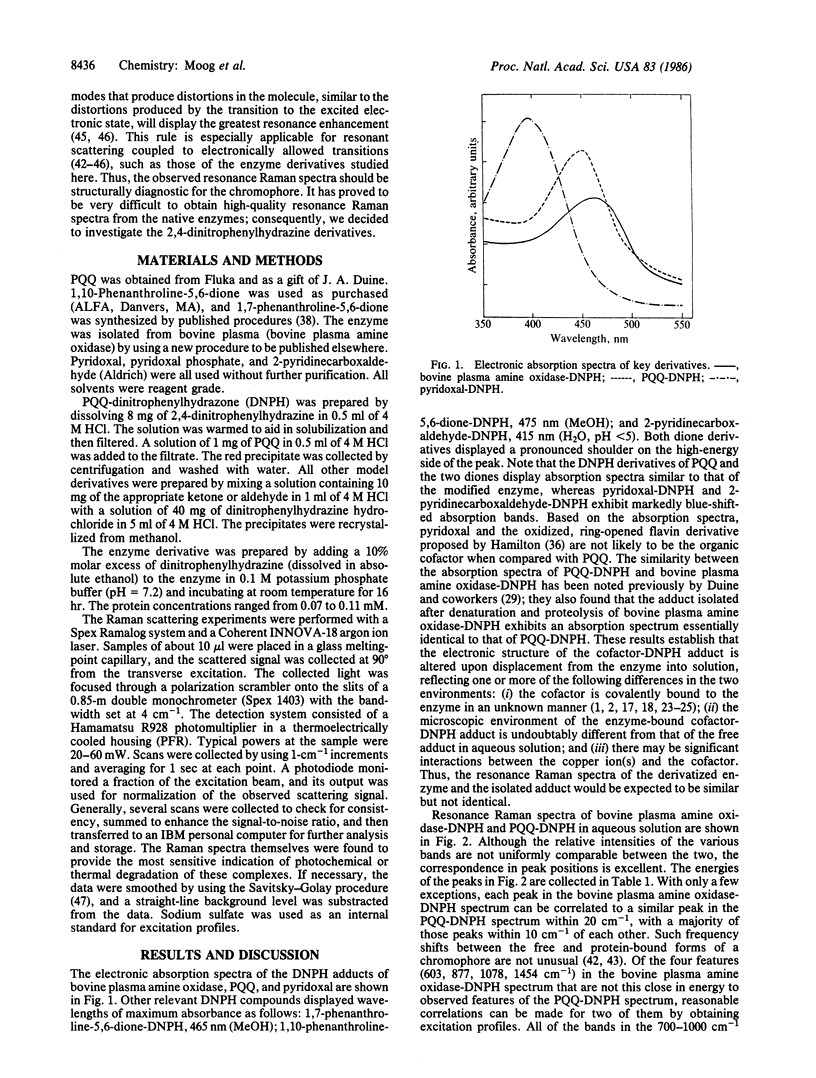
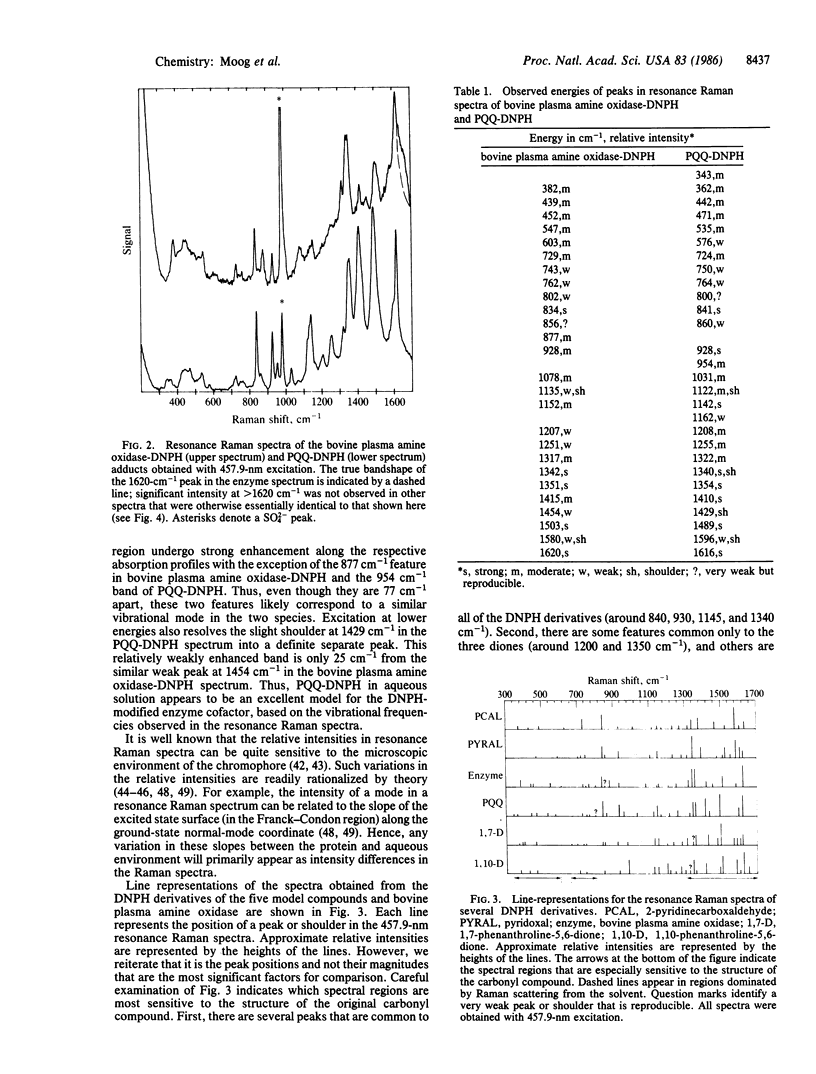
Abstract
Resonance Raman spectra of the 2,4-dinitrophenylhydrazine derivatives of bovine plasma amine oxidase [amine:oxygen oxidoreductase (deaminating) (copper-containing), EC 1.4.3.6] have been measured. Detailed comparisons to the spectra of the corresponding derivatives of methoxatin (pyrroloquinolinequinone), pyridoxal, and other aldehydes and diones provide further evidence that covalently bound methoxatin or a closely similar derivative is the organic cofactor in copper-containing amine oxidases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrò A. F., Guerrieri P., Costa M. T., Mondovì B. On the nature of chromophore in pig kidney diamine oxidase. Eur J Biochem. 1977 Apr 15;74(3):435–440. doi: 10.1111/j.1432-1033.1977.tb11409.x. [DOI] [PubMed] [Google Scholar]
- Barker R., Boden N., Cayley G., Charlton S. C., Henson R., Holmes M. C., Kelly I. D., Knowles P. F. Properties of cupric ions in benzylamine oxidase from pig plasma as studied by magnetic-resonance and kinetic methods. Biochem J. 1979 Jan 1;177(1):289–302. doi: 10.1042/bj1770289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K. A., Abeles R. H. Mechanism of action of plasma amine oxidase products released under anaerobic conditions. Biochemistry. 1980 Jul 8;19(14):3186–3189. doi: 10.1021/bi00555a012. [DOI] [PubMed] [Google Scholar]
- Buffoni F., Della Corte L. Pig plasma benzylamine oxidase. Adv Biochem Psychopharmacol. 1972;5:133–149. [PubMed] [Google Scholar]
- Eckert T. S., Bruice T. C., Gainor J. A., Weinreb S. M. Some electrochemical and chemical properties of methoxatin and analogous quinoquinones. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2533–2536. doi: 10.1073/pnas.79.8.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk M. C. Stoichiometry of phenylhydrazine inactivation of pig plasma amine oxidase. Biochemistry. 1983 Aug 2;22(16):3740–3745. doi: 10.1021/bi00285a004. [DOI] [PubMed] [Google Scholar]
- Hill J. M. The inactivation of pea-seedling diamine oxidase by peroxidase and 1,5-diaminopentane. Biochem J. 1967 Sep;104(3):1048–1055. doi: 10.1042/bj1041048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa A. Y., Tsuboi M. Molecular geometry in an excited electronic state and a preresonance Raman effect. Science. 1975 Apr 25;188(4186):359–361. doi: 10.1126/science.188.4186.359. [DOI] [PubMed] [Google Scholar]
- Inamasu M., Yasunobu K. T. Cofactor investigation of bovine plasma amine oxidase. NaBH4 reduction of enzyme-substrate mixture. J Biol Chem. 1974 Aug 25;249(16):5265–5268. [PubMed] [Google Scholar]
- Lindström A., Pettersson G. Active-site titration of pig-plasma benzylamine oxidase. Eur J Biochem. 1978 Feb 1;83(1):131–135. doi: 10.1111/j.1432-1033.1978.tb12076.x. [DOI] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Mondovi B., Rotilio G., Agro A. F., Vallogini M. P., Malmström B. G., Antonini E. Copper reduction by substrate in diamine oxidase. FEBS Lett. 1969 Jan;2(3):182–184. doi: 10.1016/0014-5793(69)80013-x. [DOI] [PubMed] [Google Scholar]
- Nylen U., Szybek P. Kinetics and other characteristics of diamine oxidase of pea seedlings. Acta Chem Scand B. 1974;28(10):1153–1160. doi: 10.3891/acta.chem.scand.28b-1153. [DOI] [PubMed] [Google Scholar]
- Olsson B., Olsson J., Pettersson G. Effects on enzyme activity of ligand-binding to copper in pig-plasma benzylamine oxidase. Eur J Biochem. 1978 Jun 1;87(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12345.x. [DOI] [PubMed] [Google Scholar]
- Olsson B., Olsson J., Pettersson G. Stopped-flow spectrophotometric characterization of enzymic reaction intermediates in the anaerobic reduction of pig-plasma benzylamine oxidase by amine substrates. Eur J Biochem. 1976 Dec 11;71(2):375–382. doi: 10.1111/j.1432-1033.1976.tb11124.x. [DOI] [PubMed] [Google Scholar]
- Rinaldi A., Floris G., Sabatini S., Finazzi-Agrò A., Giartosio A., Rotilio G., Mondovì B. Reaction of beef plasma and lentil seedlings Cu-amine oxidases with phenylhydrazine. Biochem Biophys Res Commun. 1983 Sep 30;115(3):841–848. doi: 10.1016/s0006-291x(83)80011-4. [DOI] [PubMed] [Google Scholar]
- Suva R. H., Abeles R. H. Studies on the mechanism of action of plasma amine oxidase. Biochemistry. 1978 Aug 22;17(17):3538–3545. doi: 10.1021/bi00610a018. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sakurai T., Nakahara A., Manabe T., Okuyama T. Effect of metal substitution on the chromophore of bovine serum amine oxidase. Biochemistry. 1983 Mar 29;22(7):1630–1635. doi: 10.1021/bi00276a016. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sakurai T., Nakahara A., Oda O., Manabe T., Okuyama T. Spectroscopic aspects of copper binding site in bovine serum amine oxidase. FEBS Lett. 1980 Jul 11;116(1):17–20. doi: 10.1016/0014-5793(80)80519-9. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Smith R. A., Inamasu M., Yasunobu K. T. Recent investigations on the prosthetic group of beef plasma amine oxidase. Adv Biochem Psychopharmacol. 1972;5:107–117. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. MONOAMINE OXIDASE. IV. NATURE OF THE SECOND PROSTHETIC GROUP OF PLASMA MONOAMINE OXIDASE. J Biol Chem. 1963 Aug;238:2669–2675. [PubMed] [Google Scholar]



