Abstract
Background: Inflammation is considered to be a major initiator to angioplasty-induced vascular restenosis. Proinflammatory cytokines stimulate vascular smooth muscle cell (VSMC) migration and proliferation leading to neointimal hyperplasia. It has been reported that chronic caffeine use suppresses the production of proinflammatory cytokine TNF-α (tumor necrosis factor Alpha) and alters adenosine receptor expression in human neutrophils, indicating that caffeine may attenuate vascular injury-induced inflammation and subsequent neointimal hyperplasia. Our current study was designed to test the hypothesis that chronic caffeine treatment decreases vascular injury-induced neointimal hyperplasia by suppressing VSMC migration and proliferation.
Methods and Results: The experiments were carried out using both in vivo (rat carotid artery injury model) and in vitro (VSMCs isolated from rat aorta) models. Male Sprague-Dawley rats that received chronic caffeine treatment (10 and 20 mg/kg per day, through oral gavage) showed a significant decrease in neointimal hyperplasia when compared to rats that received vehicle. To understand the underlying mechanisms, we tested if caffeine inhibits fetal bovine serum (FBS)-induced VSMC migration and proliferation. We found that caffeine substantially suppressed FBS-induced VSMC migration and proliferation. The attenuation of FBS-stimulated cell migration is dose dependent.
Conclusion: Together, our results suggest that chronic treatment with high concentrations of caffeine attenuates vascular injury-induced neointimal hyperplasia by suppressing smooth muscle cell migration and proliferation in rats.
Introduction
Angioplasty is a vascular intervention to mechanically open narrow or obstructed coronary arteries caused by atherosclerotic plaque formation. A major drawback to angioplasty is the recurrence of vascular narrowing (restenosis) in up to 60% of patients that undergo the procedure.1 Angioplasty-induced vascular restenosis is a hypertrophic wound healing process resulting from an interaction between inflammatory cells and the normal elements of the arterial wall in response to unintentional damage to the vessel wall by the procedure.2–4 The enhanced vascular smooth muscle cell (VSMC) proliferation and migration as the result of this interaction are the key events in injury-induced intimal hyperplasia and vascular restenosis.5
Caffeine is the most widely used active substance in the world6 and is found in many different sources such as coffee, tea, chocolate, and soft drinks. It has been estimated that caffeine intake per day in the United States and Canada is anywhere from 200 to 250 mg/person. In Nordic countries and Great Britain that number may be as high as 300–400 mg/person per day. Caffeine and its metabolites act in the body's cells by different mechanisms of action and on a wide range of molecular targets.7 Although extensively studied, the effect of caffeine on the cardiovascular system remains inconclusive. Some have found that the consumption of caffeine increases cardiovascular risk,8–10 while others have described the beneficial or neutral effects.11–13 Previous findings indicate that caffeine may promote atherosclerosis by inhibiting vascular cell apoptosis,14–16 whereas a most recent study found that chronic consumption (6–12 weeks) of 400 mg/kg of caffeine significantly reduced fat-fed-induced atherosclerotic plaque area in C57Bl/6 apolipoprotein E (ApoE) knockout mouse in a lipid-independent manner,17 indicating that chronic treatment with high concentrations of caffeine may produce direct effects on vascular components. However, the effect of caffeine on vascular injury-induced neointimal hyperplasia is not very clear.
Our current study found that chronic caffeine treatment significantly attenuated vascular injury-induced neointimal hyperplasia and substantially suppressed fetal bovine serum (FBS)-induced VSMC migration and proliferation.
Materials and Methods
Collagenase type I and elastase were obtained from Worthington (Lakewood, NJ). All other chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise.
In vivo studies
Experimental animals
Male Sprague-Dawley rats (Hilltop Lab Animals, Scottsdale, PA) (n=30) weighing 100–120 g were used in this study. Rats were randomly assigned to a high caffeine group (20 mg/kg; n=10), a moderate caffeine group (10 mg/kg; n=10), or a noncaffeine control group (n=10). The rats received caffeine (20 or 10 mg/kg, dissolved in distilled water) or vehicle (distilled water) daily through gastric gavage at an equal volume per body mass starting 2 weeks before and ending 2 weeks after carotid artery injury was induced using a balloon catheter (see below). The chosen dose and duration of the treatment were based on the evidence that chronic caffeine treatment (10 and 20 mg/kg per day for 8 weeks) improves the erectile function in diabetic rats, and that consumption of caffeine (400–600 mg/day) for 2 weeks upregulates adenosine receptor A2a expression in humans.18,19
For consistency, all rats were treated at the same time each morning. Rats were housed in approved hanging wire cages in the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-Certified, U.S. Department of Agriculture (USDA)-Approved Animal Care Facility with constant temperature maintained at 23°C and controlled 12-hour light/12-hour dark cycles. The experimental protocols in this study were reviewed and approved by the Kirksville College of Osteopathic Medicine Institutional Animal Care and Use Committee (IACUC).
Carotid artery injury model
To determine if caffeine inhibits vascular injury-induced neointimal hyperplasia, male Sprague-Dawley rats underwent balloon catheterization of the right common carotid artery to induce vascular neointimal hyperplasia as described previously.20 The rats were anesthetized by intraperitoneal injection of ketamine (60 mg/kg, i.p.; JHP Pharmaceuticals, Rochester, MI) and xylazine (5 mg/kg i.p.; AGRI Labs, St. Joseph, MO). Common carotid artery injury was generated by using a 2F Fogarty arterial embolectomy balloon catheter (Edward Lifesciences, Irvine, CA). Topical ketoprofen (Med Depot Pharmacy, Kirksville, MO) was applied for analgesia. The animal was allowed to recover from anesthesia and returned to the Animal Care Facility.
Intimal hyperplasia analysis
Two weeks after surgery, the rats were anesthetized by injection of an overdose of sodium pentobarbital (120 mg/kg i.p.). The rats were fixed with 3.7% formaldehyde (Fisher Scientific, Fair Lawn, NJ) through the apex of the heart at physiological pressure (100 mmHg). Arterial grafts were processed for paraffin embedding using a Leica TP1020 semienclosed benchtop tissue processor (Leica Microsystems, Nussloch, Germany), paraffin embedded, cross sectioned (8 μm thick) using a Leica RM2255 microtome (Leica Microsystems), and stained with hematoxylin and eosin for morphometric analysis. Images of the resulting sections were captured using a Leica DFC400 digital microscope camera (Leica Microsystems) mounted on a Leica DM4000B microscope (Leica Microsystems) utilizing Surveyor Software (Objective Imaging Ltd., Cambridge, United Kingdom). The media and neointima area of the vessel wall were traced carefully utilizing a Wacom Bamboo tablet (CTH-460) and quantified using ImageJ software (National Institute of Health). The cross-sectional area of the neointima was defined as the area confined by the internal elastic lamina and the arterial lumen. The cross-sectional area of the media was defined as the area surrounded by the internal and external elastic laminae. Neointimal formation was defined as the ratio of intima to media.
In vitro studies
Cell culture
VSMCs were isolated from the thoracic aorta of 100–125 g Sprague-Dawley male rats (Hilltop Lab Animals) by following a previously described protocol.21 Cells were cultured in the DMEM/F12 (Mediatech, Inc., Manassas, VA) containing 10% of FBS, 50 U/mL penicillin, 50 μg/mL streptomycin, and 0.5 μg/mL amphotericin. Experiments utilized subcultured cells of passages 5–9.
Measurement of cell migration
Cell migration was performed by using a wound healing assay.21 Images were then taken 24 hours before and after the treatment using a Scion Corporation Color Digital Camera (Model CFW-1312C) mounted to a Bausch & Lomb PhotoZoom Inverted Microscope (Cat. No. 31-29-14). Cell migration was measured using NIH ImageJ software and determined by comparing the percentage changes of the mean cell-free gap area at 0 and 24 hours after treatments. Results were expressed as the percentage changes of gap area±SE.
Dose–response effects of FBS on VSMC motility
To determine the optimal concentration of FBS for use in later FBS-stimulated migration and proliferation studies, VSMCs were pretreated with serum-free media for 24 hours followed by stimulation with different concentrations of FBS (0%, 0.0375%, 0.075%, 0.15%, 0.3%, 0.625%, 1.25%, 2.5%, and 5%) for 24 hours. We chose 1.25% FBS for all subsequent motogenic and mitogenic experiments as this percentage of FBS caused a moderate, yet significant, increase in cellular migration as compared to control (3.6 times greater decrease in cell-free area as compared to control, data not shown).
Time course and dose response of caffeine treatment
For time course, VSMCs were pre-exposed to 2.5 mM caffeine for 0, 24, and 48 hours, followed by stimulation with 1.25% of FBS in the presence or absence of caffeine for 24 hours. This experiment utilized 2.5 mM caffeine as the previous study indicates that this concentration of caffeine may suppress VSMC proliferation.17 Both acute (no pretreatment) and chronic (with 24- and 48-hour pretreatment) caffeine treatment produced significant inhibition of FBS-stimulated VSMC migration. Therefore, pretreatment for a period of 24 hours with caffeine was selected for the subsequent experiments as this treatment period allowed for complete blockade of FBS-induced cell migration (p<0.001). No significant difference was seen between 24 and 48 hours of pretreatment groups, p=0.721 (data not shown).
For dose–response experiment, VSMCs were pretreated with serum-free media containing different concentrations of caffeine (0, 0.5, 1.0, 2.5, 5.0 mM, D5385; Sigma-Aldrich) for 24 hours followed by stimulation with 0 or 1.25% of FBS for 24 hours in the continued presence or absence of caffeine. This concentration range has been indicated to inhibit VSMC proliferation isolated from Apo E knockout mice.17
Measurement of cell proliferation
Cell proliferation was measured by following a modified version of instructions provided by the manufacturer of the 5-bromo-2′-deoxyuridine (BrdU) labeling reagent (00-0103; Invitrogen, Grand Island, NY). Sixty percent confluent VSMCs in an 8-chamber slide (Nalge Nunc International, Naperville, IL) were cultured in serum-free media (with or without caffeine treatment) for 24 hours followed by incubating in media containing BrdU labeling reagent (1:100 dilution from concentrate, 00-0103; Invitrogen) in the presence or absence of 1.25% of FBS (with or without caffeine treatment) for 24 hours. Cells were fixed in methanol:acetone (1:1) for 30 minutes, air-dried, and then treated with 4 N HCl for 15 minutes at room temperature.22 Following the denaturation of the DNA with HCl, cells were neutralized by rinsing in the borax-borate buffer (pH 9.0) for 5 minutes followed by phosphate-buffered saline (PBS), blocked in 0.2% bovine serum albumin (BSA) in PBS for 30 minutes, and incubated with an unconjugated BrdU mouse monoclonal antibody (1:100 dilution from concentrate, B35128; Invitrogen) in PBS containing 0.2% BSA overnight at 4°C. The unbound primary antibody was discarded and the cells were washed three times with PBS for 10 minutes each. Cells were then labeled with anti-mouse IgG conjugated with FITC (1:100 dilution, F0257; Sigma-Aldrich) for 30 minutes and then washed with PBS three times for 10 minutes each. Nuclei were stained with 5 μM DRAQ5 (#4084; Cell Signaling Technology, Danvers, MA) in PBS for 3 minutes. All chambers were then washed three times with PBS. Images were taken in four random fields of view per chamber using a Leica DMI6000B confocal laser scanning microscope, visualized, and photographed with Leica Application Suite Advanced Fluorescence (LAS AF) software (Leica Microsystems). Cells were counted by two independent blinded observers using LAS AF Lite, and cell proliferation was expressed as the ratio of the cells labeled with BrdU to the cells labeled with DRAQ5.
Effects of caffeine on VSMC proliferation
VSMCs were pretreated with serum-free media containing 1.0 mM of caffeine for 24 hours followed by stimulation with 0 or 1.25% of FBS plus a BrdU labeling reagent for an additional 24 hours in the continued presence or absence of caffeine.
Statistical analysis
Statistical analyses of results were performed using Sigma Plot (version 11.0; Systat Software, Inc., San Jose, CA). A one-way ANOVA followed by a Holm-Sidak pairwise comparison post hoc test was used to analyze the data. p<0.05 was considered as a significant difference. Data are expressed as mean±standard error.
Results
Chronic caffeine treatment attenuates vascular injury-induced neointimal hyperplasia
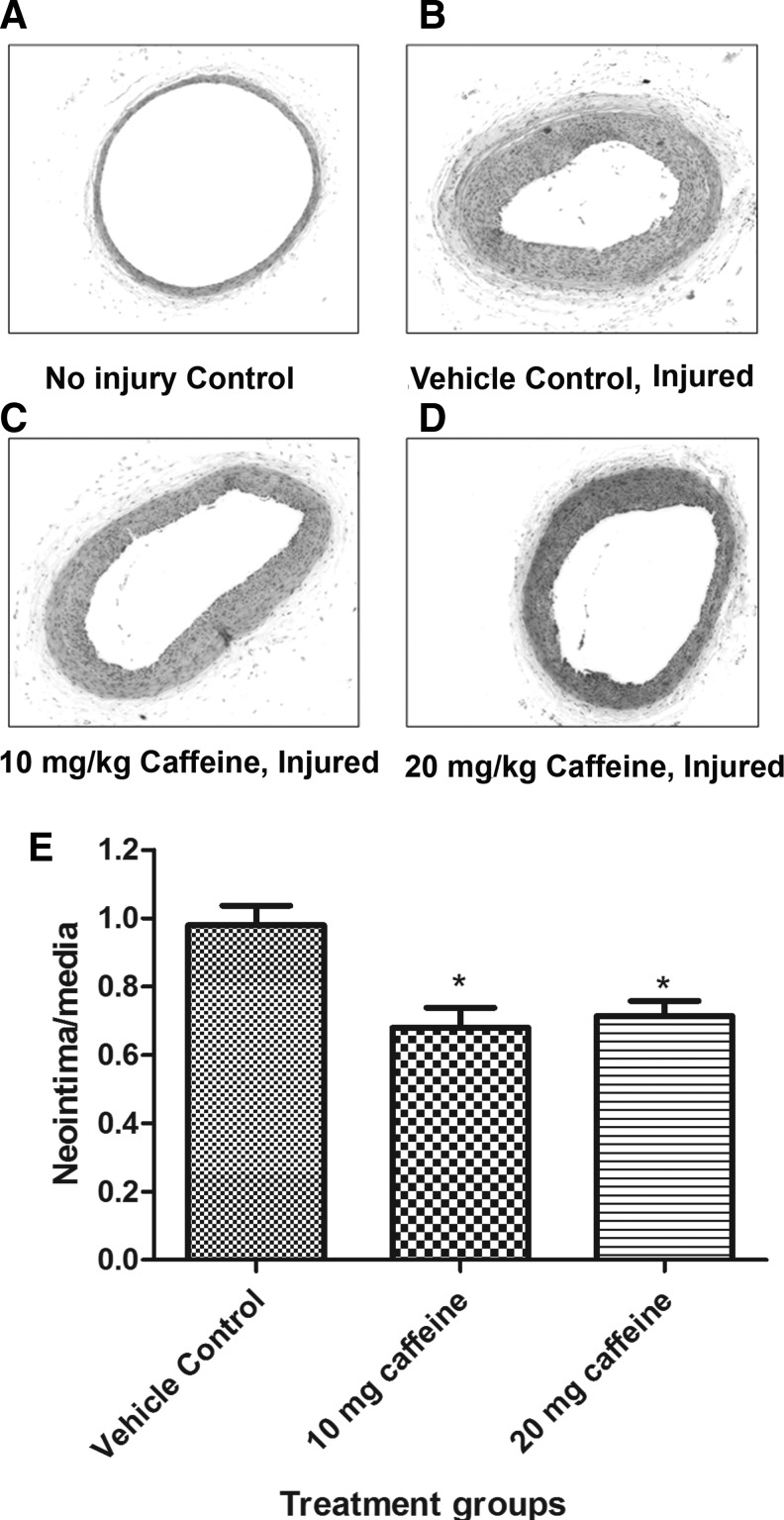
As shown in Figure 1, 2 weeks after injury, rats that received 10 or 20 mg/kg caffeine showed an attenuation in the ratio of neointima to media by 28% and 24%, respectively, as compared to control, p=0.034. The control (0 mg/kg caffeine), the 10 mg/kg caffeine and the 20 mg/kg caffeine groups had average neointima to media ratios of 0.94, 0.68, and 0.72, respectively. No significant difference was observed between 10 and 20 mg/kg caffeine treatment groups.
FIG. 1.
Effect of caffeine on the neointima-media ratio of the common carotid artery. Rats were treated with vehicle (control) or caffeine 2 weeks before and after vascular injury. Carotid arteries were collected, fixed, sectioned, and stained with hematoxylin and eosin. Hematoxylin and eosin-stained cross sections (×40) from control uninjured (A), injured without caffeine treatment (B), injured with 10 mg/kg caffeine treatment (C), and injured with 20 mg/kg caffeine treatment (D). (E) Graph shows the values of mean±SEM. *p<0.05 when compared to the untreated injured group; n=10 per group.
Chronic treatment with caffeine suppresses FBS-induced VSMC migration in a dose-independent manner
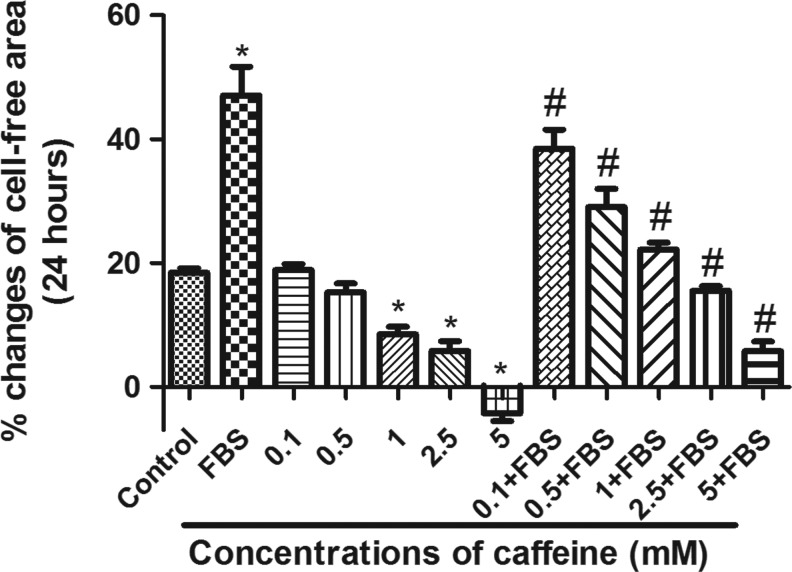
Vascular injury-induced neointimal hyperplasia is characterized by the accumulation of intimal smooth muscle cells (SMC) as the result of enhanced VSMC migration and proliferation,23,24 which is triggered by inflammatory cytokines and growth factors released following vascular injury. Inhibition of migration and proliferation of VSMCs after vascular injury has been considered as a major approach to suppress angioplasty-induced vascular restenosis in both research and clinical practice.25,26 Therefore, our next experiments were designed to test if the effect of caffeine on vascular injury-induced neointimal hyperplasia occurs through inhibition of VSMC migration. As shown in Figure 2, pretreatment with caffeine for 24 hours attenuated FBS-induced VSMC migration in a dose dependent manner. Pretreatment with 0.1, 0.5, 1.0, 2.5, and 5 mM of caffeine, respectively, caused inhibition of 29.95%, 62.9%, 86.87%, 110.13%, and 144.08% in FBS-stimulated VSMC migration as compared to FBS treatment alone (expressed as the percentage decrease in cell-free area after 24 hours, p<0.05). High concentrations of caffeine (5 and 10 mM) induced significant cell death (data not shown). Therefore, 1.0 mM of caffeine was used for the subsequent VSMC proliferation studies as this concentration produced significant inhibition of FBS-induced VSMC migration without causing cell death.
FIG. 2.
Dose–response effect of caffeine on fetal bovine serum (FBS)-stimulated vascular smooth muscle cell (VSMC) migration. Cells were pretreated with different concentrations of caffeine in a serum-free medium for 24 hours followed by stimulation with 1.25% FBS in the presence or absence of same concentrations of caffeine for 24 hours. Cell migration was measured before and after FBS treatment. The percentage of changes in the cell-free area was compared between different groups. Values are mean±SEM. *p<0.05 when compared to control (con). #p<0.05 when compared to FBS alone; n=4.
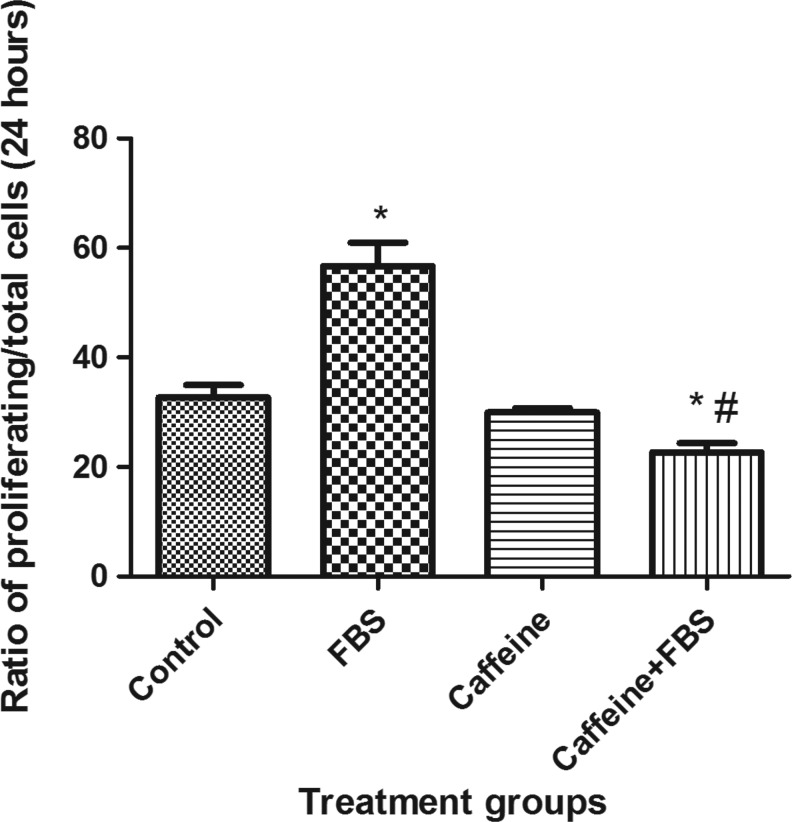
Chronic treatment with caffeine suppressed FBS-induced VSMC proliferation
Our next experiments were designed to test if inhibition of VSMC proliferation also contributes to the effect of caffeine on vascular injury-induced neointimal hyperplasia. As shown in Figures 3 and 4, pretreatment with 1 mM of caffeine completely abolished nonstimulated and FBS-induced VSMC proliferation.
FIG. 3.
Effect of caffeine on FBS-induced VSMC proliferation. Cells were pretreated with 1 mM caffeine in a serum-free medium for 24 hours followed by stimulation with 1.25% FBS in the presence or absence of the same concentration of caffeine for 24 hours. The ratio of cells labeled with 5-bromo-2′-deoxyuridine (BrdU) to the cells labeled with DRAQ5 was compared between different groups. Values are mean±SEM. *p<0.05 when compared to control. #p<0.05 when compared to FBS alone; n=4.
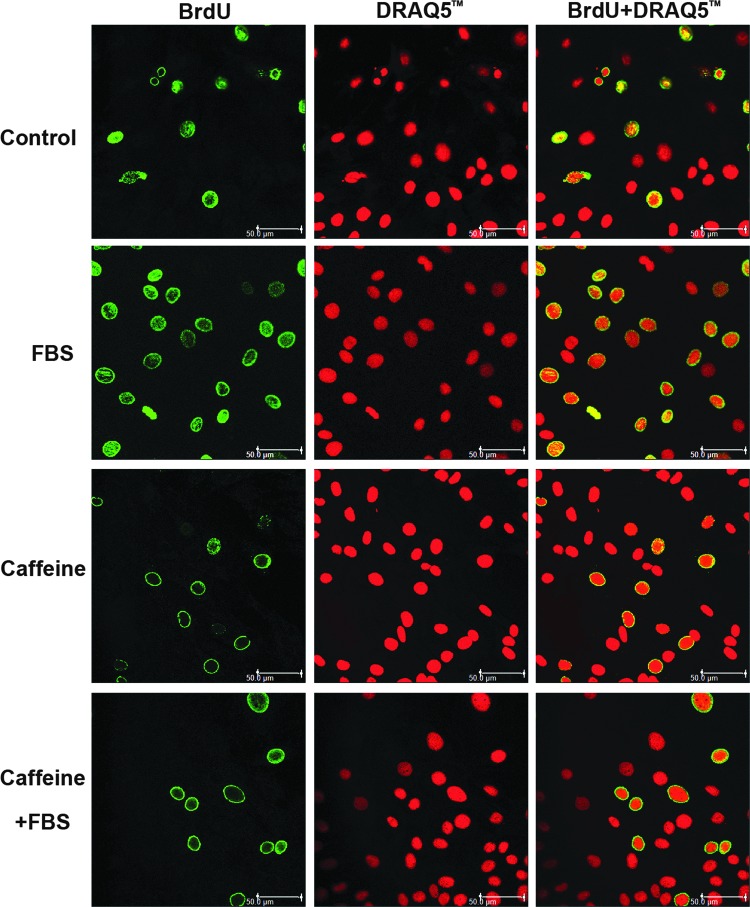
FIG. 4.
Representative confocal microscopy images from different treatment groups.
Discussion
This is the first study to investigate the direct effects of chronic caffeine use on vascular injury-induced neointimal hyperplasia. Our results indicate that chronic caffeine administration to rats (through oral gavage) at either 10 or 20 mg/kg per day suppresses vascular injury-induced neointimal hyperplasia. VSMCs treated with caffeine showed significant inhibition of FBS-induced VSMC migration. The attenuation of FBS-stimulated VSMC migration was dose dependent. Pretreatment with 1 mM of caffeine completely blocked FBS-induced VSMC proliferation.
Mercer et al. recently found that chronic treatment (14 weeks) with a high concentration of caffeine (400 mg/kg) significantly reduced DNA damage and reactive oxygen species generation in nonfat-fed ApoE−/− mice and that the same concentration of caffeine for 6 to 20 weeks of treatment decreased high-fat-diet-induced atherosclerotic plaque area in a lipid- and VSMC-independent manner.17 Our results showed that rats that received 10 and 20 mg/kg of caffeine for total of 4 weeks exhibited a significant reduction in injury-induced intima thickening compared to untreated rats. Although the concentrations we used in our study were much lower and the duration of the treatment was much shorter when compared to those used by Mercer et al.,17 the inhibition of vascular injury-induced neointimal hyperplasia was pronounced. It should be noted that, in our experiments, caffeine was administered through oral gavage instead of being given in drinking water as done by Mercer et al. This route avoided the variation of caffeine amount ingested by the animals and ensured the designated amount of caffeine was received by each rat. Thus, our results suggest that even with caffeine concentrations of 10 and 20 mg/kg for 4 weeks, vascular injury-induced neointimal hyperplasia was significantly reduced, suggesting that high concentrations of caffeine may prevent angioplasty-induced vascular restenosis.
Vascular injury induces cells in the media and intima to enter the cell cycle, resulting in proliferation, migration, and synthesis of extracellular matrix and collagen similar to that seen in neointimal hyperplasia.27 To elucidate the mechanism responsible for the attenuation of vascular injury-induced neointimal hyperplasia due to chronic caffeine consumption, we examined the effect of caffeine on VSMC migration and proliferation, the key contributors to the intimal thickening observed in restenosis.5 We found that FBS-induced VSMC migration was substantially reduced following pretreatment with caffeine. This inhibition was dose dependent. Chronic treatment of VSMCs with 1 mM of caffeine completely blocked FBS-induced VSMC proliferation, indicating that inhibition of VSMC migration and proliferation by chronic treatment with caffeine may contribute to the attenuation of vascular injury-induced neointimal hyperplasia. Further studies need to be conducted to address the underlying mechanisms.
In summary, our results showed that chronic caffeine consumption suppressed vascular injury-induced neointimal hyperplasia in rats. These results are consistent with our in vitro studies in which we found that pretreatment with caffeine inhibited FBS-stimulated VSMC migration and proliferation, suggesting that chronic caffeine treatment attenuates vascular injury-induced neointimal hyperplasia by inhibiting VSMC migration and proliferation. Chronic consumption of caffeine may be beneficial in reducing the risk of restenosis after arterial angioplasty.
Acknowledgments
This work was supported by funding from the Graduate Program Committee of the Kirksville College of Osteopathic Medicine, A.T. Still University of Health Sciences. The authors would like to thank Dr. Keith Elmslie for his constructive criticism on the manuscript and experimental design, Nicholas Quinn and Alex Bolton for their assistance during these experiments, and Lena Jia for serving as a blinded observer. We are grateful for the technical contributions provided by Michael Cramberg, Stephanie Smith, and Erica Stanley.
Author Disclosure Statement
The authors have nothing to disclose. No competing financial interests exist.
References
- 1.Schillinger M, Minar E. Restenosis after percutaneous angioplasty: the role of vascular inflammation. Vasc Health Risk Manag. 2005;1:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695 [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175 [PMC free article] [PubMed] [Google Scholar]
- 4.Serrano CV, Jr., Ramires JA, Venturinelli M, Arie S, D'Amico E, Zweier JL, Pileggi F, da Luz PL. Coronary angioplasty results in leukocyte and platelet activation with adhesion molecule expression. Evidence of inflammatory responses in coronary angioplasty. J Am Coll Cardiol. 1997;29:1276–1283 [DOI] [PubMed] [Google Scholar]
- 5.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varani K, Portaluppi F, Gessi S, Merighi S, Ongini E, Belardinelli L, Borea PA. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors: functional and biochemical aspects. Circulation. 2000;102:285–289 [DOI] [PubMed] [Google Scholar]
- 7.Echeverri D, Montes FR, Cabrera M, Galan A, Prieto A. Caffeine's vascular mechanisms of action. Int J Vasc Med. 2010;2010:834060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaCroix AZ, Mead LA, Liang KY, Thomas CB, Pearson TA. Coffee consumption and the incidence of coronary heart disease. N Engl J Med. 1986;315:977–982 [DOI] [PubMed] [Google Scholar]
- 9.Happonen P, Voutilainen S, Salonen JT. Coffee drinking is dose-dependently related to the risk of acute coronary events in middle-aged men. J Nutr. 2004;134:2381–2386 [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg L, Palmer JR, Kelly JP, Kaufman DW, Shapiro S. Coffee drinking and nonfatal myocardial infarction in men under 55 years of age. Am J Epidemiol. 1988;128:570–578 [DOI] [PubMed] [Google Scholar]
- 11.Sofi F, Conti AA, Gori AM, Eliana Luisi ML, Casini A, Abbate R, Gensini GF. Coffee consumption and risk of coronary heart disease: a meta-analysis. Nutr Metab Cardiovasc Dis. 2007;17:209–223 [DOI] [PubMed] [Google Scholar]
- 12.Cornelis MC, El-Sohemy A. Coffee, caffeine, and coronary heart disease. Curr Opin Lipidol. 2007;18:13–19 [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Garcia E, van Dam RM, Willett WC, Rimm EB, Manson JE, Stampfer MJ, Rexrode KM, Hu FB. Coffee consumption and coronary heart disease in men and women: a prospective cohort study. Circulation. 2006;113:2045–2053 [DOI] [PubMed] [Google Scholar]
- 14.Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382 [PubMed] [Google Scholar]
- 15.Sinn B, Tallen G, Schroeder G, Grassl B, Schulze J, Budach V, Tinhofer I. Caffeine confers radiosensitization of PTEN-deficient malignant glioma cells by enhancing ionizing radiation-induced G1 arrest and negatively regulating Akt phosphorylation. Mol Cancer Ther. 2010;9:480–488 [DOI] [PubMed] [Google Scholar]
- 16.Bode AM, Dong Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett. 2007;247:26–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercer JR, Gray K, Figg N, Kumar S, Bennett MR. The methyl xanthine caffeine inhibits DNA damage signaling and reactive species and reduces atherosclerosis in ApoE(-/-) mice. Arterioscler Thromb Vasc Biol. 2012;32:2461–2467 [DOI] [PubMed] [Google Scholar]
- 18.Varani K, Portaluppi F, Gessi S, Merighi S, Vincenzi F, Cattabriga E, Dalpiaz A, Bortolotti F, Belardinelli L, Borea PA. Caffeine intake induces an alteration in human neutrophil A2A adenosine receptors. Cell Mol Life Sci. 2005;62:2350–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang R, Wang J, Chen Y, Sun Z, Wang R, Dai Y. Effect of caffeine on erectile function via up-regulating cavernous cyclic guanosine monophosphate in diabetic rats. J Androl. 2008;29:586–591 [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Yang J, Jennings LK. Attenuation of neointima formation through the inhibition of DNA repair enzyme PARP-1 in balloon-injured rat carotid artery. Am J Physiol Heart Circ Physiol. 2004;287:H659–H666 [DOI] [PubMed] [Google Scholar]
- 21.Chang Y, Ceacareanu B, Dixit M, Sreejayan N, Hassid A. Nitric oxide-induced motility in aortic smooth muscle cells: role of protein tyrosine phosphatase SHP-2 and GTP-binding protein Rho. Circ Res. 2002;91:390–397 [DOI] [PubMed] [Google Scholar]
- 22.Sasaki K, Adachi S, Yamamoto T, Murakami T, Tanaka K, Takahashi M. Effects of denaturation with HCl on the immunological staining of bromodeoxyuridine incorporated into DNA. Cytometry. 1988;9:93–96 [DOI] [PubMed] [Google Scholar]
- 23.Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992;86:723–729 [DOI] [PubMed] [Google Scholar]
- 24.Pickering JG, Weir L, Jekanowski J, Kearney MA, Isner JM. Proliferative activity in peripheral and coronary atherosclerotic plaque among patients undergoing percutaneous revascularization. J Clin Invest. 1993;91:1469–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang Y, Ceacareanu B, Zhuang D, Zhang C, Pu Q, Ceacareanu AC, Hassid A. Counter-regulatory function of protein tyrosine phosphatase 1B in platelet-derived growth factor- or fibroblast growth factor-induced motility and proliferation of cultured smooth muscle cells and in neointima formation. Arterioscler Thromb Vasc Biol. 2006;26:501–507 [DOI] [PubMed] [Google Scholar]
- 26.Seedial SM, Ghosh S, Saunders RS, Suwanabol PA, Shi X, Liu B, Kent KC. Local drug delivery to prevent restenosis. J Vasc Surg. 2013;57:1403–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett MR. In-stent stenosis: pathology and implications for the development of drug eluting stents. Heart. 2003;89:218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]