Abstract
Significance: Evidence is emerging that parenteral administration of high-dose vitamin C may warrant development as an adjuvant therapy for patients with sepsis. Recent Advances: Sepsis increases risk of death and disability, but its treatment consists only of supportive therapies because no specific therapy is available. The characteristics of severe sepsis include ascorbate (reduced vitamin C) depletion, excessive protein nitration in microvascular endothelial cells, and microvascular dysfunction composed of refractive vasodilation, endothelial barrier dysfunction, and disseminated intravascular coagulation. Parenteral administration of ascorbate prevents or even reverses these pathological changes and thereby decreases hypotension, edema, multiorgan failure, and death in animal models of sepsis. Critical Issues: Dehydroascorbic acid appears to be as effective as ascorbate for protection against microvascular dysfunction, organ failure, and death when injected in sepsis models, but information about pharmacodynamics and safety in human subjects is only available for ascorbate. Although the plasma ascorbate concentration in critically ill and septic patients is normalized by repletion protocols that use high doses of parenteral ascorbate, and such doses are tolerated well by most healthy subjects, whether such large amounts of the vitamin trigger adverse effects in patients is uncertain. Future Directions: Further study of sepsis models may determine if high concentrations of ascorbate in interstitial fluid have pro-oxidant and bacteriostatic actions that also modify disease progression. However, the ascorbate depletion observed in septic patients receiving standard care and the therapeutic mechanisms established in models are sufficient evidence to support clinical trials of parenteral ascorbate as an adjuvant therapy for sepsis. Antioxid. Redox Signal. 19, 2129–2140.
Introduction
The purpose of this review is to critically examine the role of vitamin C in sepsis. The cardinal characteristics of this disease are a suspected or proved infection and a systemic inflammatory response. Sepsis is a major public health issue. In the United States alone, the combination of sepsis and acute organ dysfunction (i.e., severe sepsis) occurs with an incidence of 3.0 cases per 1000 population, its hospital costs total $24.3 billion annually, its mortality is 28.6%, and survivors are at elevated risk of long-term disability (33). Death or disability often occurs despite multiple supportive therapies. These include source control (e.g., removal of an infectious nidus), antibiotic therapy, fluid resuscitation, vasopressor and inotropic-vasopressor therapy, glycemic control, prophylaxis for deep-vein thrombosis, prophylaxis for stress ulcer, and supplemental oxygen and mechanical ventilation. Although oxidative stress is elevated in sepsis, antioxidant therapy has not become a mainstay of care.
Sepsis care bundles often include bactericidal antibiotics that acutely increase inflammation by stimulating the release of bacterial toxins, such as Escherichia coli lipopolysaccharide (LPS) (1, 60). The inflammation may adversely affect the prognosis for patients exposed to septic insults, for example, intensive care unit patients in whom abdominal infection elevates the risks of septic peritonitis, severe sepsis, and death (55). For ampicillin/sulbactam, the effect has been studied by controlled experiments in animals subjected to cecal ligation and puncture (CLP). Ampicillin/sulbactam is a bactericidal antibiotic used against abdominal infections that cause septic peritonitis; for example, it is administered prophylactically before elective colorectal surgery. CLP is the most clinically relevant model of septic peritonitis, because of inflammatory responses to both the polymicrobial infections induced by cecal puncture and the ischemic tissue injury resulting from cecal ligation (8). Ampicillin/sulbactam treatment of CLP rats temporarily increases inflammation, as indicated by elevated serum interleukin-6 concentration (44). Ampicillin/sulbactam also exacerbates the CLP-induced acute liver and kidney dysfunction, as shown by increased serum levels of alanine aminotransferase and creatinine, respectively (44). These findings suggest that a need exists for adjuvant therapies that prevent the inflammatory and injurious effects of bactericidal antibiotics in sepsis.
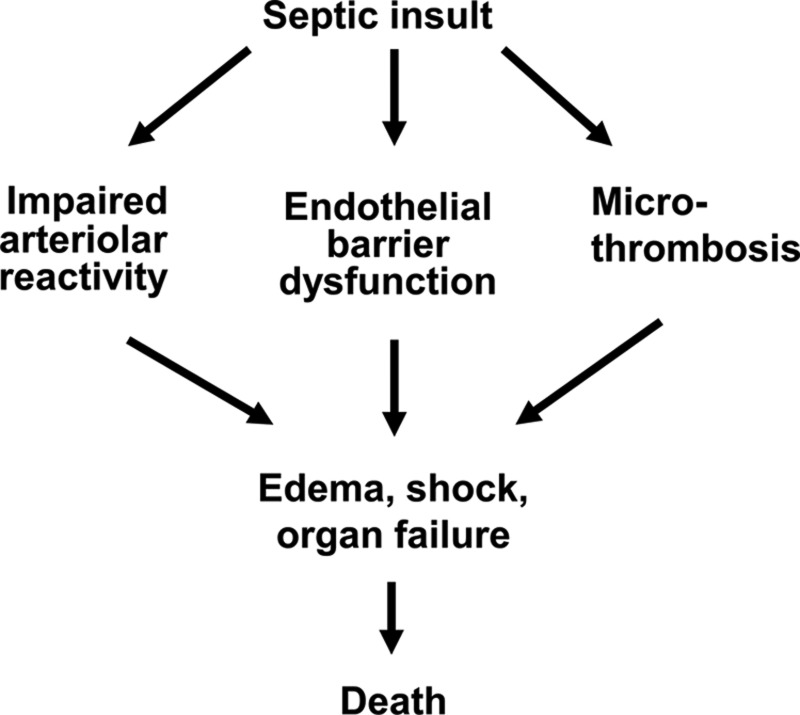
Another reason to search for novel adjuvant therapies is that the deaths of septic patients often are attributable to microvascular dysfunction for which no specific therapy exists (61). The microvascular dysfunction can be considered to have 3 components, namely, arteriolar reactivity impairment, endothelial barrier dysfunction, and capillary plugging by microthrombi (Fig. 1).
FIG. 1.
Microvascular dysfunction during sepsis progression. A septic insult, such as infection by virulent bacteria, triggers a systemic inflammatory response that can cause microvascular dysfunction if it is too exuberant. One component of this dysfunction is an impairment of arteriolar reactivity to vasoconstrictors (e.g., norepinephrine and angiotensin II) that contributes to loss of blood pressure control and eventually to shock. Another component is a disruption of the endothelial barrier that allows extravasation of plasma proteins and fluid from capillaries; this leakage leads to the development of tissue edema and it may also contract blood volume and thereby contribute to shock. A third component of septic microvascular dysfunction is an accumulation of platelets and microthrombi in capillaries that alters the distribution of blood flow, as has been observed in skeletal muscles of septic mice. The edema and maldistribution of capillary blood flow cause tissue hypoxia that may lead to organ failure. Death may result from multiorgan failure even if shock is prevented by interventions such as fluid resuscitation and vasopressor or inotropic-vasopressor therapy.
Impairments of arteriolar reactivity and cardiac function compromise the regulation of blood pressure and consequently patients with sepsis may develop shock. Also contributing to the risk of shock in these patients is endothelial barrier dysfunction that increases the permeability of the capillary wall to macromolecules and thereby accelerates plasma protein extravasation (i.e., capillary leakage) and loss of circulating blood volume (34).
When shock is prevented by supportive therapies, many patients with severe sepsis nevertheless die of multiorgan failure, despite adequate cardiac output and arterial blood oxygenation (49). The progression from infection to organ failure is precipitated by excessive accumulation of interstitial fluid (edema) and increased heterogeneity (maldistribution) of capillary blood flow, both of which lengthen the diffusion distance for oxygen and allow tissues to become hypoxic (23, 34). The edema and blood flow maldistribution are consequences of capillary leakage and plugging, respectively. Capillary plugging, in turn, is associated with formation of microthrombi and is an example of disseminated intravascular coagulation (Fig. 1).
It is clear that shock, edema, organ failure, and premature death remain major issues in sepsis. Reviewed below are recent findings that relate these pathologies to changes in vitamin C levels in patients and preclinical models.
Vitamin C Depletion and Repletion in Sepsis
Infection is one of the most common health problems for which complementary and alternative medicine (CAM) practitioners recommend injection of high doses of ascorbate (reduced vitamin C) (43). One possible reason for the prevalence of this practice is that infection may trigger a systemic inflammatory response that depletes endogenous ascorbate. Plasma ascorbate concentrations fall very low in patients with sepsis or traumatic injury, and they are not corrected by parenteral nutrition containing a moderate amount of ascorbate (200 mg/day) (50). Plasma ascorbate concentration does return to normal upon resolution of the inflammatory illness (50). The latter observation may reflect accelerated destruction of ascorbate (e.g., oxidation at a rate that exceeds reduction). Ferritin, redox-reactive (i.e., catalytic) iron, and ascorbate oxidation rate are elevated in systemic blood collected from septic patients (19–21, 56). Extracellular ferritin or damaged extracellular proteins may be sources of redox-reactive iron that oxidizes ascorbate in blood and interstitial fluid (12, 14). The use of exogenous ascorbate to raise plasma ascorbate concentration is reviewed immediately below and the potential for ascorbate repletion to alter oxidative damage in septic patients is discussed afterward, particularly in the section entitled “Vitamin C dosage and safety.”
Parenteral supplementation can restore plasma ascorbate concentrations to normal in critically ill patients if the administered dose of ascorbate is sufficiently high. For example, parenteral ascorbate at a dose of 300 mg/day fails, but 1000 mg/day is minimally effective on average (35). Since 200 mg/day is the amount of ascorbate administered by parenteral nutrition typically (50), 1000 mg/day mg obviously qualifies as a high dose. Although parenteral administration of high-dose ascorbate achieves a level of repletion that is statistically significant, the plasma ascorbate response to this adjuvant therapy may be confounded by unknown factors. The variability of the response to parenteral ascorbate is exemplified by the interindividual differences in septic shock patients after the injection of a mixture of antioxidants containing a nominal dose of 1000 mg ascorbate (21). Serial blood sampling at 30 min intervals shows that i.v. injection of this mixture raises the plasma ascorbate concentration in most of these patients but has no detectable effect in some (21). It is not known if this variability is due to interindividual differences in the rates of urinary excretion of ascorbate, extracellular oxidation of ascorbate, or cellular uptake of ascorbate and its oxidation products (e.g., dehydroascorbic acid [DHAA]). However, it is clear that clinical testing of ascorbate treatment for sepsis should include monitoring of plasma or serum ascorbate concentration.
Mortality and Morbidity
Oral administration of ascorbate (1500 mg/day) in combination with other antioxidants (vitamin E, beta carotene, zinc, and selenium) failed to decrease mortality in critically ill adults who had multiorgan failure, but it is not known if plasma ascorbate concentration was restored to normal by the enteral ascorbate administration (27). Parenteral ascorbate administration can normalize plasma ascorbate (21, 35), but little is known about the clinical outcomes of this intervention. Two small, randomized clinical trials have given parenteral ascorbate at high doses as an adjuvant therapy to patients at high risk of becoming septic. The first of these 2 trials found that infusion i.v. of ascorbate [1584 mg/(kg.day)] decreases edema and improves respiratory function in severely burned patients (57). This trial is relevant because, first, there are similarities between the systemic inflammatory responses to septic insults and burn injury, and second, a major cause of death in severely burned patients is multiorgan failure due to sepsis. The other trial reported that a combination of ascorbate (3000 mg/day i.v. for up to 28 days) and vitamin E decreases organ failure incidence and shortens intensive care unit stay in patients after traumatic injury or major surgery (39). However, the effect of parenteral vitamin C on clinical outcome has not been tested in patients with sepsis who do not have burn injury or vitamin E supplementation.
Preclinical studies of sepsis models indicate that mortality depends on vitamin C. For example, when mice deficient in l-gulono-γ-lactone oxidase (Gulo−/−), the rate-limiting enzyme in ascorbate synthesis, are depleted of ascorbate and then infected with the virulent bacterium Klebsiella pneumoniae, they are three times more likely as ascorbate-replete Gulo−/− mice to die from infection (22). This apparent effect of endogenous ascorbate on mortality was not accompanied by any detectable change in the levels of oxidized amino acids and of F2-isoprostanes (markers of lipid oxidation) in peritoneal lavage fluid (22). Further evidence of the importance of vitamin C is that its parenteral administration decreases mortality in wild-type mice that are ascorbate-replete before exposure to septic insults. For example, bolus injection of ascorbate (10–200 mg/kg) improves survival in mice made septic by either CLP or fecal stem solution injection into peritoneum (FIP) (18, 59, 64) (Fig. 2). Injection of DHAA (i.e., the oxidized state of vitamin C that cells take up and reduce to ascorbate) (200 mg/kg) also prolongs survival in FIP mice (18). Similarly, injection of ascorbate or DHAA (200 mg/kg) increases survival in mice made endotoxemic by exposure to LPS (17).
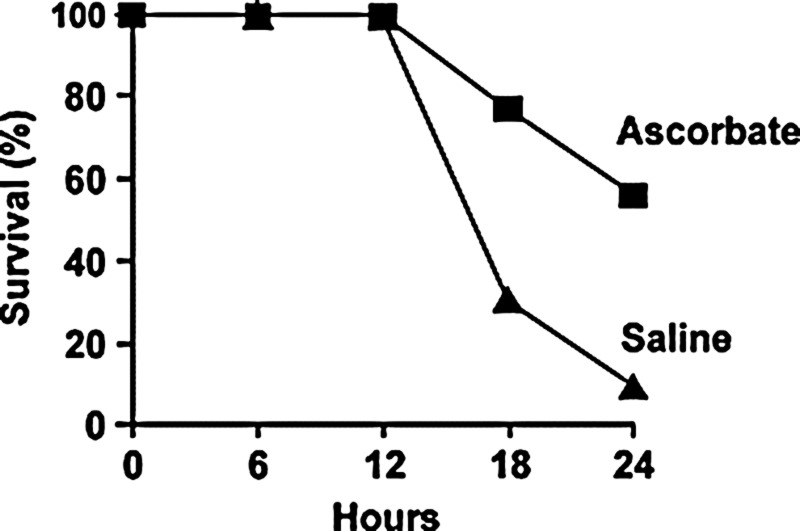
FIG. 2.
Parenteral ascorbate increases survival in experimental sepsis. Sepsis was induced by CLP in anesthetized mice that also received analgesia with buprenorphine and fluid resuscitation with saline. Ascorbate (200 mg/kg) or saline vehicle was injected i.v. at 30 min before CLP. Subsequently, Kaplan–Meier survival analysis showed the survival rate was greater in the mice that were administered ascorbate than in the septic controls (p<0.05). Reprinted by permission (63). CLP, cecal ligation and puncture.
While the results of these experiments are dramatic, they are derived from studies of mice and not humans. Further, a major experimental design issue that affects extrapolation to clinical settings is the omission of antibiotics and other supportive interventions that are typical of sepsis care bundles. For example, the effect of ascorbate on survival after CLP has been tested in mice receiving buprenorphine analgesia and fluid resuscitation but not antibiotic therapy (64) (Fig. 2). Addition of antibiotic therapy increases survival in CLP mice (29) but it is not known if this addition decreases the survival benefit conferred by ascorbate treatment. Nevertheless, the results currently available provide a strong impetus for investigations of the mechanism of action of vitamin C. The results of published mechanistic studies are reviewed in the following sections.
Bacterial Replication
Ascorbate protects microvascular function and improves survival in septic mice (18, 59, 64). It is not certain to what extent, if any, this protection is an indirect result of bacteriostasis. Ascorbate (5 mM) completely inhibits the growth of Staphylococcus aureus in vitro, even when any effect of the vitamin on extracellular acidity is prevented by neutralization (31). A lower concentration of ascorbate (100 μM), which protects endothelial cells from injury by exogenous hydrogen peroxide, is sufficient to inhibit replication in vitro of bacteria obtained from the cecum (2). It is possible that the bacteriostatic mechanism may involve production of hydrogen peroxide during oxidation of ascorbate in culture medium. Ascorbate reduces the valence of free transition metals and then the reduced metals catalyze the production of hydrogen peroxide, which is a potent antibacterial agent. For instance, E. coli replication is inhibited by 25–50 μM hydrogen peroxide (bacteriostatic action) and significant killing of the bacteria occurs at 500 μM hydrogen peroxide (bactericidal action) (30). It remains to be determined if the amount of hydrogen peroxide generated during ascorbate oxidation is sufficient to explain the vitamin's bacteriostatic effect.
Incubation of ascorbate with dilute fecal samples obtained from rat cecum shows that the vitamin inhibits bacterial replication in vitro, with the maximal inhibitory concentration being 100 μM ascorbate (2). In contrast, incubation with 100–1000 μM ascorbate does not kill microvascular endothelial cells but, on the contrary, confers protection against the injurious actions of exogenous hydrogen peroxide (2). These findings suggest the bacteriostatic and curative effects of ascorbate may be related.
CLP rats have an accumulation of purulent peritoneal fluid and inflammation of the intestine, evident by swelling of the intestinal wall, at 24 h after surgery. In contrast, nonseptic control rats and ascorbate-injected (76 mg/kg i.v.) CLP rats have a normal peritoneal cavity (2). These autopsy results suggest that ascorbate may exert wound-healing and bacteriostatic actions in septic animals. Although prophylactic injection i.v. of ascorbate (200 mg/kg) in mice does not alter the number of bacterial colony forming units in peritoneal lavage fluid at 6 h post-CLP (6.5 h after injection of ascorbate) (64), it is possible that the vitamin may cause bacteriostasis at other times or locations.
Most patients with severe sepsis have bacteria circulating in their blood (7). It is unlikely that vitamin C acts through blood-borne neutrophils to enhance bacterial killing, since injection of 2000 mg ascorbate in human subjects does not alter superoxide production by neutrophils isolated from blood (16). However, infected patients often have bacteria in their interstitial fluid, too. It is in interstitial fluid that the concentrations of ascorbate and hydrogen peroxide rise most after parenteral administration of an extremely high dose of ascorbate (4000 mg/kg) (10). Perhaps the same increase in interstitial fluid hydrogen peroxide concentration may be achieved with lower ascorbate doses in septic patients because of an abundance of redox-reactive transition metals that may generate hydrogen peroxide through oxidation of ascorbate. Another possible source of hydrogen peroxide is extravasated neutrophils because this cell type accumulates ascorbate and raises the extracellular concentration of reactive oxygen specis (9). Ascorbate and DHAA (300 μM) do not alter phagocytosis of E. coli by isolated neutrophils but do enhance subsequent generation of reactive oxygen species (52), which may include hydrogen peroxide. Further investigation is required to determine if bacteriostatic levels of hydrogen peroxide are produced in the interstitial fluid after injection of ascorbate in patients whose rate of ascorbate oxidation is accelerated, as it is in septic patients (19). Meanwhile, the alternative possibility that the improvement in survival induced by vitamin C in sepsis models is principally due to protection against nitric oxide (NO) imbalances, protein nitration, and microvascular dysfunction will be discussed next.
Ascorbate Preserves Arteriolar Reactivity
One component of microvascular dysfunction in sepsis is diminished arteriolar reactivity to vasoconstrictors. This defect causes refractory vasodilation that contributes to hypotension and shock. The mechanism underlying the impairment of vasoconstriction in sepsis includes activation of soluble guanylate cyclase, activation of cyclic guanosine 3′,5′-monophosphate-dependent protein kinase, stimulation of large conductance potassium channels, and subsequent smooth muscle cell membrane hyperpolarization that counteracts the depolarizing effects of vasoconstrictors (64). A key mediator of impaired arteriolar reactivity is inducible nitric oxide synthase (iNOS), because this enzyme is the source of high levels of NO that activate soluble guanylate cyclase.
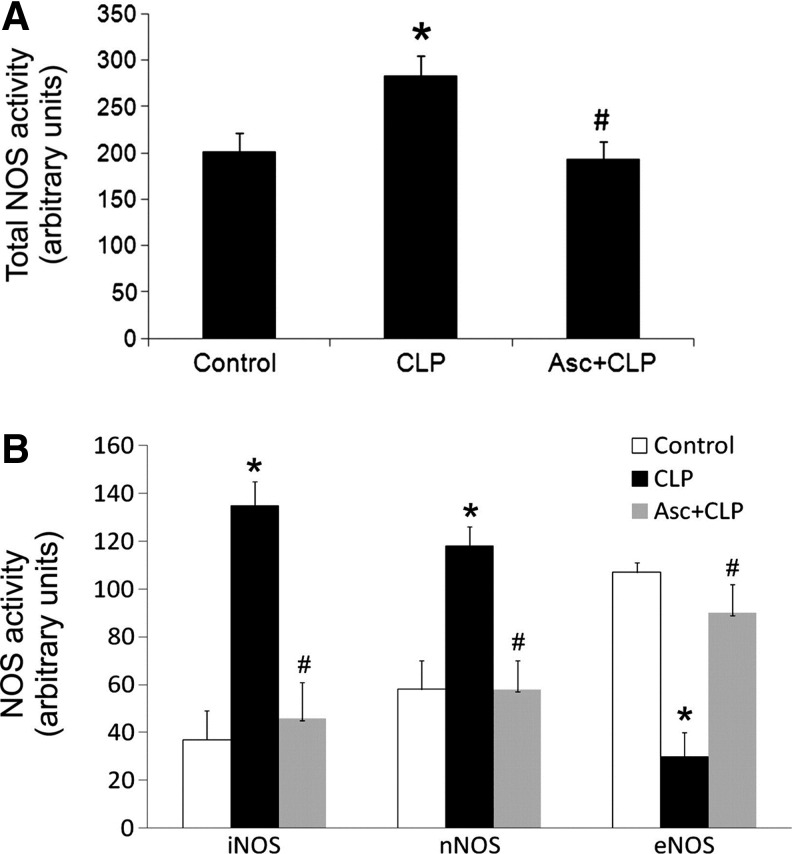
Prophylactic bolus injection i.v. of ascorbate (76–200 mg/kg) attenuates the loss of arteriolar and blood pressure responsiveness to the vasoconstrictors norepinephrine and angiotensin II in CLP rats and mice (2, 40, 63, 64). An important mechanism for the protection of arteriolar reactivity by ascorbate is inhibition of iNOS expression in endothelial cells. Part of the evidence for this mechanism is that injection of ascorbate (200 mg/kg) prevents CLP from elevating the concentration of NO metabolites in plasma (64). Further, ascorbate injection blocks the increase by CLP of iNOS mRNA expression in microvascular endothelial cells at 3 h after CLP, apparently due to inhibition by ascorbate of NADPH oxidase activity (63, 65). Parenteral ascorbate also blocks the increases in total NOS, iNOS, and neuronal NOS (nNOS) activities in skeletal muscle of CLP mice (68) (Fig. 3). Finally, iNOS deficiency (i.e., iNOS knockout) is as effective as ascorbate injection for protecting the vasoconstrictor and vasopressor responses to angiotensin II and norepinephrine in CLP mice (64).
FIG. 3.
Parenteral ascorbate prevents septic changes in NOS activities. Injection i.v. of ascorbate (200 mg/kg) in mice at 30 min before CLP prevented increase in total NOS activity in skeletal muscle at 12 h post-CLP (A). Ascorbate also blocked the increases in iNOS and nNOS activities, and the decrease in eNOS activity (B). *p<0.05 compared with control within the group. #p<0.05 compared with CLP within the group. Reprinted by permission (67). eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; nNOS, neuronal nitric oxide synthase.
Low baseline values of mean arterial blood pressure develop in animals made endotoxemic by LPS injection or made septic by CLP or FIP. Bolus injection i.v. of ascorbate blocks hypotension from developing in endotoxemic rats (53) Ascorbate also prevents hypotension for 24 h in CLP rats (2, 58), attenuates the fall in baseline blood pressure for 6 h in CLP mice (63), and fails to protect baseline blood pressure in FIP mice (59). The reasons for this variation between the different septic peritonitis models may be that, compared to saline-vehicle injected CLP rats (75% survival at 24 h), sepsis progresses more rapidly in saline vehicle-injected CLP and FIP mice (9% and 19% survival, respectively, at 24 h). Further, it is possible that myocardial depression lowers blood pressure in the septic mice despite ascorbate administration (59). It is clear from studies of CLP animals that (i) hypotension is associated with falls in plasma ascorbate concentration and arteriolar reactivity and also with increases in iNOS protein and activity levels; (ii) iNOS knockout blocks the effects of CLP on arteriolar reactivity and baseline arterial blood pressure; and (iii) injection of ascorbate prevents the CLP-induced changes in iNOS, arteriolar reactivity and blood pressure (2, 29, 40, 58, 59, 64). The identities of the molecular targets of iNOS-derived NO that mediate the effects of septic insult and ascorbate therapy on arteriolar reactivity are unknown. More information is available about the mechanisms of action of vitamin C in other components of microvascular dysfunction, and it is presented in the following sections.
Extravasation of Plasma Proteins and Fluid
A second component of septic microvascular dysfunction is increased permeability of the endothelium in perfused capillaries. This endothelial barrier dysfunction increases the extravasation of plasma proteins and fluid. By causing tissue edema, endothelial barrier dysfunction leads to tissue hypoxia and organ failure. By contracting the plasma volume, it also impairs blood volume and pressure regulation.
The lung is a critical organ in which to study this component because many septic patients develop pulmonary edema (36). In the lungs of FIP mice without vitamin C supplementation, an absence of plasma protein leakage suggests that the endothelial permeability barrier remains intact, whereas increased permeability to fluorescent dextran (average molecular mass 4 kDa) and accumulation of fluid indicate that the epithelial barrier is dysfunctional (18). Injection of either ascorbate or DHAA (200 mg/kg) lessens both dextran (4 kDa) leakage and fluid accumulation in the lungs of FIP mice (18). It is not clear if altered expression of tight junction proteins mediates these effects. In lung tissue of FIP mice, the expression of the tight junction protein claudin-4 rises but that of the cytoskeletal connector protein zona occludens-1 falls, and these responses to FIP are prevented by ascorbate but not DHAA (18). Ascorbate also prevents fluid accumulation in septic lungs by improving alveolar fluid clearance, because the infusion of the vitamin induced the expression of aquaporin 5, cystic fibrosis transmembrane conductance regulator, epithelial sodium channel, and Na+-K+-ATPase (18).
The lung injury in animal models of endotoxemia is also mitigated by vitamin C. For instance, parenteral administration of ascorbate [1000 mg/kg bolus injection followed by 200 mg/(kg.h) continuous infusion] decreases protein extravasation in the lungs of LPS-exposed sheep (15). Also, bolus injection of ascorbate or DHAA (200 mg/kg) decreases the concentration of protein in bronchoalveolar lavage fluid of LPS-exposed mice (17). Prevention by vitamin C of LPS-induced endothelial barrier dysfunction is a likely explanation for these findings.
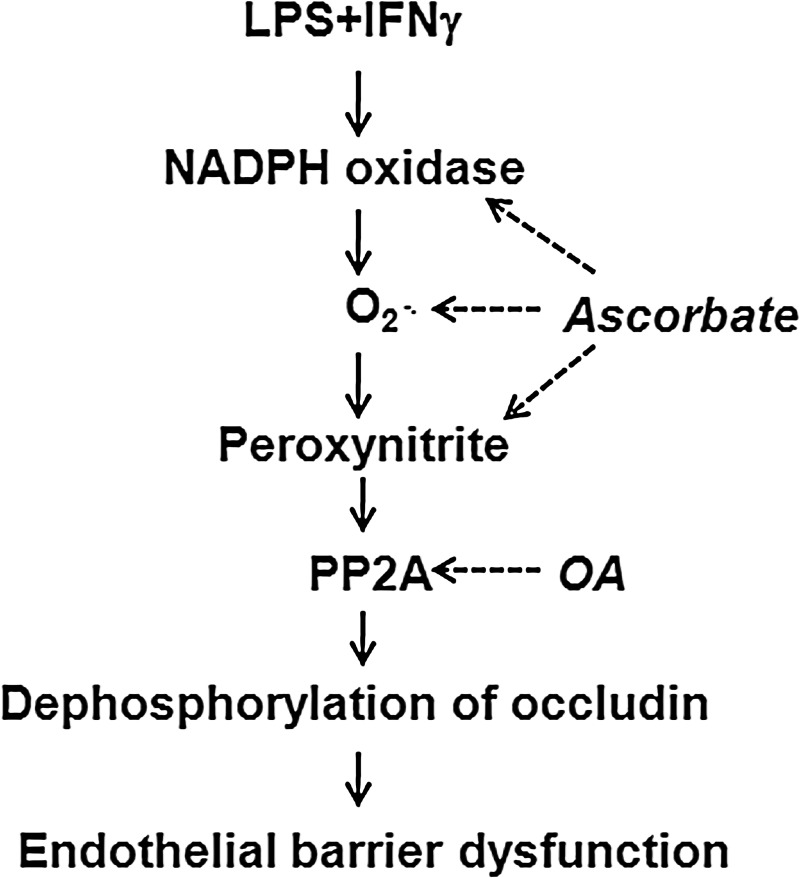
Because LPS and proinflammatory cytokines (e.g., interferon γ [IFNγ]) are mediators of the systemic inflammatory response to virulent bacterial infection, the mechanism of action of LPS+IFNγ in monolayer cultures of murine microvascular endothelial cells has been investigated. This septic insult increases the expression of NADPH oxidase 1 (Nox1) and iNOS proteins, the nitration of other proteins by peroxynitrite (assessed as 3-nitrotyrosine immunoreactivity), and the paracellular permeability to albumin (24, 62, 66) (Fig. 4). The increased production of peroxynitrite may also result from increased superoxide synthesis by uncoupled iNOS. Taken together, these findings support using cultures of microvascular endothelial cells to study in vitro the mechanisms underlying septic microvascular dysfunction, because CLP similarly raises Nox1, iNOS, peroxynitrite, protein nitration, and plasma protein extravasation levels in vivo (40, 64, 68).
FIG. 4.
Prevention by ascorbate of endothelial barrier dysfunction in sepsis. Exposure of microvascular endothelial cells to septic insult (LPS+IFNγ) causes endothelial barrier dysfunction. Ascorbate blocks septic increases in NADPH oxidase expression and activity, superoxide, and peroxynitrite. These effects of ascorbate attenuate septic stimulation of PP2A nitration and activation, so that phosphorylation and distribution of the tight junction protein occludin remain normal. OA inhibits PP2A activity and thus prevents occludin dephosphorylation. As consequences of these actions, ascorbate and OA prevent endothelial barrier dysfunction. LPS, lipopolysaccharide; IFNγ, interferon γ; PP2A, serine/threonine protein phosphatase 2A; PP2Ac, serine/threonine protein phosphatase 2A catalytic subunit; OA, okadaic acid.
An essential mediator of endothelial barrier dysfunction in sepsis is protein phosphatase 2A (PP2A). Tyrosine residues in the catalytic subunit of PP2A (PP2Ac) become nitrated in microvascular endothelial cells exposed to LPS+IFNγ, because this insult increases NADPH oxidase synthesis of superoxide, iNOS synthesis of NO, and consequently the production of peroxynitrite from superoxide and NO (62, 65). The tyrosine nitration of PP2Ac increases the phosphatase activity of PP2A, which dephosphorylates serine and threonine residues in occludin (24, 41). This dephosphorylation causes a redistribution of occludin away from tight junctions and consequently increases paracellular permeability to albumin (24, 62, 66) (Fig. 4). The essential role of PP2A is supported by observations that inhibition of this phosphatase by okadaic acid (OA) or siRNA knockdown prevents the LPS+IFNγ-induced changes in occludin and permeability (62). Occludin dephosphorylation may increase tight junction permeability independently of the changes in surface charge and actin cytoskeleton that also are associated with endothelial barrier dysfunction (28, 54).
Incubation of microvascular endothelial cells with 500 μM of either ascorbate or DHAA prevents the LPS+IFNγ-induced increase in paracellular permeability to albumin observed in ascorbate-deficient cells. This concentration is equal to the plasma ascorbate concentration achieved by an ascorbate infusion protocol that lessens edema formation in burn patients (57). For the ascorbate and DHAA treatments in cell cultures, the protection of the endothelial barrier is associated with increased intracellular ascorbate concentration and it depends on inhibition of both peroxynitrite formation and PP2A nitration/activation (24). Administration of ascorbate prevents the induction by septic insult of NAPDH oxidase, and consequently blocks induction of iNOS, in microvascular endothelial cells (65). Thus, ascorbate slows peroxynitrite formation and nitration of PP2Ac. The ascorbate-deficient endothelial cells in these experiments contained no detectable ascorbate, which is a degree of ascorbate depletion unlikely to occur in vivo. However, subsequent in vivo experiments confirmed the protective mechanism of ascorbate, as is described next.
CLP causes NADPH oxidase activation and endothelial NOS (eNOS) uncoupling that produce superoxide, and CLP also induces activations of iNOS and nNOS that generate NO, in skeletal muscles (68). The superoxide and NO combine to form peroxynitrite, which then nitrates multiple proteins (Fig. 5). PP2Ac is one of the proteins that becomes nitrated and consequently PP2A becomes activated. Immunoblot analysis of freshly harvested endothelial cells of the septic skeletal muscles reveals dephosphorylation of serine and threonine in occludin (68), similar to the observation made in microvascular endothelial cell cultures exposed to LPS+IFNγ (24). Moreover, the systemic inflammatory response of mice to CLP is associated with a very reproducible extravasation of fluorescent dextran (average molecular mass 70 kDa) in cremaster muscle and Evans blue-conjugated albumin in hindlimb skeletal muscles within 12 h, at which time survival is 100% (64, 68) (Fig. 6). Prophylactic injection i.v. of ascorbate (200 mg/kg), given 30 min prior to CLP, prevents eNOS uncoupling, iNOS and nNOS activation, peroxynitrite formation, PP2A activation, occludin dephosphorylation, and dextran (70 kDa) and plasma protein extravasation at 12 h post-CLP. A delayed ascorbate injection, given 3 h after CLP, also attenuates the endothelial barrier dysfunction (68).
FIG. 5.
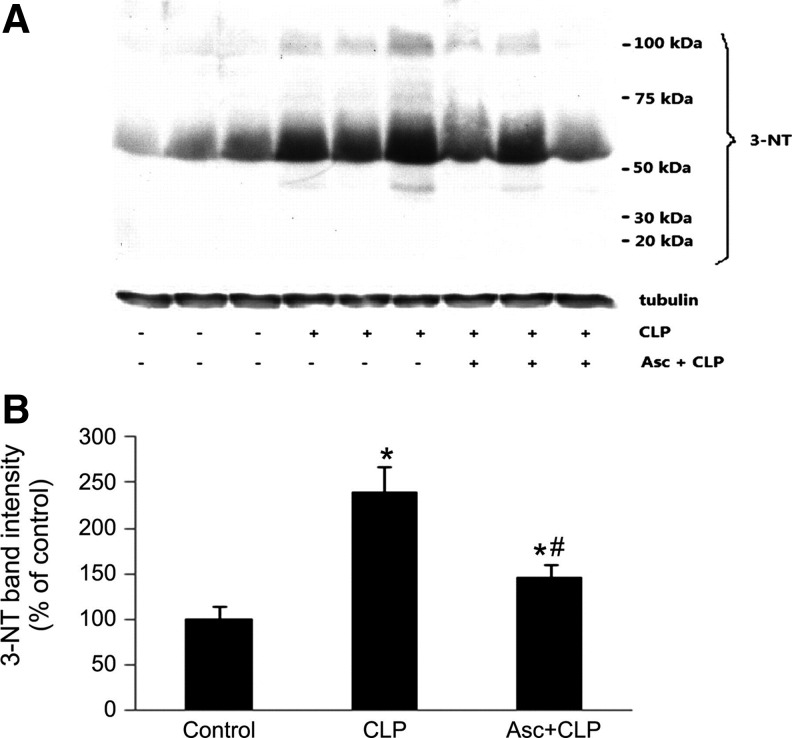
Parenteral ascorbate inhibits protein nitration in septic mouse skeletal muscle. Injection i.v. of ascorbate (200 mg/kg) in mice at 30 min before CLP prevented increase in 3-nitrotyrosine formation. Shown are representative western blots of 3-nitrotyrosine and tubulin for 3 nonseptic mice, 3 CLP mice, and 3 ascorbate-treated CLP mice at 12 h post-CLP (A). Also shown is the summary of 3-nitrotyrosine band intensities, for 6 mice per group, at 12 h post-CLP; *p<0.05 compared with control; #p<0.05 compared with CLP (B). Reprinted by permission (67).
FIG. 6.
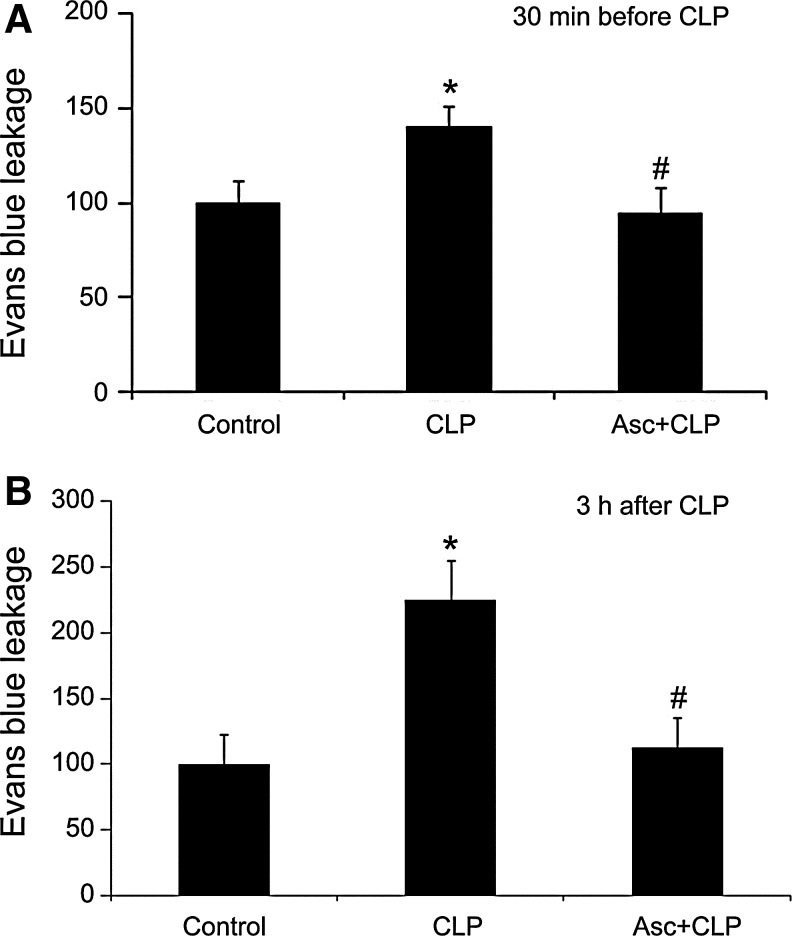
Parenteral ascorbate decreases plasma protein extravasation in septic mouse skeletal muscle. Injection i.v. of ascorbate (200 mg/kg) in mice at either 30 min before (A) or 3 h after CLP (B) prevented the increase in plasma protein extravasation assessed by Evans blue leakage at 12 h post-CLP. The leakage is expressed as percent control. *p<0.05 compared with control; #p<0.05 compared with CLP. Reprinted by permission (67).
For the effects of sepsis and ascorbate on endothelial barrier dysfunction, more is known about the role of iNOS than eNOS. Genetic or pharmacological interventions that specifically inhibit iNOS prevent the increase in vascular permeability to macromolecules in CLP mice (29). High levels of ascorbate are effective for inhibiting the induction of iNOS protein and elevation of iNOS activity by septic insult in cultures of microvascular endothelial cells (24, 62, 66). In CLP animals, too, ascorbate (200 mg/kg) injection blocks CLP-induced increases in iNOS mRNA in freshly harvested microvascular endothelial cells, iNOS protein and activity in skeletal muscles, and NO metabolites in plasma (63, 64). This may explain, at least in part, the observation that ascorbate treats septic endothelial barrier dysfunction in cell culture and animal models of sepsis.
The identity of the enzyme that synthesizes NO (whether eNOS or iNOS) may determine where NO and peroxynitrite are produced. Peroxynitrite has a short half-life of appearance and thus the site of its production dictates which tyrosine residues it can access. iNOS is known to interact with PP2Ac directly (42). If eNOS does not interact with PP2Ac similarly, then the discrepant interactions of the NOS isoforms with PP2Ac may account for the increase in PP2A nitration that occurs in sepsis, when iNOS activity is elevated and eNOS activity is depressed. Further, inhibition of iNOS expression may be the effect of ascorbate that accounts for all of the decrease in PP2A activity and defense of the endothelial barrier. Nevertheless, it remains a possibility that some of ascorbate's protection of endothelial barrier function may be due to the fact that the vitamin also attenuates the loss of eNOS activity during sepsis (68) (Fig. 3), since experiments with nonseptic endothelial cells indicate that ascorbate-dependent tightening of the endothelial barrier involves maintaining NO through the eNOS/guanylate cyclase pathway (37).
Coagulopathy and Capillary Plugging
A third component of septic microvascular dysfunction is the stoppage of blood flow in many capillaries. The extent of this maldistribution of capillary blood flow correlates with organ failure and mortality in patients with severe sepsis (49). The capillary blood flow impairment is due to coagulopathy and, more particularly, disseminated intravascular coagulation (13). Blood coagulation involves platelet aggregation and adhesion to endothelial cells, fibrin deposition, and formation of microthrombi (26). The sensitivity of these phenomena to vitamin C has been studied in endotoxemic, CLP and FIP mice (18, 45, 51, 59).
The systemic blood of septic human patients with disseminated intravascular coagulation becomes hypocoagulable because of accelerated consumption of clotting factors. Similarly, when mice are made endotoxemic by injection of LPS, blood samples obtained from them by cardiac puncture show prolonged prothrombin times and activated partial thromboplastin times (17). Ascorbate (200 mg/kg) or DHAA (200 mg/kg) injection mitigates this LPS-induced hypocoagulability in systemic blood (17). Histological analysis of the lungs shows extensive microvascular thrombosis in endotoxemic mice, except those treated with ascorbate or DHAA (17). Ascorbate and DHAA also blunt the induction by LPS of tissue factor mRNA in lung tissue, although whether they change the concentration of tissue factor protein at potential sites of blood clotting is unknown (17).
Septic peritonitis also causes hypocoagulability in the systemic blood of FIP mice, as indicated by thromboelastography that measures the viscoelastic properties of blood samples that have been collected by cardiac puncture (18). Ascorbate (200 mg/kg) injection prevents the FIP-induced hypocoagulability in systemic blood (18). To explain these changes, mRNA expression of procoagulant and anticoagulant factors has been examined in lung tissue. FIP increases mRNA expression of tissue factor and decreases that of thrombomodulin, and both these changes are countered by ascorbate (18). The mRNA expression of tissue plasminogen activator (t-PA) is not significantly affected by FIP but it is increased by the combination of FIP and ascorbate (18). These effects of ascorbate injection on mRNA expression in lung suggest the vitamin may modulate procoagulant, anticoagulant, and fibrinolytic pathways beneficially (18). However, it is not known if the changes observed in lung tissue mRNA levels are indicative of alterations in the proteins and their regulatory activities in the microvasculature.
In contrast to the hypocoagulability of systemic blood, capillary blood becomes hypercoagulable as maldistribution of capillary blood flow (e.g., increased proportion of capillaries with stopped-flow) develops in FIP mice (51). The evidence for this localized hypercoagulability comes from examination of vascular beds in skeletal muscles by intravital microscopy, which shows platelet adhesion and fibrin deposition are localized to capillaries (51, 59). Further, the capillary plugging in skeletal muscles is prevented by prophylactic depletion of platelets and also by i.v. injection of either P-selectin blocking antibody or antithrombin (51). Therefore, this component of septic microvascular dysfunction arises because a localized procoagulant state causes many capillaries to be plugged by microthrombi (51, 59), while systemic blood is depleted of clotting factors (18).
As described in an earlier section of this review, overproduction of NO by excessive iNOS activity may explain refractory arteriolar dilation in sepsis, since that component of microvascular dysfunction is absent in iNOS knockout mice (64). However, septic insult causes maldistribution of capillary blood flow to similar extents in iNOS-deficient and wild-type mice (51). Whether a capillary lumen remains patent may depend on the NO concentration at the potential site of microthrombus formation instead of on global levels of NO. Insufficient NO production by eNOS in capillaries, and increased NO consumption by reactive oxygen species there, may account for increases in platelet aggregation, platelet adhesion and microthrombus formation that plug septic capillaries (51).
The antithrombotic effects of NO in capillaries may diminish as the systemic inflammatory response to infection progresses. Part of the explanation for this change is increased production of superoxide that reacts with NO to form peroxynitrite. NADPH oxidase is the principal source of superoxide in septic microvascular endothelial cells (62). Consistently, platelet adhesion and capillary plugging in FIP mice are prevented by NADPH oxidase deficiency or injection i.v. of ascorbate (51, 59). Cell culture experiments show that high concentrations of ascorbate prevent increases in the activity of NADPH oxidase and the production of superoxide and related oxidants in microvascular endothelial cells exposed to septic insult (24, 65). By lowering reactive oxygen species levels, either NADPH oxidase deficiency or ascorbate administration may increase the effective concentration of NO within capillaries and thus prevent microthrombus formation during sepsis (59).
Ascorbate Reverses Capillary Plugging
In FIP mice, blood flow in plugged capillaries is restored by delayed treatment with ascorbate (51, 59). Injection i.v. of ascorbate (10 mg/kg) in anesthetized wild-type mice at 6 h post-FIP decreases the density of stopped-flow capillaries and increases the density of perfused capillaries within 10–30 min. The effect of ascorbate injection on capillary flow persists for at least 12 h postinjection (i.e., 18 h post-FIP) (59) (Fig. 7A). This ascorbate treatment does not reverse hypotension in FIP mice, so it is evident that reperfusion of capillaries is not caused by increased arterial pressure (59) (Fig. 7).
FIG. 7.
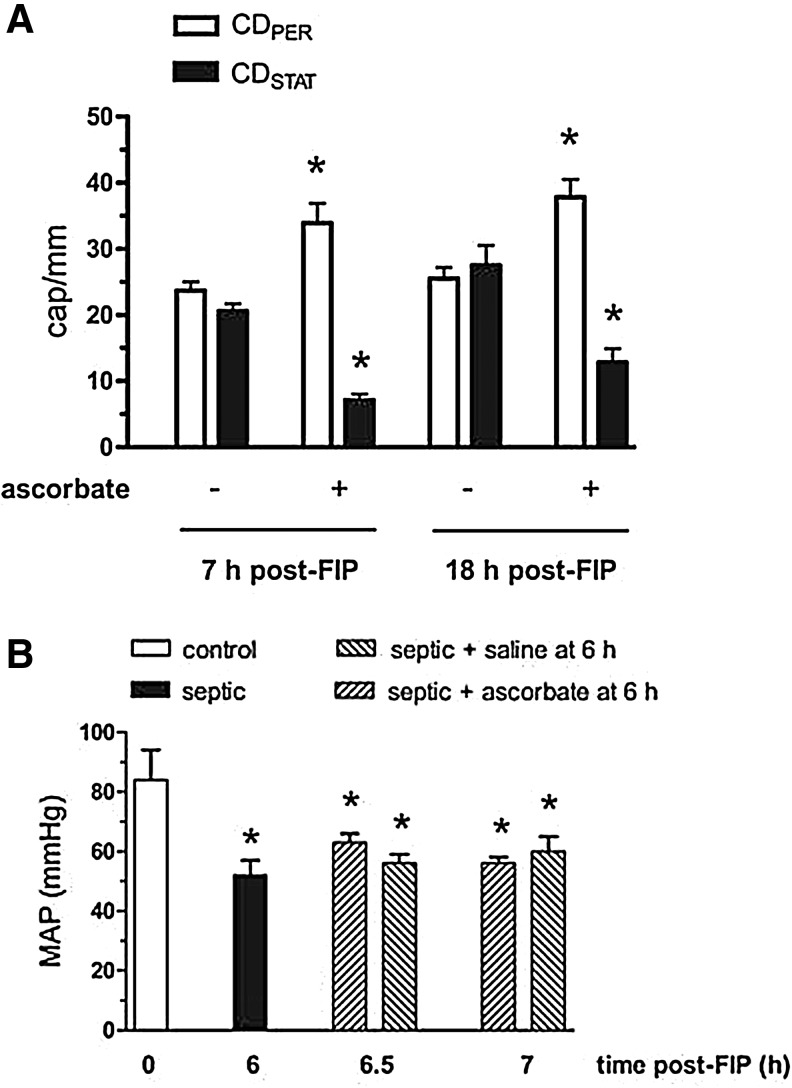
Parenteral ascorbate improves capillary blood flow distribution independently of blood pressure in septic wild-type mice. Injection i.v. of ascorbate (10 mg/kg) into anesthetized wild-type mice at 6 h post-FIP altered the densities of perfused and stopped-flow capillaries (CDPER and CDSTAT, respectively) in skeletal muscle at 7 h and 18 h post-FIP; *p<0.05 compared with respective control (A). However, ascorbate did not alter the decrease in mean arterial blood pressure (MAP) at 6.5–7 h post-FIP; *p<0.05 compared to 0 h (B). Reprinted by permission (58). FIP, fecal stem solution-injected into peritoneum.
Local superfusion of the capillary bed with either an NO donor or tetrahydrobiopterin (BH4) also improves capillary blood flow distribution after FIP, although the responses to these agents are transitory compared to the response to ascorbate injection (51). Presumably, BH4 acts as an antioxidant and eNOS cofactor to increase local concentrations of NO sufficiently to restore patency in plugged capillaries. The reversal of capillary plugging by ascorbate injection and BH4 superfusion does not occur in eNOS knockout FIP mice. In contrast, ascorbate does improve capillary blood flow distribution in nNOS knockout and iNOS knockout FIP mice (51) (Fig. 8).
FIG. 8.
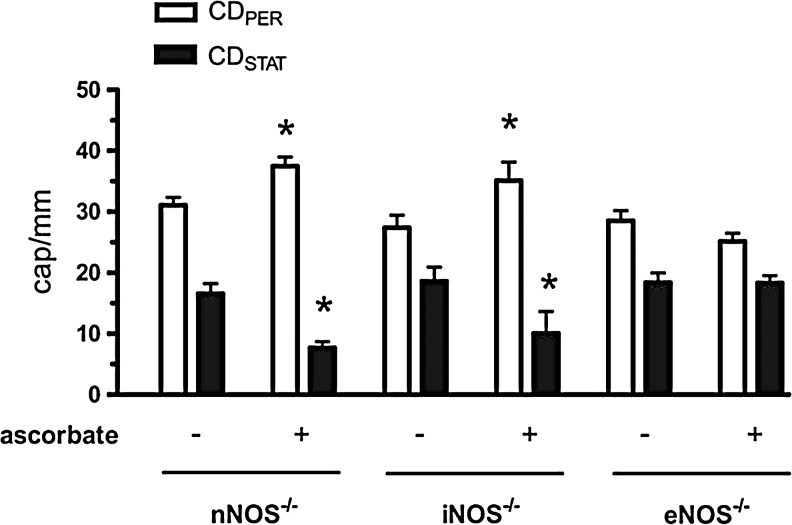
eNOS deficiency prevents improvement of septic capillary blood flow distribution by ascorbate. The densities of perfused and stopped-flow capillaries (CDPER and CDSTAT, respectively) were measured at 7 h post-FIP in skeletal muscle of anesthetized NOS-deficient mice. Ascorbate (10 mg/kg injected i.v. at 6 h post-FIP) prevented impairment of capillary blood flow in nNOS knockout and iNOS knockout mice (nNOS−/− and iNOS−/−) but not in eNOS knockout mice (eNOS−/−). *p<0.05 compared with respective control. Reprinted by permission (58).
Relevant information is also available from studies of CLP mice. Neither CLP nor ascorbate injection alters the eNOS mRNA levels measured in endothelial cells at 3 h post-CLP, which is a time when the iNOS mRNA levels in these cells are elevated (63). However, the eNOS activity in skeletal muscle is decreased by CLP and parenteral ascorbate (200 mg/kg) blocks this loss of activity (68) (Fig. 3).
Taken together, the observations reviewed here indicate that the NO synthesized by eNOS is a necessary mediator in the mechanism of action of ascorbate that regulates microvascular thrombosis in sepsis. How does ascorbate stimulate eNOS to synthesize NO? Ascorbate stabilizes the eNOS cofactor BH4 (e.g., ascorbate reduces the products of BH4 oxidation) and thus prevents eNOS uncoupling in endothelial cells (25). But ascorbate also enhances eNOS activity in these cells through a rapid phosphorylation at eNOS-Ser1177 (32).
eNOS activity is controlled by multiple kinases and phosphatases, including protein kinase C (PKC), Akt, AMP-activated kinase (AMPK), protein phosphatase 1, and PP2A (32). For the effect of ascorbate on eNOS phosphorylation, AMPK is the responsible upstream kinase (32). AMPK and eNOS are dephosphorylation targets of PP2A, and inhibition of PP2A by OA induces the same pattern of eNOS phosphorylation as does ascorbate (32). Further, PP2A overexpression prevents the effects of ascorbate on eNOS phosphorylation and activity. These observations indicate that ascorbate stimulates eNOS activity by decreasing PP2A activity and thereby inhibiting the dephosphorylation of eNOS by PP2A (32).
Ascorbate alters eNOS phosphorylation in endothelial cells within 5 min (32), which is fast enough to support the rapid, eNOS-dependent reversal of capillary plugging that is observed when ascorbate is injected into septic mice (59). The effect of ascorbate on eNOS phosphorylation does not require the production of hydrogen peroxide, because it is not prevented by the peroxide-reducing enzyme catalase, and it is specific for ascorbate because it is not elicited by other antioxidants (e.g., N-acetylcysteine, trolox) (32). These observations indicate a mechanism through which ascorbate is more effective than other antioxidants for treatment of septic microvascular dysfunction.
Fibrinolysis may be decreased in septic patients (26). Experiments with mice show that bolus i.v. injection of ascorbate at 6 h post-FIP (i.e., when capillary plugging is prevalent) improves capillary blood flow distribution during the next 10 min to 18 h (51, 59) (Fig. 7). Since FIP increases fibrin deposition in capillaries as microthrombi form (51), it is possible that ascorbate restores capillary patency by enhancing fibrinolysis locally.
Fibrinolysis involves regulatory factors, such as the pro-fibrinolytic t-PA and urokinase plasminogen activator (u-PA), and the anti-fibrinolytic plasminogen activator inhibitor 1 that inhibits t-PA and u-PA. If ascorbate affects these regulatory factors in septic capillaries selectively then it may enhance fibrinolysis there, without having a pro-fibrinolytic effect in systemic blood or parenchymal cells of tissues. However, the hypothesis that ascorbate stimulates fibrinolysis in capillaries selectively is hard to determine because it is technically difficult to access capillaries without greatly modifying them. For example, to analyze regulatory factors expressed selectively in microvascular endothelial cells, it may be necessary to isolate the cells by immunoseparation procedures that introduce confounding variables. To the best of our knowledge, there are no published studies of ascorbate on either fibrinolysis in capillaries or fibrinolytic regulators in freshly isolated microvascular endothelial cells.
Parenteral ascorbate is a useful means to rebalance NO production and protein nitration, and thereby restore capillary patency in the lungs and skeletal muscles of septic mice. However, confirmation that ascorbate exerts a similar action throughout the body awaits future studies of additional organs.
Vitamin C Dosage and Safety
The low plasma ascorbate concentration in septic patients (6, 20, 35) and the decreased morbidity and mortality observed in animal models of sepsis (18, 59, 64) indicate that parenteral vitamin C may be a beneficial adjuvant therapy in sepsis. Allometric dose translation is useful for extrapolating findings from animals to humans. A published formula for normalization to body surface area (47) indicates that the mouse dose of 200 mg/kg is equivalent to a human dose of 16 mg/kg, which corresponds to 1120 mg for a typical 70 kg adult person. This approximates the minimal dose of parenteral ascorbate (1000 mg ascorbate/day) that raises the plasma ascorbate concentration of septic patients into the range of normal plasma levels (35). The dose should be tolerated well, judging from clinical trials that injected 1000–2000 mg ascorbate every 8–24 h for up to 7 days in critically ill surgical or acutely injured patients (5, 11, 16, 39).
Generally, ascorbate is not a dangerous intervention for most subjects. However, concerns have been raised that high-dose ascorbate may induce pro-oxidant effects in patients (38). One basis for this concern is that ascorbate reduces the valence of free transition metals, such as iron (e.g., ascorbate reduces FeIII to FeII), which then catalyze the formation of hydrogen peroxide. Hydrogen peroxide is generated in the interstitial fluid by oxidation of large amounts of exogenous ascorbate (10) and this may alter the function of some cells. Another source of hydrogen peroxide is extravasated neutrophils, because ascorbate enhances reactive oxygen species production by neutrophils exposed to E. coli (52). Indeed, ascorbate kills cancer cells in culture and the underlying mechanism involves promotion hydrogen peroxide formation in the culture medium, because the cell killing can be suppressed by extracellular catalase (46). However, the validity of these concerns is not supported by observations in most of the subjects and patients who have been studied. Repeated i.v. injection of 750–7500 mg/day of vitamin C for 6 days in healthy volunteers does not induce a pro-oxidant change in plasma markers (38). Further, i.v. infusion of high-dose ascorbate [1584 mg/(kg.day) for 3 days] lowers serum malondialdehyde concentration in severely burned patients, indicating that the vitamin decreases the oxidative stress associated with the systemic inflammatory response to burn injury (57). High-dose ascorbate also decreases postsurgical oxidative stress. The evidence for the latter statement is, in patients who undergo gastrointestinal surgery, the urinary excretion of 8-isoprostane (another marker of oxidative stress) is lower after infusion of ascorbate at a high dose (500 mg/day) compared to a low dose (100 mg/day) (67). Nevertheless, because the removal of hydrogen peroxide from blood requires NADPH generated by glucose-6-phosphate, high-dose ascorbate increases the risk of intravascular hemolysis in patients with glucose-6-phosphate dehydrogenase deficiency (14).
Formation of calcium oxalate stones in the kidneys is a potential adverse effect of long-term administration of high-dose ascorbate (3). However, less than 0.5% of the i.v. dose of ascorbate is recovered as oxalate in the urine of human subjects with normal renal function (48). Septic patients may require high-dose vitamin C therapy for only a few days, until source control and antibiotic therapy have eliminated virulent bacteria and the adverse effects of bactericidal antibiotics have ceased. Such a brief course of ascorbate therapy may not elevate the risk of oxalate stone formation, except in patients with impaired kidney function. However, clinical trial will be necessary to determine the safety of ascorbate in sepsis. Until clinical testing is completed, large doses of ascorbate should not be given to patients with glucose-6-phosphate dehydrogenase deficiency, renal disease, renal insufficiency or renal failure, a history of oxalate nephrolithiasis, or paroxysmal nocturnal hemoglobinuria (43).
Conclusions and Future Directions
Sepsis remains a major cause of death and long-term disability despite multiple supportive therapies. Poor outcome is associated with ascorbate depletion, unreactive arterioles, and leaky or plugged capillaries. Ascorbate repletion in septic patients occurs only after resolution of the disease or parenteral ascorbate administration at higher doses than current clinical practice. Studies of animal models of sepsis have discovered that parenteral ascorbate improves survival. Ascorbate also prevents refractory vasodilation and plasma protein extravasation in experimental sepsis, although its ability to avert hypotension greatly varies between rat and mouse models. Prophylactic ascorbate prevents, and delayed ascorbate reverses, the maldistribution of capillary blood flow caused by disseminated intravascular coagulation in experimental sepsis.
The therapeutic mechanism of ascorbate involves rebalancing NO production and utilization. It is clear that changes in arteriolar reactivity are mediated by septic stimulation of iNOS and the inhibition of that process by ascorbate, although the molecular targets of iNOS-derived NO that drive this component of microvascular dysfunction remain to be identified.
The mechanistic understanding of other components of septic microvascular dysfunction is very detailed already. During the systemic inflammatory response to infection, expression of NADPH oxidase and iNOS accelerates the production of peroxynitrite. The latter nitrates PP2Ac and consequently activates PP2A. This phosphatase dephosphorylates occludin and thus increases the paracellular permeability of the microvascular endothelium, which permits extravasation of plasma proteins and fluid. PP2A also inhibits eNOS and thereby increases blood coagulability, microthrombus formation, and capillary plugging. By attenuating the septic increases in NADPH oxidase and reactive oxygen species, high levels of ascorbate normalize the NOS enzymes, peroxynitrite, PP2A, occludin, endothelial barrier function, and capillary patency.
Future experiments with sepsis models may determine if ascorbate improves outcome through pro-oxidant and bacteriostatic actions localized to the interstitial fluid, and through the microvascular effects reviewed in detail here. Also, since most information about those microvascular effects is derived from investigations of lung and skeletal muscles, confirmation that ascorbate exerts similar actions throughout the body awaits future studies of other organs.
Although repletion protocols that require high doses of ascorbate have been described for critically ill and septic patients, and similar doses are tolerated well by most healthy subjects, whether such doses have adverse effects in patients is uncertain. Nevertheless, enough facts are known about the ascorbate depletion in septic patients receiving standard care, and the therapeutic mechanisms established in models, to support the design of clinical trials of parenteral ascorbate as an adjuvant therapy for sepsis. Preclinical studies indicate that ascorbate and DHAA each protect against microvascular dysfunction, organ failure, and death in experimental sepsis. Presumably, DHAA is reduced to ascorbate without lowering the concentrations of electron donors (e.g., NADPH, glutathione) below critical levels. However, clinical trials should focus on ascorbate because of the relative abundance of information about pharmacodynamics and potential adverse effects for this redox state of vitamin C.
Abbreviations Used
- AMPK
AMP-activated kinase
- BH4
tetrahydrobiopterin
- CAM
complementary and alternative medicine
- CLP
cecal ligation and puncture
- DHAA
dehydroascorbic acid
- eNOS
endothelial nitric oxide synthase
- FIP
fecal stem solution-injected into peritoneum
- Gulo−/−
l-gulono-γ-lactone oxidase
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- Nox1
NADPH oxidase 1
- OA
okadaic acid
- PKC
protein kinase C
- PP2A
serine/threonine protein phosphatase 2A
- PP2Ac
serine/threonine protein phosphatase 2A catalytic subunit
- t-PA
tissue plasminogen activator
- u-PA
urokinase plasminogen activator
Acknowledgments
This publication was made possible by grant number 5R01AT003643 from the National Center for Complementary and Alternative Medicine (NCCAM) at National Institutes of Health. Its contents are solely the responsibility of the author and do not necessarily represent the official views of NCCAM.
References
- 1.Alkharfy KM, Kellum JA, and Matzke GR. Unintended immunomodulation: part II. Effects of pharmacological agents on cytokine activity. Shock 13: 346–360, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Armour J, Tyml K, Lidington D, and Wilson JX. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J Appl Physiol 90: 795–803, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin North Am 31: 927–949, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Barreiro E, Comtois AS, Gea J, Laubach VE, and Hussain SN. Protein tyrosine nitration in the ventilator muscles: role of nitric oxide synthases. Am J Respir Cell Mol Biol 26: 438–446, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Berger MM, Soguel L, Shenkin A, Revelly JP, Pinget C, Baines M, and Chiolero RL. Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Crit Care 12: R101, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrelli E, Roux-Lombard P, Grau GE, Girardin E, Ricou B, Dayer J, and Suter PM. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med 24: 392–397, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, and Regnier B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis JAMA 274: 968–974, 1995 [PubMed] [Google Scholar]
- 8.Buras JA, Holzmann B, and Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4: 854–865, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Carr AC. and Frei B. Human neutrophils oxidize low-density lipoprotein by a hypochlorous acid-dependent mechanism: the role of vitamin C. Biol Chem 383: 627–636, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, and Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A 105: 11105–11109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collier BR, Giladi A, Dossett LA, Dyer L, Fleming SB, and Cotton BA. Impact of high- dose antioxidants on outcomes in acutely injured patients. JPEN J Parenter Enteral Nutr 32: 384–388, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Deubzer B, Mayer F, Kuçi Z, Niewisch M, Merkel G, Handgretinger R, and Bruchelt G. H(2)O(2)-mediated cytotoxicity of pharmacologic ascorbate concentrations to neuroblastoma cells: potential role of lactate and ferritin. Cell Physiol Biochem 25: 767–774, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Dhainaut JF, Shorr AF, Macias WL, Kollef MJ, Levi M, Reinhart K, and Nelson DR. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med 33: 341–348, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Du J, Cullen JJ. and Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta 1826: 443–457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dwenger A, Pape HC, Bantel C, Schweitzer G, Krumm K, Grotz M, Lueken B, Funck M, and Regel G. Ascorbic acid reduces the endotoxin-induced lung injury in awake sheep. Eur J Clin Invest 24: 229–235, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Ellis GR, Anderson RA, Lang D, Blackman DJ, Morris RH, Morris-Thurgood J, McDowell IF, Jackson SK, Lewis MJ, and Frenneaux MP. Neutrophil superoxide anion- generating capacity, endothelial function and oxidative stress in chronic heart failure: effects of short- and long-term vitamin C therapy. J Am Coll Cardiol 36: 1474–1482, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Fisher BJ, Seropian IM, Kraskauskas D, Thakkar JN, Voelkel NF, Fowler AA, 3rd, and Natarajan R. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med 39: 1454–1460, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Ward KR, Voelkel NF, Fowler AA, 3rd, and Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol 303: L20–L32, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Galley HF, Davies MJ, and Webster NR. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radic Biol Med 20: 139–143, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Galley HF, Davies MJ, and Webster NR. Xanthine oxidase activity and free radical generation in patients with sepsis syndrome. Crit Care Med 24: 1649–1653, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Galley HF, Howdle PD, Walker BE, and Webster NR. The effects of intravenous antioxidants in patients with septic shock. Free Radic Biol Med 23: 768–774, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Gaut JP, Belaaouaj A, Byun J, Roberts LJ, 2nd, Maeda N, Frei B, and Heinecke JW. Vitamin C fails to protect amino acids and lipids from oxidation during acute inflammation. Free Radic Biol Med 40: 1494–1501, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Goldman D, Bateman RM, and Ellis CG. Effect of sepsis on skeletal muscle oxygen consumption and tissue oxygenation: interpreting capillary oxygen transport data using a mathematical model. Am J Physiol Heart Circ Physiol 287: H2535–H2544, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Han M, Pendem S, Teh SL, Sukumaran DK, Wu F, and Wilson JX. Ascorbate protects endothelial barrier function during septic insult: Role of protein phosphatase type 2A. Free Radic Biol Med 48: 128–135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, and Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 276: 40–47, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Helling H, Schenk HJ, Pindur G, Weinrich M, Wagner B, and Stephan B. Fibrinolytic and procoagulant activity in septic and haemorrhagic shock. Clin Hemorheol Microcirc 45: 295–300, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, and Day AG; Canadian Critical Care Trials Group A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 368: 1489–1497, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Hirase T, Kawashima S, Wong EY, Ueyama T, Rikitake Y, Tsukita S, Yokoyama M, and Staddon JM. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J Biol Chem 276: 10423–10431, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Hollenberg SM, Guglielmi M, and Parrillo JE. Discordance between microvascular permeability and leukocyte dynamics in septic inducible nitric oxide synthase deficient mice. Crit Care 11: R125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyslop PA, Hinshaw DB, Scraufstatter IU, Cochrane CG, Kunz S, and Vosbeck K. Hydrogen peroxide as a potent bacteriostatic antibiotic: implications for host defense. Free Radic Biol Med 19: 31–37, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Kallio J, Jaakkola M, Mäki M, Kilpeläinen P, and Virtanen V. Vitamin C inhibits Staphylococcus aureus growth and enhances the inhibitory effect of quercetin on growth of Escherichia coli in vitro. Planta Med 78: 1824–1830, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Ladurner A, Schmitt CA, Schachner D, Atanasov AG, Werner ER, Dirsch VM, and Heiss EH. Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic Biol Med 52: 2082–2090, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, and Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 40: 754–761, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Lee WL. and Slutsky AS. Sepsis and endothelial permeability. N Engl J Med 363: 689–691, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghesi L, Franks W, Lawson TC, and Sauberlich HE. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res 109: 144–148, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Martin GS, Mannino DM, Eaton S, and Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554, 2003 [DOI] [PubMed] [Google Scholar]
- 37.May JM. and Qu ZC. Nitric oxide mediates tightening of the endothelial barrier by ascorbic acid. Biochem Biophys Res Commun 404: 701–705, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mühlhöfer A, Mrosek S, Schlegel B, Trommer W, Rozario F, Böhles H, Schremmer D, Zoller WG, and Biesalski HK. High-dose intravenous vitamin C is not associated with an increase of pro-oxidative biomarkers. Eur J Clin Nutr 58: 1151–1158, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, Radella F, Garcia I, and Maier RV. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 236: 814–822, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nin N, El-Assar M, Sánchez C, Ferruelo A, Sánchez-Ferrer A, Martínez-Caro L, Rojas Y, Paula M, Hurtado J, Esteban A, and Lorente JA. Vascular dysfunction in sepsis: effects of the peroxynitrite decomposition catalyst MnTMPyP. Shock 36: 156–161, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, 3rd, and Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol 158: 967–978, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohama T. and Brautigan DL. Endotoxin conditioning induces VCP/p97-mediated and inducible nitric-oxide synthase-dependent Tyr284 nitration in protein phosphatase 2A. J Biol Chem 285: 8711–8718, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, and Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One 5: e11414, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng ZY, Wang HZ, Srisawat N, Wen X, Rimmelé T, Bishop J, Singbartl K, Murugan R, and Kellum JA. Bactericidal antibiotics temporarily increase inflammation and worsen acute kidney injury in experimental sepsis. Crit Care Med 40: 538–543, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raeven P, Feichtinger GA, Weixelbaumer KM, Atzenhofer S, Redl H, Van Griensven M, Bahrami S, and Osuchowski MF. Compartment-specific expression of plasminogen activator inhibitor-1 correlates with severity/outcome of murine polymicrobial sepsis. Thromb Res 129: e238–e245, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Ranzato E, Biffo S, and Burlando B. Selective ascorbate toxicity in malignant mesothelioma: a redox Trojan mechanism. Am J Respir Cell Mol Biol 44: 108–117, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Reagan-Shaw S, Nihal M, and Ahmad N. Dose translation from animal to human studies revisited. FASEB J 22: 659–661, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Robitaille L, Mamer OA, Miller WH, Jr., Levine M, Assouline S, Melnychuk D, Rousseau C, and Hoffer LJ. Oxalic acid excretion after intravenous ascorbic acid administration. Metabolism 58: 263–269, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakr Y, Dubois MJ, De Backer D, Creteur J, and Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 32: 1825–1831, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Schorah CJ, Downing C, Piripitsi A, Gallivan L, Al-Hazaa AH, Sanderson MJ, and Bodenham A. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr 63: 760–765, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Secor D, Li F, Ellis CG, Sharpe MD, Gross PL, Wilson JX, and Tyml K. Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Med 36: 1928–1934, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma P, Raghavan SA, Saini R, and Dikshit M. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: modulatory effect of nitric oxide. J Leukoc Biol 75: 1070–1078, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Shen KP, Lo YC, Yang RC, Liu HW, Chen IJ, and Wu BN. Antioxidant eugenosedin-A protects against lipopolysaccharide-induced hypotension, hyperglycaemia and cytokine immunoreactivity in rats and mice. J Pharm Pharmacol 57: 117–125, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Shostak A. and Gotloib L. Increased mesenteric, diaphragmatic, and pancreatic interstitial albumin content in rats with acute abdominal sepsis. Shock 9: 135–137, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Solomkin JS. and Mazuski J. Intra-abdominal sepsis: newer interventional and antimicrobial therapies. Infect Dis Clin North Am 23: 593–608, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Spada PL, Rossi C, Alimonti A, Bocca B, Cozza V, Ricerca BM, Bocci MG, Vulpio C, and De Sole P. Ferritin iron content in haemodialysis patients: comparison with septic and hemochromatosis patients. Clin Biochem 41: 997–1001, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, and Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg 135: 326–331, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Tyml K, Li F, and Wilson JX. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit Care Med 33: 1823–1828, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Tyml K, Li F, and Wilson JX. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Crit Care Med 36: 2355–2362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vianna RC, Gomes RN, Bozza FA, Amâncio RT, Bozza PT, David CM, and Castro- Faria-Neto HC. Antibiotic treatment in a murine model of sepsis: impact on cytokines and endotoxin release. Shock 21: 115–120, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Vincent JL, Nelson DR, and Williams MD. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit Care Med 39: 1050–1055, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Wu F. and Wilson JX. Peroxynitrite-dependent activation of protein phosphatase type 2A mediates microvascular endothelial barrier dysfunction. Cardiovasc Res 81: 38–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu F, Wilson JX, and Tyml K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am J Physiol Regul Integr Comp Physiol 285: R50–R56, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Wu F, Wilson JX, and Tyml K. Ascorbate protects against impaired arteriolar constriction in sepsis by inhibiting inducible nitric oxide synthase expression. Free Radic Biol Med 37: 1282–1289, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Wu F, Tyml K, and Wilson JX. iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J Cell Physiol 217: 207–214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu F, Han M, and Wilson JX. Tripterine prevents endothelial barrier dysfunction by inhibiting endogenous peroxynitrite formation. Br J Pharmacol 157: 1014–1023, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamazaki E, Horikawa M, and Fukushima R. Vitamin C supplementation in patients receiving peripheral parenteral nutrition after gastrointestinal surgery. Nutrition 27: 435–439, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Zhou G, Kamenos G, Pendem S, Wilson JX, and Wu F. Ascorbate protects against vascular leakage in cecal ligation and puncture-induced septic peritonitis. Am J Physiol Regul Integr Comp Physiol 302: R409–R416, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]