracellular space after ischemia. Mol Chem Neuropathol 1993;18:27–33 [DOI] [PubMed] [Google Scholar]
- 46.Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS One 2008;3:e1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burgoyne RD, Weiss JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J 2001:353:1–12 [PMC free article] [PubMed] [Google Scholar]
- 48.Király R, Demény M, Fésüs L. Protein transamidation by transglutaminase 2 in cells: A disputed Ca2+-dependent action of a multifunctional protein. FEBS J 2011;278:4717–4739 [DOI] [PubMed] [Google Scholar]
- 49.Achyuthan KE, Greenberg CS. Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J Biol Chem 1987;262:1901–1906 [PubMed] [Google Scholar]
- 50.Begg GE, Carrington L, Stokes PH, Matthews JM, Wouters MA, Husain A, Lorand L, Iismaa SE, Graham RM. Mechanism of allosteric regulation of transglutaminase 2 by GTP. Proc Natl Acad Sci USA 2006;103:19683–19688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol 2007;5;2788–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iismaa SEW, Wu MJ, Nanda N, Church WB, Graham RM. GTP binding and signaling by Gh/transglutaminase II involves distinct residues in a unique GTP-binding pocket. J Biol Chem 2000;275:18259–18265 [DOI] [PubMed] [Google Scholar]
- 53.Bergamini CM. GTP modulates calcium binding and cation-induced conformational changes in erythrocyte transglutaminase. FEBS Lett 1988;239:255–258 [DOI] [PubMed] [Google Scholar]
- 54.Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res 2010;107:117–125 [DOI] [PubMed] [Google Scholar]
- 55.Lai TS, Hausladen A, Slaughter TF, Eu JP, Stamler JS, Greenberg CS. Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase. Biochemistry 2001;40:4904–4910 [DOI] [PubMed] [Google Scholar]
- 56.Citi S. Protein kinase inhibitors prevent junction dissociation induced by low extracellular calcium in MDCK epithelial cells. J Cell Biol 1992;117:169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Supinski G, Nethery D, Stofan D, DiMarco A. Extracellular calcium modulates generation of reactive oxygen species by the contracting diaphragm. J Appl Physiol 1999;87:2177–2185 [DOI] [PubMed] [Google Scholar]
- 58.Stokes BT, Fox P, Hollinden G. Extracellular calcium activity in the injured spinal cord. Exp Neurol 1983;80:561–572 [DOI] [PubMed] [Google Scholar]
- 59.Moriya T, Hassan AZ, Young W, Chesler M. Dynamics of extracellular calcium activity following contusion of the rat spinal cord. J Neurotrauma 1994;11:255–263 [DOI] [PubMed] [Google Scholar]
- 60.Stokes B, Somerson S. Spinal cord extracellular microenvironment. Neurochem Pathol 1987;7:47–55 [DOI] [PubMed] [Google Scholar]
- 61.Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol 1989;109:1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest 1992;89:530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pillai S, Bikle DD, Mancianti M-L, Cline P, Hincenbergs M. Calcium regulation of growth and differentiation of normal human keratinocytes: Modulation of differentiation competence by stages of growth and extracellular calcium. J Cell Physiol 1990;143:294–302 [DOI] [PubMed] [Google Scholar]
- 64.Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, Riccardi D. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci USA 2004;101:5140–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balsinde J, Fernández B, Diez E. Regulation of arachidonic acid release in mouse peritoneal macrophages. The role of extracellular calcium and protein kinase C. J Immunol 1990;144:4298–4304 [PubMed] [Google Scholar]
- 66.Betsholtz C, Westermark B. Growth factor-induced proliferation of human fibroblasts in serum-free culture depends on cell density and extracellular calcium concentration. J Cell Physiol 1984;118:203–210 [DOI] [PubMed] [Google Scholar]
- 67.Kanatani M, Sugimoto T, Fukase M, Fujita T. Effect of elevated extracellular calcium on the proliferation of osteoblastic MC3T3-E1 cells: Its direct and indirect effects via monocytes. Biochem Biophys Res Commun 1991;181:1425–1430 [DOI] [PubMed] [Google Scholar]
- 68.Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995;121:1129–1137 [DOI] [PubMed] [Google Scholar]
- 69.Traut T. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem 1994;140:1–22 [DOI] [PubMed] [Google Scholar]
- 70.Stamnaes J, Pinkas DM, Fleckenstein B, Khosla C, Sollid LM. Redox regulation of transglutaminase 2 activity. J Biol Chem 2010;285:25402–25409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagy L, Thomázy VA, Saydak MM, Stein JP, Davies PJ.The promoter of the mouse tissue transglutaminase gene directs tissue-specific, retinoid-regulated and apoptosis-linked expression. Cell Death Differ 1997;4:534–547 [DOI] [PubMed] [Google Scholar]
- 72.Lee ZW, Kwon SM, Kim SW, Yi SJ, Kim YM, Ha KS. Activation of in situ tissue transglutaminase by intracellular reactive oxygen species. Biochem Biophys Res Commun 2003;305:633–640 [DOI] [PubMed] [Google Scholar]
- 73.Bergamini CM. Effects of ligands on the stability of tissue transglutaminase: Studies in vitro suggest possible modulation by ligands of protein turn-over in vivo. Amino Acids 2007;33:415–421 [DOI] [PubMed] [Google Scholar]
- 74.Casadio R, Polverini E, Mariani P, Spinozzi F, Carsughi F, Fontana A, de Laureto PP, Matteucci G, Bergamini CM. The structural basis for the regulation of tissue transglutaminase by calcium ions. Eur J Biochem 1999;262:3 (672–679) [DOI] [PubMed] [Google Scholar]
- 75.Pontremoli S, Melloni E. Extralysosomal protein degradation. Annu Rev Biochem 1986;55:455–481 [DOI] [PubMed] [Google Scholar]
- 76.Qian R-Q, Glanville RW. Alignment of fibrillin molecules in elastic microfibrils is defined by transglutaminase-derived cross-links. Biochemistry 1997;36:15841–15847 [DOI] [PubMed] [Google Scholar]
- 77.Gaudry CA, Verderio E, Aeschlimann D, Cox A, Smith C, Griffin M. Cell surface localization of tissue transglutaminase is dependent on a fibronectin-binding site in its N-terminal beta-sandwich domain. J Biol Chem 1999;274:30707–30714 [DOI] [PubMed] [Google Scholar]
- 78.Macdonald JA. Extracellular matrix assembly. Annu Rev Cell Biol 1988;4:183–207 [DOI] [PubMed] [Google Scholar]
- 79.Akimov SS, Belkin AM. Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin. J Cell Sci 2001;114: 2989–3000 [DOI] [PubMed] [Google Scholar]
- 80.Aeschlimann D, Kaupp O, Paulsson M. Transglutaminase-catalyzed matrix cross-linking in differentiating cartilage: Identification of osteonectin as a major glutaminyl substrate. J Cell Biol 1995;129: 881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kleman J-P, Aeschlimann D, Paulsson M, van der Rest M. Transglutaminase-catalyzed crosslinking of fibrils of collagen V/XI in A204 rhabdomyosarcoma cells. Biochemistry 1995;34:13768–13775 [DOI] [PubMed] [Google Scholar]
- 82.Bowness JM, Folk JE, Timpl R. Identification of a substrate site for liver transglutaminase on the aminopropeptide of type III collagen. J Biol Chem 1987;262:1022– 1024 [PubMed] [Google Scholar]
- 83.Anwar R, Miloszewski KJA. Factor XIII deficiency. Br J Haematol 1999;107:468–484 [DOI] [PubMed] [Google Scholar]
- 84.Peterson LL, Zettergren JG, Wuepper KD. Biochemistry of transglutaminases and cross-linking in the skin. J Invest Dermatol 1983;81:95s–100s [DOI] [PubMed] [Google Scholar]
- 85.Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: Assembly and structural features of the cornified cell envelope. BioEssays 2002;24:789–800 [DOI] [PubMed] [Google Scholar]
- 86.O'Regan GM, Sandilands A, McLean WHI, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol 2009;124(Suppl 2):R2–R6 [DOI] [PubMed] [Google Scholar]
- 87.Matsuki M, Yamashita F, Ishida-Yamamoto A, Yamada K, Kinoshita C, Fushiki S, Ueda E, Morishima Y, Tabata K, Yasuno H, Hashida M, Iizuka H, Ikawa M, Okabe M, Kondoh G, Kinoshita T, Takeda J, Yamanishi K. Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase). Proce Nat l Acad Sci USA 1998;95:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuramoto N, Takizawa T, Takizawa T, Matsuki M, Morioka H, Robinson JM, Yamanshi K. Development of ichthyosiform skin compensates for defective permeability barrier function in mice lacking transglutaminase 1. J Clin Invest 2002;109:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Russell LJ, DiGiovanna JJ, Rogers GR, Steinert PM, Hashem N, Compton JG, Bale SJ. Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat Genet 1995;9:279–283 [DOI] [PubMed] [Google Scholar]
- 90.Schittny JC, Paulsson M, Vallan C, Burri PH, Kedei N, Aeschlimann D. Protein cross-linking mediated by tissue transglutaminase correlates with the maturation of extracellular matrices during lung development. Am J Resp Cell Mol Bio 1997;17:334–343 [DOI] [PubMed] [Google Scholar]
- 91.Lee SK, Chi JG, Park SC, Chung SI. Transient expression of transglutaminase c during prenatal development of human muscles. J Histochem Cytochem 2000;48:1565–1574 [DOI] [PubMed] [Google Scholar]
- 92.Bowness MJ, Tarr AH, Wong T. Increased transglutaminase activity during skin wound healing in rats. Biochim Biophys Acta 1988;967:234–240 [DOI] [PubMed] [Google Scholar]
- 93.Verderio EAM, Johnson T, Griffin M. Tissue transglutaminase in normal and abnormal wound healing: Review article. Amino Acids 2004;26:387–404 [DOI] [PubMed] [Google Scholar]
- 94.Haroon ZA, Hettasch JM, Lai T-S, Dewhirst MW, Greenberg CS. Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis. FASEB J 1999;13:1787–1795 [DOI] [PubMed] [Google Scholar]
- 95.Upchurch HF, Conway E, Patterson MK, Maxwell MD.Localization of cellular transglutaminase on the extracellular matrix after wounding: Characteristics of the matrix bound enzyme. J Cell Physiol 1991;149:375–382 [DOI] [PubMed] [Google Scholar]
- 96.Linge C, Richardson J, Vigor C, Clayton E, Hardas B, Rolfe K. Hypertrophic scar cells fail to undero a form of aopotosis specific to contractile collagen—the role of transglutaminase. J Invest Dermatol 2005;125:72— 82 [DOI] [PubMed] [Google Scholar]
- 97.Yawo H, Kuno M. Calcium dependence of membrane sealing at the cut end of the cockroach giant axon. J Neurosci 1985;5:1626–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie XY, Barrett JN. Membrane resealing in cultured rat septal neurons after neurite transection: Evidence for enhancement by Ca(2+)-triggered protease activity and cytoskeletal disassembly. J Neurosci 1991;11:3257–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu J, Zern MA. Tissue transglutaminase, a key enzyme involved in liver diseases. Hepatol Res 2004;29:1–8 [DOI] [PubMed] [Google Scholar]
- 100.Fesus L, Madi A, Balajthy Z, Nemes ZS, Szondy Z. Transglutaminase induction by various cell death and apoptosis pathways. Experientia 1996;52:942–949 [DOI] [PubMed] [Google Scholar]
- 101.Fésüs L, Szondy Z. Transglutaminase 2 in the balance of cell death and survival. FEBS Lett 2005;579:3297–3302 [DOI] [PubMed] [Google Scholar]
- 102.Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: Biological significance and medical applications. Amino Acids 2009;36:659–670 [DOI] [PubMed] [Google Scholar]
- 103.Kim S-Y, Jeitner TM, Steinert PM. Transglutaminases in disease. Neurochem Int 2002;40:85–103 [DOI] [PubMed] [Google Scholar]
- 104.Piacentini M. Tissue transglutaminase: A candidate effector element of physiological cell death. Curr Top Microbiol Immunol 1995;200:163–175 [DOI] [PubMed] [Google Scholar]
- 105.Tucholski J, Johnson GVW. Tissue transglutaminase differentially modulates apoptosis in a stimuli-dependent manner. J Neurochem 2002;81:780–791 [DOI] [PubMed] [Google Scholar]
- 106.Davies PJ, Davies DR, Levitzki A, Maxfield FR, Milhaud P, Willingham MC, Pastan IH. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature 1980;283:162–167 [DOI] [PubMed] [Google Scholar]
- 107.Van Leuven F, Cassiman J-J, Van Den Berghe H. Primary amines inhibit recycling of α2M receptors in fibroblasts. Cell 1980;20:37–43 [DOI] [PubMed] [Google Scholar]
- 108.Levitzki A, Willingham M, Pastan I. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc Natl Acad Sci USA 1980;77:2706–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Szondy Z, Sarang Z, Molnár P, Németh T, Piacentini M, Mastroberardino PG, Falasca L, Aeschlimann D, Kovács J, Kiss I, Szegezdi É, Lakos G, Rajnavölgyi É, Birckbichler PJ, Melino G, Fésüs L.Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci USA 2003;100:7812–7817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM. Targeted inactivation of gh/tissue transglutaminase II. J Biol Chem 2001;276:20673–20678 [DOI] [PubMed] [Google Scholar]
- 111.De Laurenzi V, Melino G. Gene disruption of tissue transglutaminase. Mol Cell Biol 2001;21:148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tarantino U, Oliva F, Taurisano G, Orlandi A, Pietroni V, Candi E, Melino G, Maffulli N. FXIIIa and TGF-β over-expression produces normal musculo-skeletal phenotype in TG2−/− mice. Amino Acids 2009;36:679–684 [DOI] [PubMed] [Google Scholar]
- 113.Bakker ENTP, Pistea A, Spaan JAE, Rolf T, de Vries CJ, van Rooijen N, Candi E, VanBavel E. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: Possible compensation by macrophage-derived Factor XIII. Circ Res 2006;99:86–92 [DOI] [PubMed] [Google Scholar]
- 114.Stephens P, Grenard P, Aeschlimann P, Langley M, Blain E, Errington R, Kipling D, Thomas D, Aeschlimann D. Crosslinking and G-protein functions of transglutaminase 2 contribute differentially to fibroblast wound healing responses. J Cell Sci 2004;117:3389–3403 [DOI] [PubMed] [Google Scholar]
- 115.Johnson KB, Petersen-Jones H, Thompson JM, Hitomi K, Itoh M, Bakker ENTP, Johnson GVW, Colak G, Watts SW. Vena cava and aortic smooth muscle cells express transglutaminases 1 and 4 in addition to transglutaminase 2. Am J Physiol Heart Circ Physiol 2012;302:H1355–H1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bar KJ, Franke S, Wenda B, Muller S, Kientsch-Engel R, Stein G, Sauer H. Pentosidine and N-episilon-(carboxymethyl)-lysine in Alzheimer's disease and vascular dementia. Neurobiol Aging 2003;24:333–338 [DOI] [PubMed] [Google Scholar]
- 117.Sarvari M, Karpati L, Fesus L, Deli L, Muszbek L, Nemes Z. Competitive enzyme-linked immunosorbent assay for N-epsilon-(gamma-glutamyl)-lysine. Anal Biochem 2002;311:187–190 [DOI] [PubMed] [Google Scholar]
- 118.Nemes Z, Petrovski G, Fesus L. Tools for the detection and quantitation of protein transglutamination. Anal Biochem 2005;342:1–10 [DOI] [PubMed] [Google Scholar]
- 119.Hoffner G, van der Rest G, Dansette PM, Djian P. The end product of transglutaminase crosslinking: Simultaneous quantitation of [Nε-(γ-glutamyl) lysine] and lysine by HPLC–MS. Anal Biochem 2009;384:296–304 [DOI] [PubMed] [Google Scholar]
- 120.Cernadas MR, de Miguel LS, García-Durán M, González-Fernández F, Millás I, Montón M, Rodrigo J, Rico L, Fernández P, de Frutos T, Rodríguez-Feo JA, Guerra J, Caramelo C, Casado S, López-Farré A. Vena cava and aortic smooth muscle cells express transglutaminases 1 and 4 in addition to transglutaminase 2, Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res 1998;83:279–286 [DOI] [PubMed] [Google Scholar]
- 121.Nemes Z, Devreese B, Steinert PM, van Beeumen J, Fesus L. Cross-linking of ubiquitin, HSP27, parkin, and α-synuclein by γ-glutamyl-ε-lysine bonds in Alzheimer's neurofibrillary tangles. FASEB J 2004;18:1135–1137 [DOI] [PubMed] [Google Scholar]
- 122.Suzuki Y, Kuroda H, Kanbe N, Takenouchi M. Analysis of biochemical components of human skin corneocytes prepared non-invasively by tape-stripping. Nippon Koshohin Gakkaishi 2007;31:69–77 [Google Scholar]
- 123.Ghadially R, Brown BE, Sequeira-Martin SM, Feingold KE, Elias PM. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J Clin Invest 1995;95:2281–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith SM, Nillen JL, LeBlanc A, Lipton A, Demers LM, Lane HW, Leach CS. Collagen cross-link excretion during space flight and bed rest. J Clin Endocrinol Metab 1998;83:3584–3591 [DOI] [PubMed] [Google Scholar]
- 125.Johnson K, Hashimoto S, Lotz M, Pritzker K, Terkeltaub R. Interleukin-1 induces pro-mineralizing activity of cartilage tissue transglutaminase and Factor XIIIa. Am J Pathol 2001;159:149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park SC, Yeo EJ,. Han JA, Hwang YC, Choi JY, Park JS, Park YH, Kim KO, Kim I.-G, Seong SC, Kwak SJ. Aging process is accompanied by increase of transglutaminase C. The J Gerontol Ser A: Biol Sci Med Sci 1999;54:B78–B83 [DOI] [PubMed] [Google Scholar]
- 127.Enderlin V, Pallet V, Alfos S, Dargelos E, Jaffard R, Garcin H, Higueret P. Age-related decreases in mRNA for brain nuclear receptors and target genes are reversed by retinoic acid treatment. Neurosci Lett 1997;229:125–129 [DOI] [PubMed] [Google Scholar]
- 128.Rosenthal AK, Derfus BA, Henry LA. Transglutaminase activity in aging articular chondrocytes and articular cartilage vesicles. Arthritis Rheum 1997;40:966–970 [DOI] [PubMed] [Google Scholar]
- 129.Lavie L, Weinreb O. Age- and strain-related changes in tissue transglutaminase activity in murine macrophages: the effects of inflammation and induction by retinol. Mech Ageing Dev 1996;90:129–143 [DOI] [PubMed] [Google Scholar]
- 130.Singhal PC, Reddy K, Franki N, Sanwal V, Kapasi A, Gibbons N, Mattana J, Valderrama E. Age and sex modulate renal expression of SGP-2 and transglutaminase and apoptosis of splenocytes, thymocytes, and macrophages. J Investig Med 1997;45:567–575 [PubMed] [Google Scholar]
- 131.Robinson NE. Protein deamidation. Proc Natl Acad Sci USA 2002;99:5283–5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Robinson NE, Robinson AB. Prediction of primary structure deamidation rates of asparaginyl and glutaminyl peptides through steric and catalytic effects. J Peptide Res 2004;63:437–448 [DOI] [PubMed] [Google Scholar]
- 133.Sivan S-S, Van El B, Merkher Y, Schmelzer CEH, Zuurmond A-M, Heinz A, Wachtel E, Varga P-P, Lazary A, Brayda-Bruno M, Maroudas A. Longevity of elastin in human intervertebral disc as probed by the racemization of aspartic acid. Biochim Biophys Acta 2012;1820:1671–1677 [DOI] [PubMed] [Google Scholar]
- 134.Vivó M, Vera Nd, Cortés R, Mengod G, Camón Ls, Martínez E. Polyamines in the basal ganglia of human brain. Influence of aging and degenerative movement disorders. Neurosci Lett 2001;304:107–111 [DOI] [PubMed] [Google Scholar]
- 135.Morrison LD, Becker L, Ang LC, Kish SJ. Polyamines in human brain: Regional distribution and influence of aging. J Neurochem 1995;65:636–642 [DOI] [PubMed] [Google Scholar]
- 136.Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp Gerontol 2009;44:727–732 [DOI] [PubMed] [Google Scholar]
- 137.Lee C-K, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science 1999;285:1390–1393 [DOI] [PubMed] [Google Scholar]
- 138.Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: Longevity, growth, lean body mass and disease. J Gerontol 1982;37:130–141 [DOI] [PubMed] [Google Scholar]
- 139.Mohan S, Radha E. Age-related changes in rat muscle collagen. Gerontology 1980;26:61– 67 [DOI] [PubMed] [Google Scholar]
- 140.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 2007;103:2068–2076 [DOI] [PubMed] [Google Scholar]
- 141.Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung J, Wang Z, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol 2000;114:480–486 [DOI] [PubMed] [Google Scholar]
- 142.Wachtel E, Maroudas A, Schneiderman R. Age-related changes in collagen packing of human articular cartilage. Biochimi Biophys Acta 1995;1243:239–243 [DOI] [PubMed] [Google Scholar]
- 143.Oakley AJ, Coggan M, Board PG. Identification and characterization of γ-glutamylamine cyclotransferase, an enzyme responsible for γ-glutamyl-ε-lysine catabolism. J Biol Chem 2010;285:9642–9648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fink ML, Chung SI, Folk JE. Gamma-glutamylamine cyclotransferase: Specificity toward epsilon-(L-gamma-glutamyl)-L-lysine and related compounds. Proc Natl Acad Sci USA 1980;77:4564–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bowser T, Trawick M. Probing the specificity of gamma-glutamylamine cyclotransferase: An enzyme involved in the metabolism of transglutaminase-catalyzed protein crosslinks. Amino Acids 2013;44:143–150 [DOI] [PubMed] [Google Scholar]
- 146.Shi M, Gozal E, Choy HA, Forman HJ. Extracellular glutathione and gamma-glutamyl transpeptidase prevent H2O2-induced injury by 2,3-dimethoxy-1,4-naphthoquinone. Free Radic Biol Med 1993;15:57–67 [DOI] [PubMed] [Google Scholar]
- 147.Seguro K, Kumazawa Y, Ohtsuka T, Ide H, Nio N, Motoki M, Kubota K. Epsilon-(gamma-glutamyl)-lysine: Hydrolysis by gamma-glutamyltransferase of different origins, when free or protein bound. J Agric Food Chem 1995;43:1977–1981 [Google Scholar]
- 148.Obrador E, Carretero J, Ortega A, Medina I, Rodilla V, Pellicer JA, Estrela JM. Gamma-glutamyl transpeptidase overexpression increases metastatic growth of B16 melanoma cells in the mouse liver. Hepatology 2002;35:74–81 [DOI] [PubMed] [Google Scholar]
- 149.Hiramatsu K, Asaba Y, Takeshita S, Nimura Y, Tatsumi S, Katagiri N, Niida S, Nakajima T, Tanaka S, Ito M, Karsenty G, Ikeda K. Overexpression of gamma-glutamyltransferase in transgenic mice accelerates bone resorption and causes osteoporosis. Endocrinology 2007;148:2708–2715 [DOI] [PubMed] [Google Scholar]
- 150.Loewy AG, Blodgett JK, Blasé FR, May MH. Synthesis and use of a substrate for the detection of isopeptidase activity. Anal Biochem 1997;246:111–117 [DOI] [PubMed] [Google Scholar]
- 151.Balin BJ, Loewy AG, Appelt DM. Analysis of transglutaminase-catalyzed isopeptide bonds in paired helical filaments and neurofibrillary tangles from Alzheimer's disease. Methods Enzymol 1999;309:172–186 [DOI] [PubMed] [Google Scholar]
- 152.Reyes Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 2009;78:363–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS One 2008;3:e1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maroudas A, Palla G, Gilav E. Racemization of aspartic acid in human articular cartilage. Conn Tiss Res 1992;28:161–169 [DOI] [PubMed] [Google Scholar]
- 155.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JWJ, Lafeber FPJG, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 2000;275:39027–39031 [DOI] [PubMed] [Google Scholar]
- 156.Sivan SS, Tsitron E, Wachtel E, Roughley PJ, Sakkee N, van der Ham F, DeGroot J, Roberts S, Maroudas A. Aggrecan turnover in human intervertebral disc as determined by the racemization of aspartic acid. J Biol Chem 2006;281:13009–13014 [DOI] [PubMed] [Google Scholar]
- 157.Ritz-Timme S, Laumeier I, Collins MJ. Aspartic acid racemization: Evidence for marked longevity of elastin in human skin. Br J Dermatol 2003;149:951–959 [DOI] [PubMed] [Google Scholar]
- 158.Ritz S, Turzynski A, Schütz HW, Hollmann A, Rochholz G. Identification of osteocalcin as a permanent aging constituent of the bone matrix: Basis for an accurate age at death determination. Forensic Sci Int 1996;77:13–26 [DOI] [PubMed] [Google Scholar]
- 159.Kosky AA, Dharmavaram V, Ratnaswamy G, Manning MC. Multivariate analysis of the sequence dependence of asparagine deamidation rates in peptide. Pharmaceut Res 2009;26:2417–2428 [DOI] [PubMed] [Google Scholar]
- 160.Kossiakoff AA. Tertiary structure is a principal determinant to protein deamidation. Science 1988;240:191–194 [DOI] [PubMed] [Google Scholar]
- 161.Furber JD. Repairing extracellular aging and glycation. In: Fahy GM, ed. The Future of Aging, Springer: Dordrecht, Germany, 2010, pp. 587–621 [Google Scholar]
- 162.Shringarpure R, Davies KJA. Protein turnover by the proteasome in aging and disease. Free Radic Biol Med 2002;32:1084–1089 [DOI] [PubMed] [Google Scholar]
- 163.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 1996;98:996–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sivan S-S, Wachtel E, Tsitron E, Sakkee N, van der Ham F, DeGroot J, Roberts S, Maroudas A. Collagen turnover in normal and degenerate human intervertebral discs as determined by the racemization of aspartic acid. J Biol Chem 2008;283:8796–8801 [DOI] [PubMed] [Google Scholar]
- 165.DeGroot J, Verzijl N, Jacobs KMG, Budde M, Bank RA, Bijlsma JWJ, TeKoppele J.M, Lafeber FPJG. Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoerthritis Cartilage 2001;9:720–726 [DOI] [PubMed] [Google Scholar]
- 166.Babraj JA, Cuthbertson DJR, Smith K, Langberg H, Miller B, Krogsgaard MR, Kjaer M, Rennie MJ. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab 2005;289:E864–E869 [DOI] [PubMed] [Google Scholar]
- 167.Riley GP. Chronic tendon pathology: molecular basis and therapeutic implications. Exp Rev Mol Med 2005;7:1–25 [DOI] [PubMed] [Google Scholar]
- 168.Kjær M, Magnusson P, Krogsgaard M, Møller JB, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 2006;208:445–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Akar U, Ozpolat B, Mehta K, Fok J, Kondo Y, Lopez-Berestein G. Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol Cancer Re 2007;5:241–249 [DOI] [PubMed] [Google Scholar]
- 170.Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy 2010;6:322–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Butcher DT, Alliston T, Weaver VM. A tense situation: Forcing tumour progression. Nat Rev Cancer 2009;9:108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005;8:241–254 [DOI] [PubMed] [Google Scholar]
- 173.Lentini A, Abbruzzese A, Provenzano B, Tabolacci C, Beninati S. Transglutaminases: Key regulators of cancer metastasis. Amino Acids 2013;44:25–32 [DOI] [PubMed] [Google Scholar]
- 174.Mehta K, Kumar A, Kim HI. Transglutaminase 2: A multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem Pharmacol 2010;80:1921–1929 [DOI] [PubMed] [Google Scholar]
- 175.Kim DY, Park BS, Hong GU, Lee BJ, Park JW, Kim SY, Ro JY. Anti-inflammatory effects of the R2 peptide, an inhibitor of transglutaminase 2, in a mouse model of allergic asthma, induced by ovalbumin. Br J Pharmacol 2011;162:210–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ientile R, Caccamo D, Griffin M. Tissue transglutaminase and the stress response. Amino Acids 2007;33:385–394 [DOI] [PubMed] [Google Scholar]
- 177.Lampasona V, Bonfanti R, Bazzigaluppi E, Venerando A, Chiumello G, Bosi E, Bonifacio E. Antibodies to tissue transglutaminase C in type I diabetes. Diabetologia 1999;42:1195–1198 [DOI] [PubMed] [Google Scholar]
- 178.Bao F, Yu L, Babu S, Wang T, Hoffenberg EJ, Rewers M, Eisenbarth GS. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun 1999;13:143–148 [DOI] [PubMed] [Google Scholar]
- 179.Picarelli A, Di Tola M, Sabbatella L, Vetrano S, Anania MC, Spadaro A, Sorgi ML, Taccari E. Anti-tissue transglutaminase antibodies in arthritic patients: a disease-specific finding? Clin Chem 2003;49:2091–2094 [DOI] [PubMed] [Google Scholar]
- 180.Choi Y-C, Kim T-S, Kim S-Y. Increase in transglutaminase 2 in idiopathic inflammatory myopathies. Eur Neurol 2004;51:10–14 [DOI] [PubMed] [Google Scholar]
- 181.Macaione V, Aguennouz M, Mazzeo A, De Pasquale MG, Russo M, Toscano A, De Luca G, Di Giorgio RM, Vita G, Rodolico C. Expression of transglutaminase 2 does not differentiate focal myositis from generalized inflammatory myopathies. Acta Neurol Scand 2008;117:393–398 [DOI] [PubMed] [Google Scholar]
- 182.Gendek EG, Kędziora J, Gendek-Kubiak H. Can tissue transglutaminase be a marker of idiopathic inflammatory myopathies? Immunol Lett 2005;97:245–249 [DOI] [PubMed] [Google Scholar]
- 183.Huebner JL, Johnson KA, Kraus VB, Terkeltaub RA. Transglutaminase 2 is a marker of chondrocyte hypertrophy and osteoarthritis severity in the Hartley guinea pig model of knee OA. Osteoarthritis Cartilage 2009;17:1056–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rosenthal AK, Gohr CM, Uzuki M, Masuda I. Osteopontin promotes pathologic mineralization in articular cartilage. Matrix Biol 2007;26:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hallstrand ES, Wurfel MM, Lai Y, Ni Z, Gelb MH, Altemeier WA, Beyer RP, Aitken ML, Henderson WRJ. Transglutaminase 2, a novel regulator of eicosanoid production in asthma revealed by genome-wide expression profiling of distinct asthma phenotypes. PLoS One 2010;5:e8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Arjomandi M, Frelinger J, Donde A, Wong H, Yellamilli A, Raymond W. Secreted osteopontin is highly polymerized in human airways and fragmented in asthmatic airway secretions. PLoS One 2011;6:e25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Roberts CR. Is asthma a fibrotic disease? Chest 1995;107(Suppl3):111S–117S [DOI] [PubMed] [Google Scholar]
- 188.Esmann J, Voorhees JJ, Fisher GJ. Increased membrane-associated transglutaminase activity in psoriasis. Biochem Biophys Res Commun 1989;164:219–224 [DOI] [PubMed] [Google Scholar]
- 189.Widgerow AD. Current concepts in scar evolution and control. Aesth Plast Surg 2011;35:628–635 [DOI] [PubMed] [Google Scholar]
- 190.Wilgus TA, Bergdall VK, Tober KL, Hill KJ, Mitra S, Flavahan NA, Oberyszyn TM. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am J Pathol 2004;165:753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shin D-M, Jeon J-H, Kim C-W, Cho S-Y, Lee H-J, Jang G-Y, Jeong EM, Lee D-S, Kang J-H, Melino G, Park S-C, Kim I-G. TGFβ mediates activation of transglutaminase 2 in response to oxidative stress that leads to protein aggregation. FASEB J 2008;22:2498–2507 [DOI] [PubMed] [Google Scholar]
- 192.Shin D-M, Jeon J-H, Kim C-W, Cho S-Y, Kwon J-C, Lee H-J, Choi K-H, Park S-C, Kim I-G. Cell Type-specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation. J Biol Chem 2004;279:15032–15039 [DOI] [PubMed] [Google Scholar]
- 193.Sohn J, Chae JB, Lee SY, Kim S-Y, Kim J-G. A novel therapeutic target in inflammatory uveitis: transglutaminase 2 inhibitor. Korean J Ophthamol 2010;24:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sohn J, Kim S-Y. Peptide with the amino acid sequence of KVLDGQDP having anti-inflammatory properties, US Patent Office; 2006, US Patent 7,037,502 [Google Scholar]
- 195.Bowness JM, Tarr AH. Increase in transglutaminase and its extracellular products in response to an inflammatory stimulus by lipopolysaccharide. Mol Cell Biochem 1997;169:157–163 [DOI] [PubMed] [Google Scholar]
- 196.Yoo H, Ahn E-R, Kim S-J, Lee S-H, Oh S, Kim S-Y. Divergent results induced by different types of septic shock in transglutaminase 2 knockout mice. Amino Acids 2013;44:189–197 [DOI] [PubMed] [Google Scholar]
- 197.Dafik L, Albertelli M, Stamnaes J, Sollid LM, Khosla C. Activation and inhibition of transglutaminase 2 in mice. PLoS One 2012;7:e30642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997;3:797–801 [DOI] [PubMed] [Google Scholar]
- 199.Iacono OL, Petta S, Venezia G, Di Marco V, Tarantino G, Barbaria F, Mineo C, De Lisi S, Almasio PL, Craxi A. Anti-tissue transglutaminase antibodies in patients with abnormal liver tests: Is it always coeliac disease? Am J Gastroenterol 2005;100:2472–2477 [DOI] [PubMed] [Google Scholar]
- 200.Bonamico M, Ferri M, Nenna R, Verrienti A, Di Mario U, Tiberti C. Tissue transglutaminase autoantibody detection in human saliva: A powerful method for celiac disease screening. J Pediatr 2004;144:632–636 [DOI] [PubMed] [Google Scholar]
- 201.Bonamico M, Tiberti C, Picarelli A, Mariani P, Rossi D, Cipolletta E, Greco M, Di Tola M, Sabbatella L, Carabba B, Magliocca FM, Strisciuglio P, Di Mario U. Radioimmunoassay to detect antitransglutaminase autoantibodies is the most sensitive and specific screening method for celiac disease. Am J Gastroenterol 2001;96:1536–1540 [DOI] [PubMed] [Google Scholar]
- 202.Sulkanen S, Halttunen T, Laurila K, Kolho K-L, Korponay-Szabó IR, Sarnesto A, Savilahti E, Collin P, Mäki M. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 1998;115:1322–1328 [DOI] [PubMed] [Google Scholar]
- 203.Biagi F, Ellis HJ, Yiannakou JY, Brusco G, Swift GL, Smith PM, Corazza GR, Ciclitira PJ. Tissue transglutaminase antibodies in celiac disease. Am J Gastroenterol 1999;94:2187–2192 [DOI] [PubMed] [Google Scholar]
- 204.Teesalu K, Panarina M, Uibo O, Uibo R, Utt M.Autoantibodies from patients with celiac disease inhibit transglutaminase 2 binding to heparin/heparan sulfate and interfere with intestinal epithelial cell adhesion. Amino Acids 2012;42:1055–1064 [DOI] [PubMed] [Google Scholar]
- 205.Sánchez D, Tučková L, Šebo P, Michalak M, Whelan A, Šterzl I, Jelínková L, Havrdová E, Imramovská M, Beneš Z, Krupičková S, Tlaskalová-Hogenová H. Occurrence of IgA and IgG autoantibodies to calreticulin in coeliac disease and various autoimmune diseases. J Autoimmun 2000;15:441–449 [DOI] [PubMed] [Google Scholar]
- 206.Baldas V, Not T, Tommasini A, Ansaldi F, Demarini S, Sblattero D, Marzari R, Torelli L, Burlina A, Tiribelli C, Ventura A. Anti-transglutaminase antibodies and age. Clin Chem 2004;50:1856–1860 [DOI] [PubMed] [Google Scholar]
- 207.Rosenthal AK, Gohr CM, Mitton EM, Monnier V, Burner T. Advanced glycation end products increase transglutaminase activity in primary porcine tenocytes. J Invest Med 2009;57:460–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Skill NJ, Johnson TS, Coutts IGC, Saint RE, Fisher M, Huang L, El Nahas AM, Collighan RJ, Griffin M. Inhibition of transglutaminase activity reduces extracellular matrix accumulation induced by high glucose levels in proximal tubular epithelial cells. J Biol Chem 2004;279:47754–47762 [DOI] [PubMed] [Google Scholar]
- 209.Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Häring HU, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 2001;50:2792–2808 [DOI] [PubMed] [Google Scholar]
- 210.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier J.L.Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 2001;280:E685–E694 [DOI] [PubMed] [Google Scholar]
- 211.Verzijl N, DeGroot J, Zaken CB, Braun-Benjamin O, Maroudas A, Bank RA, Mizrahi J, Schalkwijk CG, Thorpe SR, Baynes JW, Bijlsma JWJ, Lafeber FPJG, TeKoppele JM. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum 2002;46:114–123 [DOI] [PubMed] [Google Scholar]
- 212.Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM. Glucosepane is a major protein cross-link of the senescent human extracellular matrix. J Biol Chem 2005;280:12310–12316 [DOI] [PubMed] [Google Scholar]
- 213.Aspinall R, Mitchell WA. Maintenance and restoration of immune system function. In: Fahy GM, et al., eds. The Future of Aging. Springer, Dordrecht, Germany, 2010, pp. 489–520 [Google Scholar]
- 214.Niwa Y, Kasama T, Miyachi Y, Kanoh T. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: Cross-sectional and longitudinal studies. Life Sci 1989;44:1655–1664 [DOI] [PubMed] [Google Scholar]
- 215.Ginaldi L, Martinis MD, D'Ostilio A, Marini L, Loreto MF, Quaglino D. The immune system in the elderly. Immunol Res 1999;20:117–126 [DOI] [PubMed] [Google Scholar]
- 216.Diegelmann RF, Evans MC. Wound healing: An overview of acite, fibrotic and delayed healing. Front Biosci 2004;9:283–289 [DOI] [PubMed] [Google Scholar]
- 217.White ES, Mantovani AR. Inflammation, wound repair, and fibrosis: Reassessing the spectrum of tissue injury and resolution. J Pathol 2013;229:141–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nardacci R, Lo Iacono O, Ciccosanti F, Falasca L, Addesso M, Amendola A, Antonucci G, Craxì A, Fimia GM, Iadevaia V, Melino G, Ruco L, Tocci G, Ippolito G, Piacentini M, Transglutaminase type II plays a protective role in hepatic injury. Am J Pathol 2003;162:1293–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Strnad P, Harada M, Siegel M, Terkeltaub RA, Graham RM, Khosla C, Omary MB. Transglutaminase 2 regulates Mallory body inclusion formation and injury-associated liver enlargement. Gastroenterology 2007;132:1515–1526 [DOI] [PubMed] [Google Scholar]
- 220.Grenard P, Bresson-Hadni S, El Alaoui S, Chevallier M, Vuitton DA, Ricard-Blum S. Transglutaminase-mediated cross-linking is involved in the stabilization of extracellular matrix in human liver fibrosis. J Hepatol 2001;35:367–375 [DOI] [PubMed] [Google Scholar]
- 221.Tatsukawa H, Fukaya Y, Frampton G, Martinez–Fuentes A, Suzuki K, Kuo TF, Nagatsuma K, Shimokado K, Okuno M, Wu J, Iismaa S, Matsuura T, Tsukamoto H, Zern MA, Graham RM, Kojima S. Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1. Gastroenterology 2009;136:1783–1795.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Skill NJ, Griffin M, El Nahas AM, Sanai T, Haylor JL, Fisher M, Jamie MF, Mould NN, Johnson TS. Increases in renal epsilon-(gamma-glutamyl)-lysine crosslinks result from compartment-specific changes in tissue transglutaminase in early experimental diabetic nephropathy: pathologic implications. Lab Invest 2001;81:705–716 [DOI] [PubMed] [Google Scholar]
- 223.Huang L, Haylor JL, Hau Z, Jones RA, Vickers ME, Wagner B, Griffin M, Saint RE, Coutts IGC, El Nahas AM, Johnson TS. Transglutaminase inhibition ameliorates experimental diabetic nephropathy. Kidney Int 2009;76:383–394 [DOI] [PubMed] [Google Scholar]
- 224.Johnson TS, Fisher M, Haylor JL, Hau Z, Skill NJ, Jones R, Saint R, Coutts I, Vickers ME, El Nahas AM, Griffin M. Transglutaminase inhibition reduces fibrosis and preserves function in experimental chronic kidney disease. J Am Soc Nephrol 2007;18:3078–3088 [DOI] [PubMed] [Google Scholar]
- 225.Olsen KC, Sapinoro RE, Kottmann RM, Kulkarni AA, Iismaa SE, Johnson GVW, Thatcher TH, Phipps RP, Sime PJ. Transglutaminase 2 and its role in pulmonary fibrosis. Am J Resp Crit Care Med 2011;184:699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kim D-S, Kim B, Tahk H, Kim D-H, Ahn E-R, Choi C, Jeon Y, Park SY, Lee H, Oh SH, Kim S-Y. Transglutaminase 2 gene ablation protects against renal ischemic injury by blocking constant NF-κB activation. Biochem Biophys Res Commun 2010;403:479–484 [DOI] [PubMed] [Google Scholar]
- 227.Oliva F, Barisani D, Grasso A, Maffulli N. Gene expression analysis in calcific tendinopathy of the rotator cuff. Eur Cells Mater 2011;21:548–557 [DOI] [PubMed] [Google Scholar]
- 228.Oliva F, Zocchi L, Codispoti A, Candi E, Celi M, Melino G, Maffulli N, Tarantino U. Transglutaminases expression in human supraspinatus tendon ruptures and in mouse tendons. Biochem Biophys Res Commun 2009;379:887–891 [DOI] [PubMed] [Google Scholar]
- 229.Lorand L, Hsu LK, Siefring GE, Rafferty NS. Lens transglutaminase and cataract formation. Proc Natl Acad Sci 1981;78:1356–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wan XH, Lee EH, Koh HJ, Song J, Kim EK, Kim CY, Lee JB, Kim S.-Y., Yao K., Lee J.H., Enhanced expression of transglutaminase 2 in anterior polar cataracts and its induction by TGF-β in vitro. Br J Ophthalmol 2002;86:1293–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sane DC, Kontos JL, Greenberg CS. Roles of transglutaminases in cardiac and vascular diseases. Front Biosci 2007;12:2530–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cho B-R, Kim M-K, Suh D-H, Hahn J-H, Lee B-G, Choi Y-C, Kwon T-J, Kim S-Y, Kim D-J. Increased tissue transglutaminase expression in human atherosclerotic coronary arteries. Coron Artery Dis 2008;19:459–468 [DOI] [PubMed] [Google Scholar]
- 233.Bowness JM, Venditti M, Tarr AH, Taylor JR. Increase in ε(γ-glutamyl)-lysine crosslinks in atherosclerotic aortas. Atherosclerosis 1994;111:247–253 [DOI] [PubMed] [Google Scholar]
- 234.Chabot N, Moreau S, Mulani A, Moreau P, Keillor JW. Fluorescent probes of tissue transglutaminase reveal its association with arterial stiffening. Chem Biol 2010:17:1143–1150 [DOI] [PubMed] [Google Scholar]
- 235.van den Akker J, VanBavel E, van Geel R, Matlung HL, Guvenc Tuna B, Janssen GMC, van Veelen PA, Boelens WC, De Mey JGR, Bakker ENTP. The redox state of transglutaminase 2 controls arterial remodeling. PLoS One 2011;6:e23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Haroon ZA, Wannenburg T, Gupta M, Greenberg CS, Wallin R, Sane DC. Localization of tissue transglutaminase in human carotid and coronary artery atherosclerosis: Implications for plaque stability and progression. Lab Invest 2001;81:83–93 [DOI] [PubMed] [Google Scholar]
- 237.Matlung HL, Groen HC, de Vos J, van Walsum T, van der Lugt A, Niessen WJ, Wentzsel JJ, van Bavel E, Bakker ENTP. Calcification locates to transglutaminases in advanced human atherosclerotic lesions. Am J Pathol 2009;175:1374–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Williams H, Pease RJ, Newell LM, Cordell PA, Graham RM, Kearney MT, Jackson CL, Grant PJ. Effect of transglutaminase 2 (TG2) deficiency on atherosclerotic plaque stability in the apolipoprotein E deficient mouse. Atherosclerosis 2010;210:94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Matlung H, VanBavel E, van den Akker J, de Vries CJM, Bakker ENTP. Role of transglutaminases in cuff-induced atherosclerotic lesion formation in femoral arteries of ApoE3 Leiden mice. Atherosclerosis 2010;213:77–84 [DOI] [PubMed] [Google Scholar]
- 240.Zhang Z, Vezza R, Plappert T, McNamara P, Lawson JA, Austin S, Praticò D, Sutton MS-J, FitzGerald GA. COX-2–dependent cardiac failure in Gh/tTG transgenic mice. Circ Res 2003;92:1153–1161 [DOI] [PubMed] [Google Scholar]
- 241.Iismaa SE, Graham RM. Dissecting cardiac hypertrophy and signaling pathways: Evidence for an interaction between multifunctional G proteins and prostanoids. Circ Res 2003;92:1059–1061 [DOI] [PubMed] [Google Scholar]
- 242.Hwang K-C, Gray CD, Sweet WE, Moravec CS, Im M-J. α1-Adrenergic receptor coupling with Gh in the failing human heart. Circulation 1996;94:718–726 [DOI] [PubMed] [Google Scholar]
- 243.Gorza L, Menabò R, Vitadello M, Bergamini CM, Di Lisa F. Cardiomyocyte troponin T immunoreactivity is modified by cross-linking resulting from intracellular calcium overload. Circulation 1996;93:1896–1904 [DOI] [PubMed] [Google Scholar]
- 244.Battaglia G, Farrace MG, Mastroberardino PG, Viti I, Fimia GM, Van Beeumen J, Devreese B, Melino G, Molinaro G, Busceti CL, Biagioni F, Nicoletti F, Piacentini M. Transglutaminase 2 ablation leads to defective function of mitochondrial respiratory complex I affecting neuronal vulnerability in experimental models of extrapyramidal disorders. J Neurochem 2007;100:36–49 [DOI] [PubMed] [Google Scholar]
- 245.Mauro P, Grazia Farrace M, Piredda L, Matarrese P, Ciccosanti F, Falasca L, Rodolfo C, Giammarioli AM, Verderio E, Griffin M, Malorni W. Transglutaminase overexpression sensitizes neuronal cell lines to apoptosis by increasing mitochondrial membrane potential and cellular oxidative stress. J Neurochem 2002;81:1061–1072 [DOI] [PubMed] [Google Scholar]
- 246.Mastroberardino PG, Farrace MG, Viti I, Pavone F, Fimia GM, Melino G, Rodolfo C, Piacentini M. “Tissue” transglutaminase contributes to the formation of disulphide bridges in proteins of mitochondrial respiratory complexes. Biochim Biophys Acta Bioenergetics 2006;1757:1357–1365 [DOI] [PubMed] [Google Scholar]
- 247.Nemes Z, Fésüs L, Egerházi A, Keszthelyi A, Degrell IM. N(epsilon)(gamma-glutamyl)-lysine in cerebrospinal fluid marks Alzheimer type and vascular dementia. Neurobiol Aging 2001;22:403–406 [DOI] [PubMed] [Google Scholar]
- 248.Johnson GVW, Cox TM, Lockhart JP, Zinnerman MD, Miller ML, Powers RE. Transglutaminase activity is increased in Alzheimer's disease brain. Brain Res 1997;751:323–329 [DOI] [PubMed] [Google Scholar]
- 249.Grosso H, Mouradian MM. Transglutaminase 2: Biology, relevance to neurodegenerative diseases and therapeutic implications. Pharmacol Therapeut 2012;133:392–410 [DOI] [PubMed] [Google Scholar]
- 250.Sárvári M, Fésüs L, Nemes Z. Transglutaminase-mediated crosslinking of neural proteins in Alzheimer's disease and other primary dementias. Drug Dev Res 2002;56:458–472 [Google Scholar]
- 251.Bonelli RM, Aschoff A, Niederwieser G, Heuberger C, Jirikowski G. Cerebrospinal fluid tissue transglutaminase as a biochemical marker for alzheimer's disease. Neurobiol Dis 2002;11:106–110 [DOI] [PubMed] [Google Scholar]
- 252.Bonelli RM, Aschoff A, Jirikowski G. Cerebrospinal fluid tissue transglutaminase in vascular dementia. J Neurol Sci 2002;203-4:207–209 [DOI] [PubMed] [Google Scholar]
- 253.Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJL, Ratan RR, Beal MF, Hersch SM, Ferrante RJ. Therapeutic effects of cystamine in a murine model of huntington's disease. J Neurosci 2002;22:8942–8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bailey CDC, Johnson GVW. The protective effects of cystamine in the R6/2 Huntington's disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiol Aging 2006;27:871–879 [DOI] [PubMed] [Google Scholar]
- 255.Lesort M, Chun W, Johnson GVW, Ferrante RJ. Tissue transglutaminase is increased in Huntington's disease brain. J Neurochem 1999;73:2018–2027 [PubMed] [Google Scholar]
- 256.Cooper AJL, Jeitner TM, Gentile V, Blass JP. Cross linking of polyglutamine domains catalyzed by tissue transglutaminase is greatly favored with pathological-length repeats: Does transglutaminase activity play a role in (CAG)n/Qn-expansion diseases? Neurochem Int 2002;40:53–67 [DOI] [PubMed] [Google Scholar]
- 257.Fujita K, Honda M, Hayashi R, Ogawa K, Ando M, Yamauchi M, Nagata Y. Transglutaminase activity in serum and cerebrospinal fluid in sporadic amyotrophic lateral sclerosis: A possible use as an indicator of extent of the motor neuron loss. J Neurol Sci 1998;158:53–57 [DOI] [PubMed] [Google Scholar]
- 258.Fesus L. Transglutaminase-catalyzed protein cross-linking in the molecular program of apoptosis and its relationship to neuronal processes. Cell Mol Neurobiol 1998;18:683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hartley DM, Zhao C, Speier AC, Woodard GA, Li S, Li Z, Walz T. Transglutaminase induces protofibril-like amyloid beta-protein assemblies that are protease-resistant and inhibit long-term potentiation. J Biol Chem 2008;283:16790–16800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Norlund MA, Lee JM, Zainelli GM, Muma NA. Elevated transglutaminase-induced bonds in PHF tau in Alzheimer's disease. Brain Res 1999;851:154–163 [DOI] [PubMed] [Google Scholar]
- 261.Muna NA. Transglutaminase is linked to neurodegenerative diseases. J Neuropathol Exp Neurol 2007;66:258–263 [DOI] [PubMed] [Google Scholar]
- 262.Evans J. Skin elasticity may serve as potential biomarker for sclerosis. In: Skin and Allergy News (http://www.skinandallergynews.com/) July2009. IMNG Medical Media Group LLC, Rockville, MD, 2009 [Google Scholar]
- 263.Arbesman H. In Vivo Measurement of the elastic properties of the skin as a biomarker for amyotrophic lateral sclerosis. American Academy of Neurology Annual Meeting, Seattle, WA, 2009 [Google Scholar]
- 264.Dominguez C, Wityak J, Prime M, Courtney S, Yarnold C, Brookfield F, Marston R. Transglutaminase TG2 inhibitors, pharmaceutical compositions and methods of use thereof. US patent application PCT/US2010/056614, assigned to CHDI Foundation, Inc., (NY), 2010 [Google Scholar]
- 265.Prime M, Andersen OA, Barker JJ, Brooks MA, Checng RKY, Toogood-Johnson I, Courtney SM, Brookfield FA, Yarnold CJ, Marston RW, Johnson PD HJ.ohnsen SF, Palfrey JJ, Vaidya D, Erfan S, Ichihara O, Felicetti B, Palan S, Pedret-Dunn A, Schaertl S, Sternberger I, Ebneth A, Scheel A, Winkler D, Toledo-Sherman L, Beconi M, MacDonald D, Munoz-Sanjuan I, Dominguez C, Wityak J. Discovery and structure—activity relationship of potent and selective covalent inhibitors of transglutaminase 2 for Huntington's disease. J Med Chem 2012;55:1021–1046 [DOI] [PubMed] [Google Scholar]
- 266.Schaertl S, Prime M, Wityak J, Dominguez C, Munoz-Sanjuan I, Pacifici RE, Courtney S, Scheel A, MacDonald D. A profiling platform for the characterization of transglutaminase 2 (TG2) inhibitor. J Biomol Screen 2010;15:478–487 [DOI] [PubMed] [Google Scholar]
- 267.Pedersen LC, Yee VC, Bishop PD, Le Trong I, Teller DC, Stenkamp RE, Transglutaminase factor XIII uses proteinase-like catalytic triad to crosslink macromolecules. Protein Sci 1994;3:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chica RA, Gagnon P, Keillor JW, Pelletier JN. Tissue transglutaminase acylation: Proposed role of conserved active site Tyr and Trp residues revealed by molecular modeling of peptide substrate binding. Protein Sci 2004;13:979–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hausch F, Halttunen T, Mäki M, Khosla C. Design, synthesis, and evaluation of gluten peptide analogs as selective inhibitors of human tissue transglutaminase. Chem Biol 2003;10:225–231 [DOI] [PubMed] [Google Scholar]
- 270.Wityak J, Prime ME, Brookfield FA, Courtney SM, Erfan S, Johnsen S, Johnson PD, Li M, Marston RW, Reed L, Vaidya D, Schaertl S, Pedret-Dunn A, Beconi M, Macdonald D, Muñoz-Sanjuan I, Dominguez C. SAR development of lysine-based irreversible inhibitors of transglutaminase 2 for Huntington's disease. ACS Med Chem Lett 2012;3:1024–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Prime ME, Brookfield FA, Courtney SM, Gaines S, Marston RW, Ichihara O, Li M, Vaidya D, Williams H, Pedret-Dunn A, Reed L, Schaertl S, Toledo-Sherman L, Beconi M, Macdonald D, Muñoz-Sanjuan I, Dominguez C, Wityak J. Irreversible 4-aminopiperidine transglutaminase 2 inhibitors for Huntington's disease. ACS Med Chem Lett 2012;3:731–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lai T-S, Liu Y, Tucker T, Daniel KR, Sane DC, Toone E, Burke JR, Strittmatter WJ, Greenberg CS. Identification of chemical inhibitors to human tissue transglutaminase by screening existing drug libraries. Chem Biol 2008;15:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Datta S, Antonyak MA, Cerione RA. GTP-binding-defective forms of tissue transglutaminase trigger cell death. Biochemistry 2007;46:14819–14829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Noble MEM, Endicott JA, Johnson LN. Protein kinase inhibitors: Insights into drug design from structure. Science 2004;303:1800–1805 [DOI] [PubMed] [Google Scholar]
- 275.Fabbro D, Ruetz S, Buchdunger E, Cowan-Jacob SW, Fendrich G, Liebetanz J, Mestan J, O'Reilly T, Traxler P, Chaudhuri B, Fretz H, Zimmermann J, Meyer T, Caravatti G, Furet P, Manley PW. Protein kinases as targets for anticancer agents: From inhibitors to useful drugs. Pharmacol Therapeut 2002;93:79–98 [DOI] [PubMed] [Google Scholar]
- 276.Hart M, Maru Y, Leonard D, Witte O, Evans T, Cerione R. A GDP dissociation inhibitor that serves as a GTPase inhibitor for the Ras-like protein CDC42Hs. Science 1992;258:812–815 [DOI] [PubMed] [Google Scholar]
- 277.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 2004;101:7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Schelling JR. Tissue transglutaminase inhibition as treatment for diabetic glomerular scarrings: It's good to be glueless. Kidney Int 2009;76:363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cordella-Miele E, Miele L, Beninati S, Mukherjee AB. Transglutaminase-catalyzed incorporation of polyamines into phospholipase A2. J Biochem 1993;113:164–173 [DOI] [PubMed] [Google Scholar]
- 280.Sohn J, Kim TI, Yoon YH, Kim JY, Kim SY. Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J Clin Invest 2003;111:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Cordella-Miele E, Miele L, Mukherjee AB. A novel transglutaminase-mediated post-translational modification of phospholipase A2 dramatically increases its catalytic activity. J Biol Chem 1990;265:17180–17188 [PubMed] [Google Scholar]
- 282.Hardas B, Dolynchuk K, Jacob C, Griffin M, Panchal C. Reversal of ε-(γ-glutamyl)-lysine cross-linking and down-regulation of fibronectin and tissue transglutaminase (tTGase) activity in hypertrophic scars following treatment with 0.8% 1,4 DAB 2HCL. Wound Repair Regeneration 2004;12:A19 [Google Scholar]
- 283.Furber JD. Extracellular glycation crosslinks: Prospects for removal. Rejuvenation Res 2006;9:274–278 [DOI] [PubMed] [Google Scholar]
- 284.Simpson RM, Christeller JT. Identification of isopeptidase activity in the midgut of insects: Purification, properties and nutritional ecology of a Hofmannophila pseudospretella (Lepidoptera: Oecophoridae) larval enzyme. Insect Sci 2010;17:325–334 [Google Scholar]
- 285.Saber-Lichtenberg Y, Brix K, Schmitz A, Heuser JE, Wilson JH, Lorand L, Herzog V. Covalent cross-linking of secreted bovine thyroglobulin by transglutaminase. FASEB J 2000;14:1005–1014 [DOI] [PubMed] [Google Scholar]
- 286.Giraud A, Dicristofaro J, De Micco C, Lejeune P-J, Barbaria J, Mallet B. A plasminogen-like protein, present in the apical extracellular environment of thyroid epithelial cells, degrades thyroglobulin in vitro. Biochem Biophys Res Commun 2005;338:1000–1004 [DOI] [PubMed] [Google Scholar]
- 287.Tepel C, Bromme D, Herzog V, Brix K, Cathepsin K.in thyroid epithelial cells: Sequence, localization and possible function in extracellular proteolysis of thyroglobulin. J Cell Sci 2000;113:4487–4498 [DOI] [PubMed] [Google Scholar]
- 288.Oakley AJ, Coggan M, Board PG. Identification and characterization of γ-glutamylamine cyclotransferase, an enzyme responsible for γ-glutamyl-ε-lysine catabolism. J Biol Chem 2010;285:9642–9648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.de Grey ADNJ. The desperate need for a biomedically useful definition of ‘‘aging.’’ Rejuvenation Res 2013;16:89–90 [DOI] [PubMed] [Google Scholar]
- 290.Bains W. Can I have volunteers to die tomorrow? Rejuvenation Res 2007;10:648–650 [DOI] [PubMed] [Google Scholar]
- 291.Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FTD, Meals RA, Smith TM, Rodzvilla J. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. N Engl J Med 2009;361:968–979 [DOI] [PubMed] [Google Scholar]
- 292.Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N. Injectable collagenase clostridium histolyticum: A new nonsurgical treatment for dupuytren's disease. J Hand Surg 2010;35:2027–2038.e1 [DOI] [PubMed] [Google Scholar]