Abstract
Aims: Vitamin C (ascorbic acid) is thought to enhance immune function, but the mechanisms involved are obscure. We utilized an in vitro model of T-cell maturation to evaluate the role of ascorbic acid in lymphocyte development. Results: Ascorbic acid was essential for the developmental progression of mouse bone marrow-derived progenitor cells to functional T-lymphocytes in vitro and also played a role in vivo. Ascorbate-mediated enhancement of T-cell development was lymphoid cell-intrinsic and independent of T-cell receptor (TCR) rearrangement. Analysis of TCR rearrangements demonstrated that ascorbic acid enhanced the selection of functional TCRαβ after the stage of β-selection. Genes encoding the coreceptor CD8 as well as the kinase ZAP70 were upregulated by ascorbic acid. Pharmacologic inhibition of methylation marks on DNA and histones enhanced ascorbate-mediated differentiation, suggesting an epigenetic mechanism of Cd8 gene regulation via active demethylation by ascorbate-dependent Fe2+ and 2-oxoglutarate-dependent dioxygenases. Innovation: We speculate that one aspect of gene regulation mediated by ascorbate occurs at the level of chromatin demethylation, mediated by Jumonji C (JmjC) domain enzymes that are known to be reliant upon ascorbate as a cofactor. JmjC domain enzymes are also known to regulate transcription factor activity. These two mechanisms are likely to play key roles in the modulation of immune development and function by ascorbic acid. Conclusion: Our results provide strong experimental evidence supporting a role for ascorbic acid in T-cell maturation as well as insight into the mechanism of ascorbate-mediated enhancement of immune function. Antioxid. Redox Signal. 19, 2054–2067.
Introduction
The process of lymphocyte development is dependent upon specific recombination of genetic loci encoding the antigen-specific receptors that characterize both B-lymphocytes (cell surface immunoglobulin) and T-lymphocytes (T-cell receptor [TCR]). For the T-cell lineage, these specific gene recombination events occur in the thymus beginning at a stage of development just before the expression of the canonical T-cell surface antigens CD4 and CD8, which are coexpressed by the majority of thymocytes at a stage of development termed double positive (DP). TCRαβ receptors newly generated through genomic recombination of TCRα and TCRβ genes interact with thymic histocompatibility and self-antigens to select lymphocytes that will function normally with respect to recognition of foreign antigens. A series of well-defined developmental steps preceding the DP stage are defined as double-negative stages 1–4 (DN1–DN4) based on expression of the adhesion molecule CD44 and the cytokine receptor CD25. Selection of functional TCRαβ proceeds through a process of interactions between TCRαβ and thymic stromal cells (15). The OP9-DL1 model of T-cell development (Supplementary Video) recapitulates thymic maturation of T-cells in vitro (27). This culture system has been shown to efficiently promote maturation of T-cells from fetal liver-derived progenitor cells, but differentiation of mature TCRαβ+ cells from adult-derived hematopoietic progenitor cells in OP9-DL1 cultures is inefficient for unknown reasons (17).
Innovation.
Vitamin C deficiency results in a variety of clinical problems, including immunodeficiency. The mechanistic basis for immunodeficiency associated with vitamin C deficiency has not been adequately explored due to a lack of tractable model systems. We demonstrate that an innovative in vitro model of T-cell maturation depends on vitamin C, and further show that epigenetic regulation of gene expression is one likely mechanism by which vitamin C mediates immune effects.
Vitamin C (ascorbic acid) is widely regarded as an enhancer of immune function, although the mechanisms involved are largely undefined. Antioxidant activity is the most obvious potential mechanism, particularly since immune responses proceed more efficiently in reducing environments (6,40). Additional possibilities for mechanistic roles of ascorbic acid in promoting the immune response include modulation of phosphatase activity (31,41), post-translational activation of AP-1 transcription factors (1), and epigenetic regulation of gene expression (8). A number of the biological activities of ascorbic acid trace to its role as a cofactor required for optimal activity of ferrous iron- and 2-oxoglutarate (Fe2+ and 2-OG)-dependent dioxygenases, which have been implicated in regulating a wide range of processes, including gene regulation, nucleotide metabolism, and oxidative repair of DNA (30). The Fe2+- and 2-OG-dependent dioxygenase enzyme family includes members with substrates that include procollagen, histones, neurotransmitters, and transcription factors. However, establishing a mechanistic basis for the role of ascorbic acid in the immune response is complicated by the lack of a model system in which pronounced effects of ascorbate on immune function can be observed and quantitated. In addition, in vivo deficiencies in ascorbate result in serious physiological problems owing to the requirement for ascorbate as a cofactor for the prolyl hydroxylase enzymes involved in collagen biosynthesis and the integrity of blood vessels. It is therefore difficult to separate primary effects on the immune system from more systemic problems resulting from ascorbate deficiency that may influence the functioning of the immune system indirectly.
We have defined in vitro T-cell maturation as a robust model for modulatory effects of ascorbic acid on the developing immune system. Our results indicate that ascorbate plays a key role in modulating expression of genes encoding accessory molecules that are involved in signal transduction through TCRαβ, resulting in positive selection of functional, adult-type T-cells. We also demonstrate a role for ascorbate in modulating T-cell maturation in vivo. Our studies indicate an important role for ascorbic acid in regulating both development and function of T-cells.
Results
Cell culture medium formulations determine successful T-cell maturation in OP9-DL1 cultures
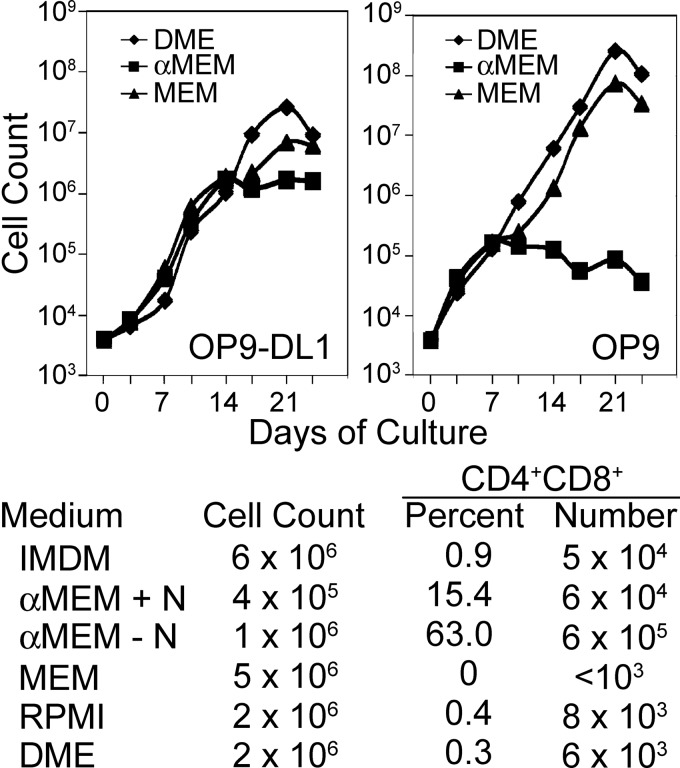
Hematopoietic progenitor cells obtained from fetal liver tissue readily differentiate as T-cells in OP9-DL1 cultures, while adult bone marrow-derived progenitors do not (17). Interestingly, we observed robust differentiation of CD4/CD8 DP cells from adult bone marrow-derived progenitors in the alpha modification of minimal essential medium (αMEM) culture medium freshly prepared from powder (36), but not in the identical medium formulation purchased in the liquid form. Of note, comparison of OP9 and OP9-DL1 cocultures established with adult-derived hematopoietic stem/progenitor cells in various cell culture medium formulations revealed that the basal formulation of minimal essential medium (MEM) and Dulbecco's modified Eagle's (DME) medium were superior to αMEM with respect to maintaining lymphoid cellular expansion (Fig. 1). In contrast, only αMEM among medium formulations tested supported robust differentiation of DP thymocytes from adult bone marrow-derived progenitor cells, and a formulation of αMEM lacking nucleosides was superior to the standard αMEM formulation with respect to both cellular expansion and differentiation of DP cells (Fig. 1). An apparent arrest in lymphocyte cellular expansion occurred at approximately the time of commitment to the lymphoid lineage in cultures maintained in αMEM, corresponding to about 2 weeks of culture under T-cell conditions and 1 week under culture in B-cell conditions (Fig. 1).
FIG. 1.
Basal cell culture medium formulations profoundly influence T-cell maturation in vitro. Top Panels: Cellular expansion of lymphocyte progenitor cells cultured in T-cell (OP9-DL1) or B-cell (OP9) conditions in the indicated basal culture medium. All cultures were supplemented with 1 ng/ml interleukin (IL)-7 and 5 ng/ml fms-like tyrosine kinase-3 ligand (Flt3L). Lower panel: Effects of basal medium formulations on T-lymphocyte progenitor expansion and differentiation in OP9-DL1 cocultures as shown in the top left panel. The alpha modification of minimal essential medium (αMEM)+N medium is a formulation containing nucleosides, while the αMEM−N formulation lacks nucleosides. All cultures were initiated by seeding 2×103 fluorescence-activated cell-sorting (FACS)-sorted LSK-Thy-1.1-neg cells onto a feeder layer of stromal cells and contained 1 ng/ml IL-7 and 5 ng/ml Flt3L with otherwise identical additives as outlined in the Materials and Methods section. Cell numbers were determined by hemocytometer counting at each passage. Cultures were passaged every 3–4 days, and appropriate dilutions were made to maintain a cell density of <5×105 cells/ml. The cell counts were corrected by a factor corresponding to the product of these dilutions. The percentage of CD4+CD8+ DP cells was determined by FACS analysis after 14 days of culture and was converted to absolute numbers based on the cell counts.
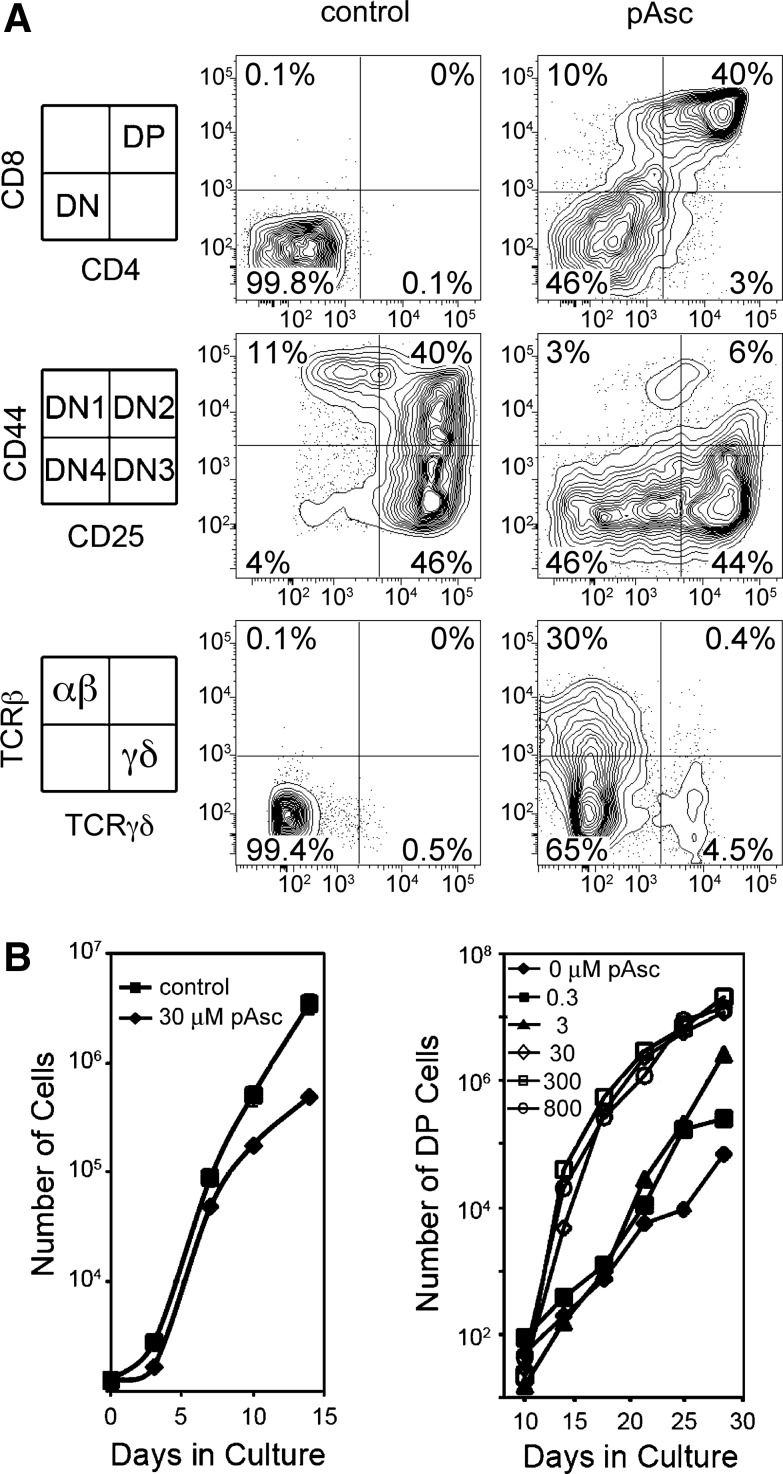
To establish what components of αMEM were critical for differentiation of DP thymocytes from mouse bone marrow-derived progenitor cells, we compared basal culture medium formulations to determine that αMEM contains five unique components (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/ars). By systematically supplementing each of these components into MEM, we found that ascorbic acid alone could account for the unique ability of αMEM to support TCRαβ+ DP T-cell differentiation in OP9-DL1 cultures. Ascorbic acid is labile in solution, which accounts for the lack of T-cell differentiation in cultures established in αMEM purchased in liquid form. L-Ascorbic acid-2-phosphate (pAsc) is a phosphorylated version of ascorbic acid that has been shown to resist spontaneous oxidation, resulting in enhanced stability relative to ascorbic acid (13). We found that both ascorbic acid and pAsc promoted T-cell maturation in a similar concentration range, and that pAsc was less toxic at high doses compared to ascorbic acid. As shown in Figure 2, T-cell development proceeded in a markedly different manner in the presence or absence of pAsc. Few lymphocytes in cultures maintained without pAsc progressed to the DP stage after 17 days of culture, and lymphoid developmental intermediates accumulated at the DN2 and DN3 stages. In contrast, maintaining cultures in pAsc resulted in progression through the DN3 stage to the DN4 and DP stages by 17–21 days of culture, and a relative increase in the number of cells expressing cell surface TCRαβ (Fig. 2A). A dose–response to pAsc was clearly apparent (Fig. 2B), with 30 μM producing a maximal effect, while doses as low as 0.3 μM were effective at promoting T-cell differentiation relative to control cultures. Plasma levels of ascorbate in most species range from 15 to 40 μM; however, lymphocytes accumulate ascorbate through the high-affinity sodium-dependent vitamin C transporter 2 (Svct2, encoded by Slc23a2) and normally achieve intracellular concentrations in the millimolar range (18). Supplementation of pAsc to high concentrations (800 μM) produced results equivalent to those seen at 30 μM (Fig. 2B). Also apparent in Figure 2B is a decrease in lymphocyte cellular expansion in cultures established and maintained in pAsc at a dose of 30 μM relative to control cultures lacking pAsc. We observed a preferential outgrowth of cells expressing CD8 in the absence of CD4 (CD8 single-positive cells, CD8SP) compared to CD4 single-positive cells (CD4SP) in this and other experiments. The magnitude of this observation varied between experiments and was more pronounced after longer times in culture. Previous studies have established that OP9-DL1 cultures favor the CD8SP subset, possibly due to persistent Notch stimulation, high concentrations of IL-7, and/or the absence of major histocompatibility complex (MHC) class II molecule expression by OP9-DL1 stromal cells (28).
FIG. 2.
Modulation of T-cell maturation by I-ascorbic acid 2-phosphate (pAsc). (A) Cultures maintained for 17 days with 5 ng/ml each of Flt3L and IL-7 in the presence or absence of pAsc (800 μM) as indicated were evaluated for the expression of cell surface antigens by flow cytometry. (B) Cultures established as above in the presence of the indicated concentrations of pAsc were evaluated for total cellular expansion and for the number of double-positive (DP) cells.
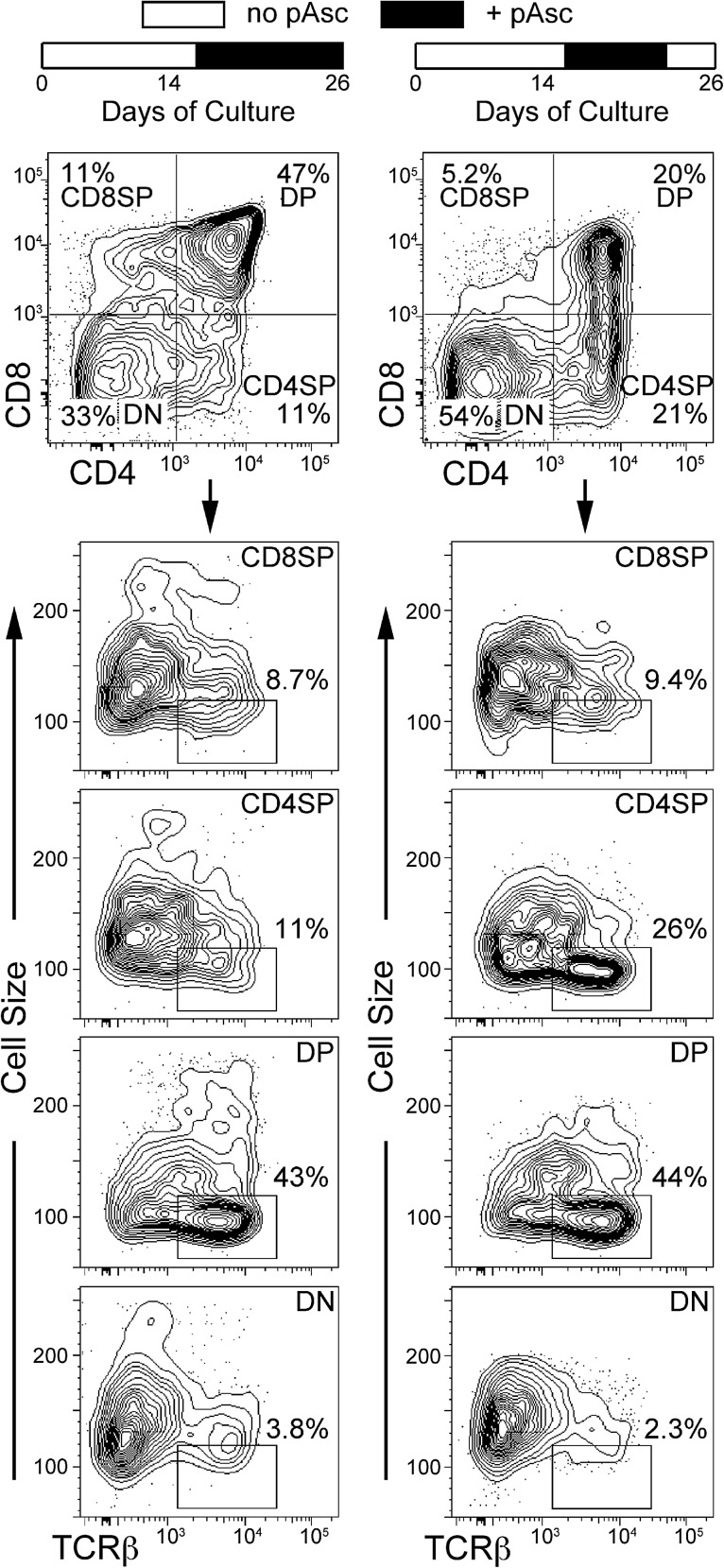
To optimize both lymphocyte expansion as well as differentiation, we established cultures with MEM in the absence of pAsc and allowed them to grow for several weeks before inducing differentiation by addition of pAsc. We found that addition of pAsc after 14 days of culture promoted robust differentiation of DP cells over the following 2 weeks (Fig. 3). Figure 3 also shows that withdrawal of pAsc for 3 days before analysis at day 26 resulted in loss of CD8, but not CD4 expression. This result suggests that pAsc plays a role in regulating CD8 expression. We found that cultures supplemented with 30 μM pAsc as late as day 33 after initiation retained the ability to progress to the DP stage of development (data not shown). As noted by others (17), we observed that decreased concentrations of IL-7 also promoted DP maturation. However, addition of pAsc to the cultures promoted differentiation of TCRαβ+ DP cells regardless of the concentration of IL-7, and maintaining IL-7 at 5 ng/ml resulted in greater lymphoid cellular expansion relative to cultures maintained at 1 ng/ml IL-7.
FIG. 3.
Addition of pAsc at later stages of OP9-DL1 cultures induces T-cell maturation, and the DP population requires continual exposure to pAsc for maintenance. LSK-Thy-1.1-neg cells cultured in an MEM with 1 ng/ml IL-7 and 5 ng/ml Flt3L were maintained for 14 days in the absence of ascorbate, with passages every 3–4 days as described in the Materials and Methods section. Cultures were supplemented with 30 μM pAsc beginning at 14 days. On the final passage at day 23, replicate cultures were established in which pAsc was either included or not for the final 3 days of culture. All cultures were analyzed after 26 days.
The lower panels of Figure 3 illustrate the cell surface expression of TCRαβ by the four subsets of cells defined by CD4 and CD8 in cultures maintained in pAsc from days 14–26 compared to cultures in which pAsc was withdrawn at day 23. Of note, TCRβ staining was detected primarily on small cells in the DP population in cultures maintained in pAsc, while a marked increase in the number of small TCRβ+ CD4SP cells was noted when pAsc was withdrawn for 3 days. This result, along with the decrease in DP frequency shown in the top panels, suggests that the small TCRβ+ CD4SP population that arises after pAsc withdrawal is derived from the small TCRβ+ DP population.
Ascorbate regulates T-cell development in vivo
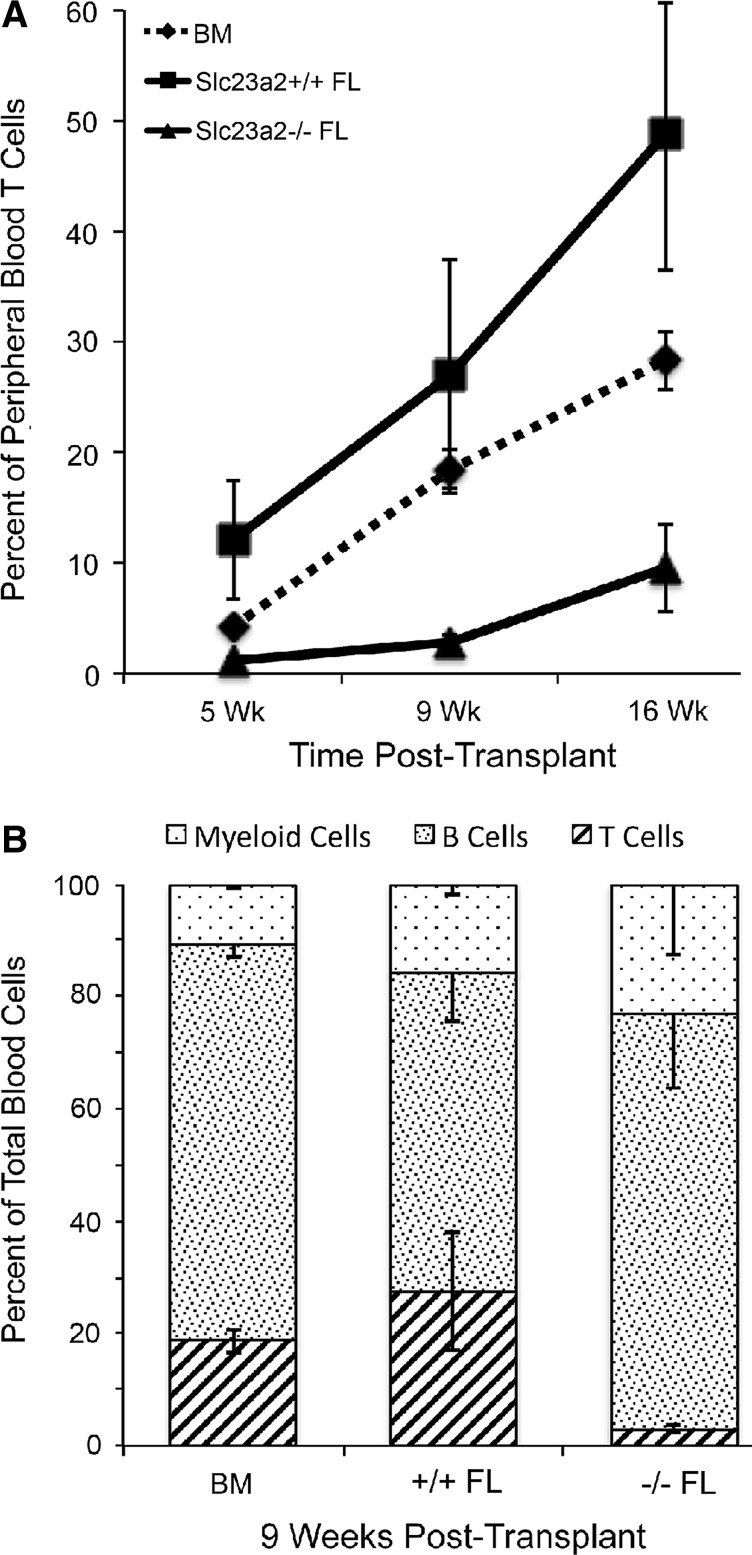
To determine the predictive value of our culture experiments with respect to the biology of T-cell maturation in vivo, we utilized a transplantation model in which fetal liver cells obtained from Slc23a2−/−-targeted mutant mice (32) were transplanted in competition with wild-type C57BL/6 bone marrow cells into lethally irradiated recipient mice. The lack of functional Slc23a2 results in an inability of hematopoietic cells to concentrate ascorbic acid. Previous studies have shown that the splenic content of ascorbic acid in mice transplanted with the Slc23a2−/− fetal liver to be one-third of that in mice transplanted with wild-type fetal liver cells (2). As shown in Figure 4, Slc23a2−/− fetal liver cells exhibited a specific defect in T-cell maturation compared to either control fetal liver or bone marrow cells. This experiment establishes a role for Slc23a2, and by extension, ascorbic acid, in T-cell development in vivo and further implicates intracellular accumulation of ascorbic acid via Slc23a2 in lymphocyte progenitor cells as a central component in lineage choice and efficient T-cell maturation.
FIG. 4.
Fetal liver cells lacking the ability to accumulate ascorbic acid due to targeted mutation of Slc23a2 have defective T-cell maturation when transplanted into irradiated recipients. Fetal liver cells (Ly-5.2) identified as Slc23a2+/+ or Slc23a2−/− by polymerase chain reaction (PCR) were mixed with equal numbers of normal bone marrow cells (Ly-5.1/Ly-5.2) for injection at a dose of 2×106 total cells into four lethally irradiated recipient mice (Ly-5.1). Fetal liver-derived peripheral blood cells were identified by flow cytometry based on Ly-5.1 and Ly-5.2 antibody staining and phenotyped as myeloid, T-cell, or B-cell lineages at the indicated times after transplantation. Panel (A) shows T-lineage engraftment at three time points post-transplantation. Panel (B) shows myeloid, T, and B lineages 9 weeks post-transplantation. BM indicates the lineage contributions by the Ly-5.1/Ly-5.2 bone marrow cells in the same recipient mice, pooling data from both Slc23a2+/+ and Slc23a2−/− FL recipients. At 16 weeks post-transplantation, the frequency of Slc23a2−/− T cells was 20% of that seen with Slc23a2+/+ T cells (p=0.023 by two-tailed t-test). Values are mean±standard error.
The role of ascorbic acid in T-cell maturation is cell intrinsic
To address the question of whether the effect of ascorbate on T-cell maturation was mediated indirectly by effects on the OP9-DL1 stromal cells versus directly by influencing the developing lymphocytes, we introduced two experimental manipulations into the OP9-DL1 model. First, we maintained cultures of OP9-DL1 cells in the presence or absence of 800 μM pAsc for several weeks. Other than an increase in the resistance of OP9-DL1 cells to trypsin when maintained in the presence of pAsc, we observed no changes in the growth rate or morphology of the stromal cells under these two conditions. We then used these cells as stromal monolayers for lymphoid progenitor cells that had been maintained in coculture as before for 14 days in the absence of pAsc. Analysis at day 21 of culture, with one passage at day 17 onto fresh OP9-DL1 cells maintained with or without pAsc, revealed no differentiation to the DP stage under either condition (data not shown). This experiment suggests that the effect of pAsc on T-cell differentiation in OP9-DL1 cultures depends on active accumulation of ascorbate by the developing lymphocytes and not by the stromal cells.
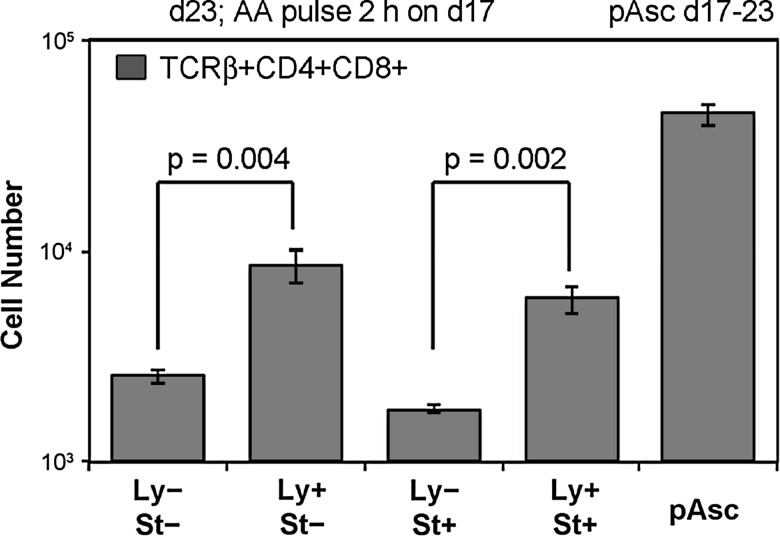
To further evaluate this hypothesis, we maintained lymphoid progenitor cells in OP9-DL1 cultures for 17 days in the absence of ascorbate and harvested the cells with a subsequent plastic adherence step to deplete OP9-DL1 stromal cells. We then incubated these lymphoid progenitor cells for 2 h with or without 250 μM ascorbic acid, washed the cells, and seeded cultures in which either the stromal cells or the lymphoid progenitor cells had been pre-exposed to ascorbic acid. After 6 days, quadruplicate cultures were harvested for cell counts and phenotypic analysis. Cultures with no pre-exposure to ascorbate and cultures incubated in the presence of 250 μM pAsc for the 6-day culture period served as negative and positive controls, respectively. As shown in Figure 5, the frequency and absolute number of TCRαβ+ DP cells increased in cultures in which lymphoid cells were pre-exposed to ascorbic acid and in cultures maintained in pAsc compared to cultures in which no ascorbate pulse was given or in which only the OP9-DL1 stromal cells were exposed to ascorbate. We confirmed this observation in five separate experiments, and consistently found that pre-exposure of the lymphocytes, but not the stromal component of the culture, promoted maturation to the TCRαβ+ DP stage of development. Lymphoid cellular expansion was consistently lower in cultures where the stromal cells were exposed to ascorbate or pAsc, suggesting that an optimal approach may be to expose only the lymphoid cells to ascorbate at each passage (every 3–4 days).
FIG. 5.
The effect of ascorbic acid on T-cell maturation is cell intrinsic. OP9-DL1 stromal cells were cultured with 2×103 FACS-sorted LSK-Thy-1.1-neg cells in the presence of 5 ng/ml each of IL-7 and Flt3L for 17 days, with passages every 3–4 days. On the 17th day of culture, nonadherent lymphoid cells were harvested by gentle rinsing of the OP9-DL1 monolayer and transferred to a new culture plate for 30 min to deplete residual OP9-DL1 cells by adherence. The lymphoid cells were then incubated at 37°C for 2 h with cytokines as above in the absence (Ly−) or presence (Ly+) of ascorbic acid (250 μM). In parallel, established monolayers of OP9-DL1 cells were also incubated in the absence (St−) or presence (St+) of the same concentration of ascorbic acid. After 2 h, all cell populations were washed, and cultures were established in which lymphocytes, stromal cells, both cell populations, or neither population was exposed to ascorbic acid. As a positive control, pAsc was added at a concentration of 250 μM to one set of cultures. After 6 days, quadruplicate cultures were harvested for cell counting and phenotypic analysis as indicated. Values represent mean±standard error. A two-tailed t-test was used for statistical analysis.
TCR rearrangement is independent of ascorbic acid
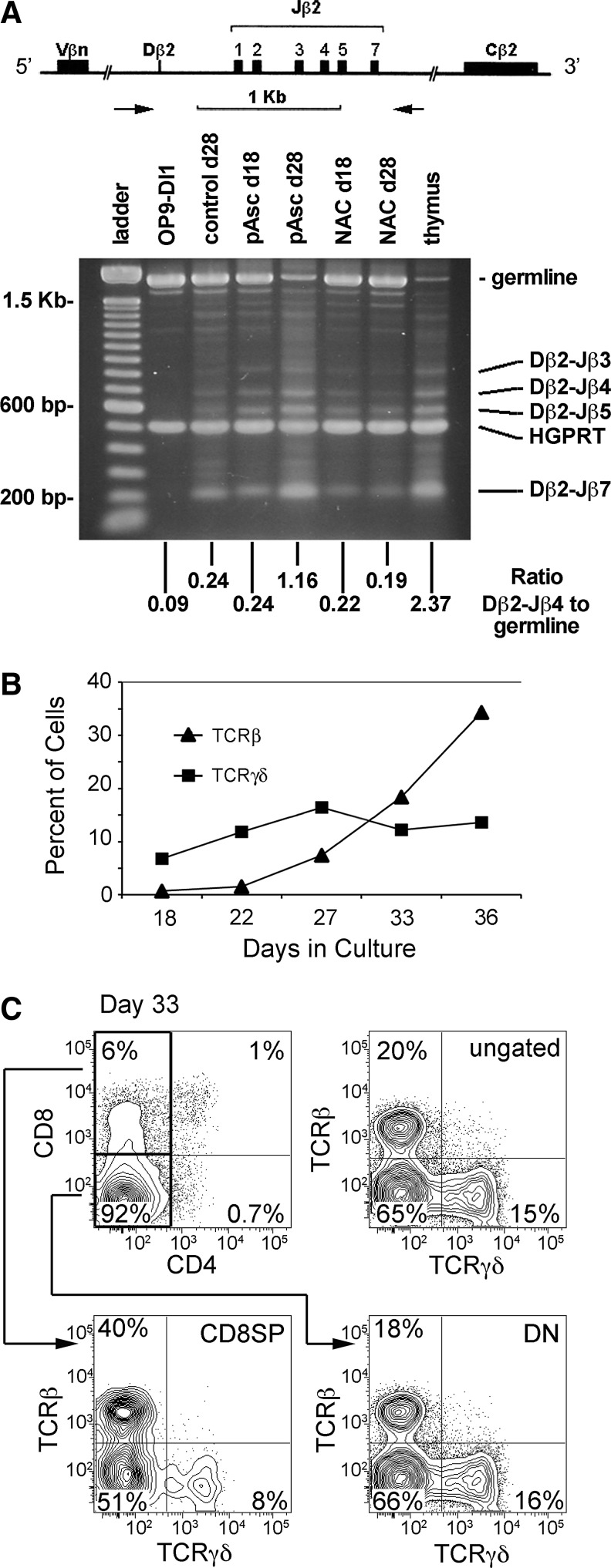
Ascorbic acid mediates a variety of biological effects as a cofactor for Fe2+- and 2-OG-dependent dioxygenases, a family of enzymes that are involved in nucleotide metabolism and DNA/RNA repair (30). Since T-cell maturation involves the regulated introduction of DNA double-strand breaks followed by directed recombination to generate diverse families of functional TCRαβ, an attractive possibility is that ascorbic acid serves as a cofactor in the DNA recombination process. To address the potential role of pAsc in mediating TCR rearrangement, we interrogated the TCRβ locus rearrangement status using polymerase chain reaction (PCR) (Fig. 6). We observed evidence of TCRβ rearrangements regardless of ascorbate supplementation and selective expansion of cells lacking a germline version of the TCRβ locus in cultures supplemented with pAsc (Fig. 6A). The ratio of Dβ2-Jβ4 amplicons to germline amplicons in cultures maintained in pAsc for 28 days (1.16) was fivefold higher than parallel control cultures (0.24) and approached the value seen in thymus (2.37). Figure 6A also illustrates that cultures maintained in N-acetylcysteine, a precursor for glutathione, fail to progress through TCR selection. Additionally, supplementation of cultures with the antioxidants lipoic acid, cysteine, or β-mercaptoethanol, or maintaining cultures in hypoxic conditions (5% O2), did not reproduce the rescue of T-cell development that was seen with both ascorbic acid and pAsc (Supplementary Fig. S1). This suggests that the mechanism of action in this biological system is not due to a broadly active antioxidant activity, in agreement with studies of the role of ascorbic acid in modulating the efficiency and quality of pluripotent stem cells induced from mature B-lymphocytes by expression of transcription factors (33). We also found that B-cell maturation, in which the same mechanisms for DNA recombination are utilized, is not enhanced by ascorbic acid (data not shown). Collectively, our results suggest that DNA rearrangement is not the aspect of T-cell maturation enhanced by pAsc.
FIG. 6.
Analysis of the effects of ascorbic acid and pAsc on T-cell receptor-β (TCRβ) rearrangement. (A) Analysis of TCRβ rearrangements by PCR. The primer set amplifies a germline fragment of 1.8 kb in non-T cells. During Dβ-to-Jβ recombination, the germline fragment is lost and is replaced by smaller fragments, depending on the particular Jβ segment that is joined to Dβ. Subsequently, Vβ-to-DβJβ rearrangement results in the loss of both germline and Dβ-to-Jβ-specific bands. Band ratios were determined using ImageJ software. (B) Cultures established as described in Figure 5 legend were maintained without ascorbate for up to 36 days to evaluate cell surface expression of TCRαβ and TCRγδ over time by FACS analysis. (C) A representative FACS analysis of the day-33 data shown in Panel (B), showing a relative lack of maturation based on CD4/CD8 staining. The distribution of TCRβ+ and TCRγδ+ cells in the total population (ungated) as well as in the gated CD8-single-positive (CD8SP) and double-negative (DN) populations is shown.
We also utilized a standard recombination-activating gene (RAG) in vitro cleavage reaction (3) to analyze recombinase activity with respect to Mg2+ and ascorbate concentrations. These studies showed that when reactions were supplemented with low levels of ascorbate (0.1 and 1 mM), the total cleavage activity observed in the reaction increased slightly compared to samples lacking ascorbate. This outcome was observed in all reactions containing Mg2+, regardless of the concentration, but was most pronounced when Mg2+ was limiting in the reaction (0.04 mM), where ascorbate was found to stimulate total RAG cleavage activity approximately threefold (Supplementary Fig. S2). Thus, while ascorbic acid does modulate the activity of the RAG complex in vitro, the data do not suggest enhancement of recombination as a major factor in the promotion of T-cell maturation.
Maintenance of OP9-DL1-lymphoid progenitor cocultures for up to 36 days in the absence of ascorbate-mediated induction of differentiation revealed an ongoing increase in the frequency of cells expressing high levels of cell surface TCRαβ, while the frequency of cells expressing TCRγδ remained relatively constant (Fig. 6B, C). By day 36, the ratio of TCRαβ+ to TCRγδ+ cells was ∼2:1. Analysis of the distribution of TCRβ+ and TCRγδ+ cells with respect to CD4 and CD8 expression (Fig. 6C) showed that the two subsets were present at equal frequencies in the DN subset, but that CD8SP subset was enriched for TCRβ+ cells. The presence of both TCRβ+ and TCRγδ+ cells in cultures lacking pAsc suggests that ascorbate does not influence the decision between these two lineages of T-cells during the course of in vitro development. The expression of high levels of cell surface TCRαβ in the absence of CD4 and CD8 expression is inconsistent with what is observed during normal T-cell maturation, where productively rearranged TCRβ associates with pre-Tα coincident with the transition to the DP stage of development and the initiation of TCRα rearrangements (4). Collectively, the results of the experiments shown in Figure 6 suggest that ascorbate deficiency during T-cell maturation does not inhibit TCR rearrangement, but rather selectively inhibits the progression of developing TCRβ+ T-cells to the DP stage of maturation.
Functional rearrangement of TCRαβ in T-cells cultured with ascorbate
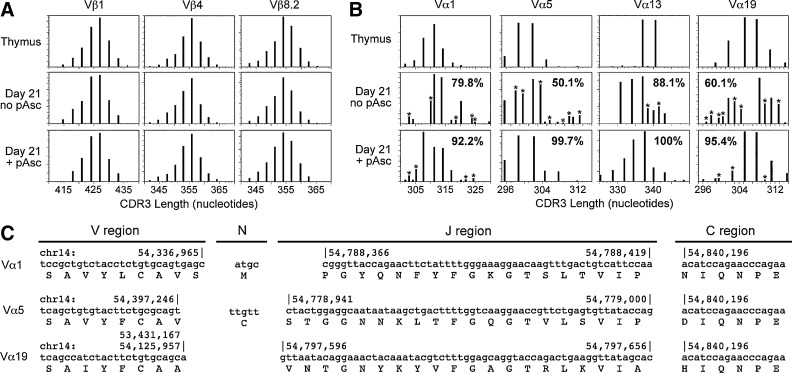
To further characterize the expression and diversity of rearranged TCR molecules in lymphocytes maturing in OP9-DL1 cultures, we utilized immunoscope analysis (23) to evaluate length polymorphisms in the CDR3 region spanning the recombination junctions between the VβDβJβCβ and VαJαCα cassettes. Representative results of this analysis for both the Vβ and Vα genes are shown in Figure 7. We compared amplicons obtained from normal thymus with cells cultured for 21 days in the presence or absence of pAsc. The results showed that the process of β-selection, where functional TCRβ chains are selected by their ability to pair with the invariant pre-Tα chain, occurred equally well in the presence or absence of pAsc (Fig. 7A). In contrast, the analysis of the TCRα CDR3 polymorphisms revealed a profound effect of pAsc on selection of properly rearranged loci, since many out-of-frame transcripts were detected in cultures lacking pAsc, but not in cultures supplemented with pAsc (Fig. 7B). Maintenance and expansion of pAsc cultures using anti-CD3/CD28 antibodies with 100 U/ml IL-2 showed preferential outgrowth of single major-size variants of several Vα genes (data not shown). To confirm and extend these results, we cloned and sequenced individual Vα1, Vα5, and Vα19 CDR3 products. In all cases, the frequency of functional sequences was higher in cultures that were maintained in pAsc. In the case of Vα1, sequence analysis revealed a recurrent CDR3 region sequence that was present in 1 of 12 in-frame clones from a control culture, 10 of 14 in-frame clones from a pAsc culture, and 27 of 27 in-frame clones from the culture expanded in IL-2 (Fig. 7C). Several other examples of functional TCRα rearrangements cloned from pAsc-supplemented OP9-DL1 cultures are also shown. This result provides strong evidence supporting the hypothesis that ascorbate supplementation enhances selection of functional TCRαβ in OP9-DL1 cultures at a stage of development that occurs after β-selection.
FIG. 7.
TCR diversity generated in OP9-DL1 cultures. (A) RNA isolated from T-cells maturing in OP9-DL1 cultures or freshly isolated thymus tissue was reverse-transcribed and evaluated by immunoscope analysis of the TCRβ transcripts as described in the Materials and Methods section. (B) As in Panel (A), except TCRα, transcripts were evaluated by immunoscope analysis. CDR3 regions with nucleotide lengths corresponding to those seen in control thymus samples were identified, and the area under each peak relative to the total peak area was calculated. The sums of these numbers shown in the lower panels indicate the percentage of CDR3 amplicons that correspond in length to those seen in thymus samples. Amplicons marked (*) do not correspond to the size of products seen in the control thymus samples. (C) Individual amplicons cloned from the reactions shown in Panel (B) were sequenced to confirm proper rearrangement of the TCRα loci. Shown are three representative sequences of ∼100 sequenced products. The numbers above the nucleotide sequences indicate the genomic location of each region. In the case of Vα19, two genomic V regions corresponding to the sequenced amplicon were identified. The genomic locations of both regions are indicated, since it is not possible to identify from which of these regions the V gene is derived.
Ascorbic acid supplementation results in gene expression changes in T-cell cultures
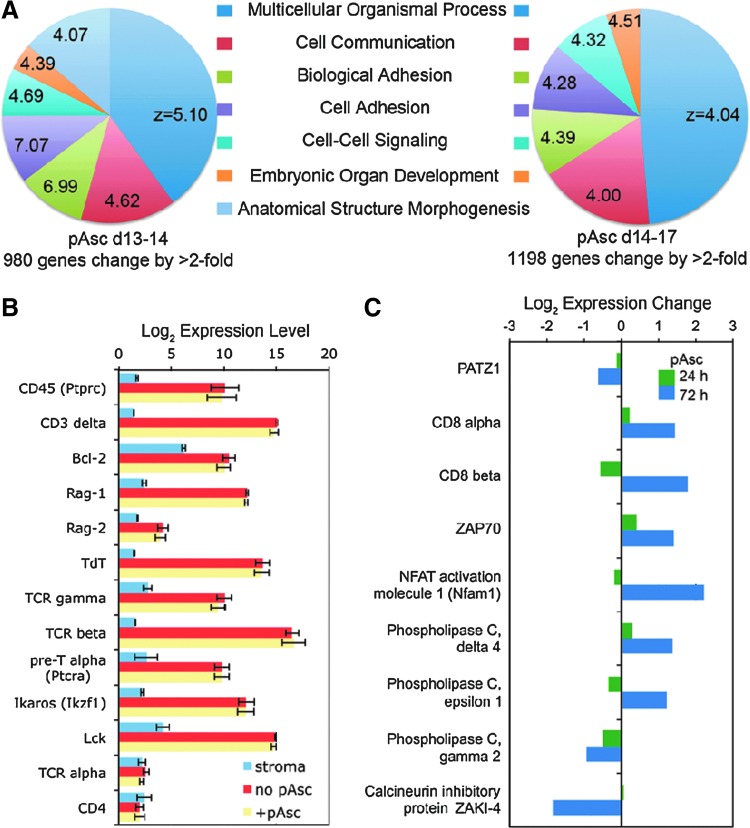
Ascorbic acid supplementation of cell cultures causes post-translational modifications of transcription factors (1,26) and epigenetic modifications, including demethylation of histones (9,33), resulting in alterations in gene expression (11). To evaluate the possibility that ascorbate functions to activate T-cell maturation through transcriptional regulation, we performed a microarray analysis of gene expression in cultures that were initiated in the absence of pAsc and switched into pAsc-containing culture conditions after 14 days (Fig. 8). RNA samples were prepared 24 and 72 h after the transition into pAsc and compared to RNA samples collected from parallel cultures maintained without pAsc. We observed greater-than twofold changes in expression of 980 and 1198 genes after 24 and 72 h, respectively (Fig. 8A). Gene ontogeny analysis (10) indicated consistent results at the two times analyzed, and the most significant biological processes influenced by pAsc in lymphocytes included genes involved in adhesion, development, cell–cell signaling, and organization of the extracellular matrix (Fig. 8A). However, expression of many of the genes associated with T-cell maturation during the DN1–4 stages was not changed in response to pAsc (Fig. 8B). Of note, we observed expression of TCRγ, TCRβ, and pre-TCRα, but not TCRα in the cultured lymphocytes, consistent with the β-selection phase of T-cell development. Figure 8C illustrates a number of candidate genes for which changes in expression were observed. Expression of the zinc-finger transcription factor Patz1, a negative regulator of Cd8 expression (25), was decreased in concert with transcriptional activation of the Cd8 locus. In contrast, Cd4 expression was not changed by ascorbate (Fig. 8B). We also observed upregulation of Zap70, a kinase that is central to signal transduction through the TCRαβ complex, but not of its upstream partner Lck (Fig. 8B, C). The microarray results for Cd8a, Patz1, and Zap70 were confirmed by quantitative real-time polymerase chain reaction (RT-PCR) analysis (data not shown). The microarray analysis also showed upregulation of an activator of NFAT transcription factors along with several isoforms of phospholipase C (PLC), and downregulation of PLCγ2 and the calcineurin inhibitory protein ZAKI-4. Collectively, the gene expression analysis supports the hypothesis that one mechanism by which pAsc modulates T-cell development is through transcriptional modulation of the coreceptor CD8 along with other components of the TCR signal transduction cascade. These data suggest that regulation of Cd8 is an early step in a multistep process required in the DN-to-DP transition.
FIG. 8.
Microarray analysis of gene expression changes in response to pAsc. Cultures established as described in the Materials and Methods section were maintained for 14 days before addition of 800 μM pAsc. Cultures with and without pAsc were harvested after 24 or 72 h for RNA isolation and microarray analysis as described in the Materials and Methods section. (A) Gene ontogeny analysis of expression changes after 24 or 72 h in pAsc. The Z values indicate significance as described by Doniger et al. (10); values >2 indicate enrichment of genes associated with a functional group of genes compared to the total set of genes. (B) Candidate genes expressed during the DN stages of T cell development that are not changed in response to pAsc. (C) Candidate genes involved in signal transduction that are altered in expression after addition of pAsc to cultures.
Ascorbic acid modulates epigenetic regulation of gene expression
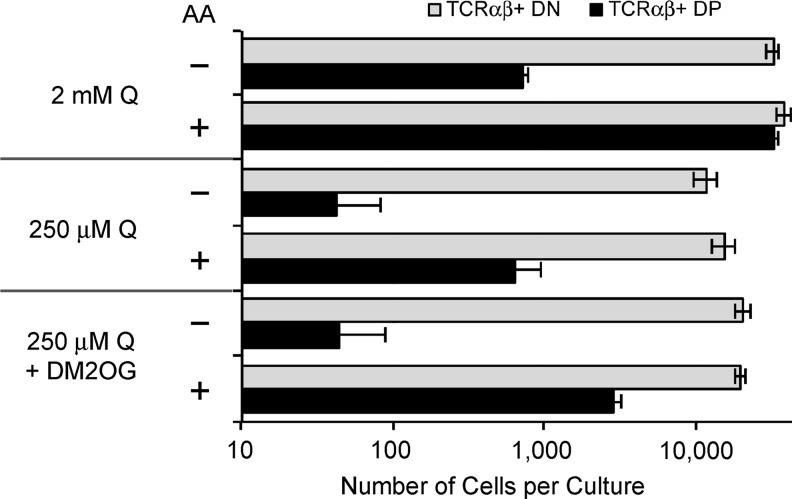
To test the hypothesis that ascorbic acid functions as a cofactor for Fe2+- and 2-OG-dependent demethylases important in epigenetic regulation of the genes we identified by microarray, we evaluated the effect of modulating intracellular 2-OG levels by limiting the availability of glutamine in the cultures. Glutamine is rapidly converted to 2-OG in cells via the sequential action of two enzymes (glutaminase, followed by glutamate dehydrogenase, alanine transaminase, or aspartate transaminase) in a process termed glutaminolysis (19). Thymocytes, activated lymphocytes, and other rapidly dividing cells utilize glutaminolysis as a mechanism to generate precursors for biosynthesis; in these cases, very little glutamine is fully oxidized via acetyl-CoA and the citric acid cycle (20). Extracellular glutamine is uniquely required for T-cell proliferation and cytokine secretion in a TCR-dependent manner (5). Figure 9 shows that a 10-fold decrease in extracellular glutamine (Q) resulted in a specific loss of the ability of ascorbic acid to promote the DN-to-DP transition, and that supplementation of the cultures with 1 mM cell-permeable dimethyl-2-OG partially reversed the inhibitory effect. Toxicity of the compound prevented us from testing higher doses. Although the number of TCRαβ+ DN cells was also decreased in cultures starved for Q, the magnitude of this effect was 5-fold compared to 100-fold for TCRαβ+ DP cells. This result is consistent with the hypothesis that ascorbic acid promotes the DN-to-DP transition through its role as an obligate cofactor for Fe2+- and 2-OG-dependent dioxygenases.
FIG. 9.
Extracellular glutamine (Q) is required for the DN-to-DP transition promoted by ascorbic acid. OP9-DL1 cocultures were established and maintained without ascorbic acid for 19 days, at which time cultures were passaged into the conditions as indicated. The cell-permeable dimethyl-2-OG (DM2OG) was added at 1 mM, while ascorbic acid (AA) was added at 100 μM. Cultures were harvested 8 days later, and the total number of lymphoid cells per culture was determined using an Accuri C6 instrument. Cells were then labeled with antibodies for analysis by flow cytometry using a B-D Canto instrument. The absolute number of TCRαβ+ DN and DP cells in triplicate cultures was calculated and is shown as mean value±standard error.
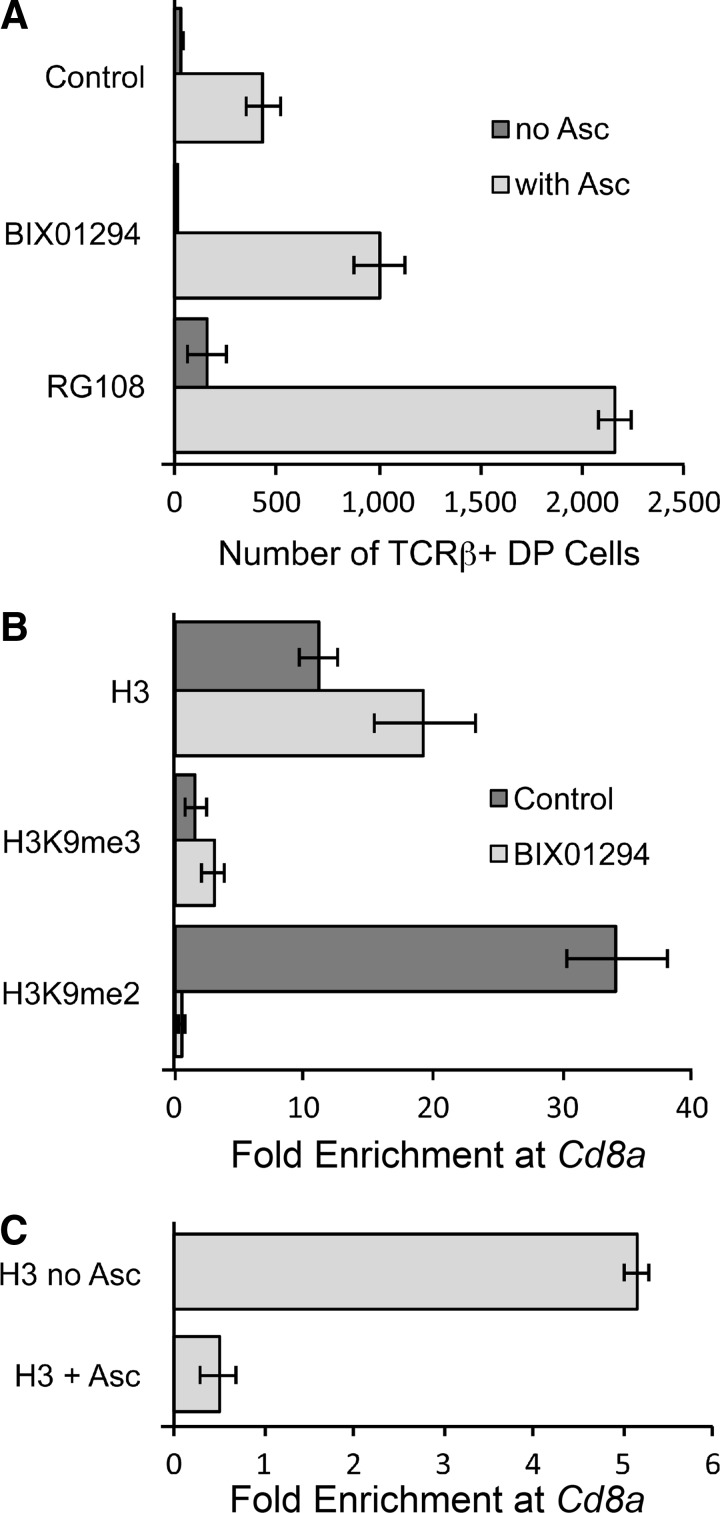
To further evaluate the hypothesis that ascorbic acid regulates gene expression by modulating epigenetic DNA and histone modifications, we tested the effect of blocking either histone lysine methyltransferase (KMT) or DNA methyltransferase (DNMT) enzymes using BIX01294 and RG108 (Fig. 10). These compounds are specific inhibitors of the H3K9 lysine methyltransferases KMT1C/D and of all DNMTs, respectively. As shown in Figure 10A, both inhibitors enhanced the DN-to-DP transition in response to ascorbic acid. The DNMT inhibitor RG108 promoted the DN-to-DP transition independently of ascorbic acid, but the transition was further enhanced in the presence of ascorbic acid. These findings strongly suggest that ascorbic acid acts, at least in part, by operating in opposition to DNA and H3K9 methyl marks. These data support the hypothesis that the critical steps enhanced by ascorbic acid include demethylation of DNA and/or H3K9. Since ascorbic acid should not directly affect KMT or DNMT enzyme function, we suspect a synergistic interaction of inhibition of histone methylation and active demethylation of DNA and/or H3K9.
FIG. 10.
Methyltransferase enzyme inhibition potentiates the ability of ascorbic acid to promote the DN-to-DP transition in OP9-DL1 cultures. (A) OP9-DL1 cocultures were maintained without ascorbic acid for 19 days, at which time RG108 (5 μM) or BIX01294 (50 nM) was added with or without 100 μM ascorbic acid. Cultures were evaluated 8 days later as described in the legend for Figure 9. Values from replicate 1 ml cultures are shown with mean±standard error. (B) Cultures maintained without ascorbic acid for 19 days were replated in the presence or absence of 30 nM BIX01294 and cultured for an additional 8 days. Lymphocytes obtained from these cultures were processed for chromatin immunoprecipitation (ChIP) using antibodies as indicated. The fold enrichment of the Cd8a promoter region relative to β-actin DNA is shown as the mean±standard error of triplicate technical replicates. (C) Cultures maintained without ascorbic acid for 21 days were replated with or without ascorbic acid and harvested for ChIP 72 h later. ChIP for total histone H3 protein was performed and analyzed as in Panel (B). Data indicate mean±standard deviation of 2 (no ascorbic acid) or 3 (+ascorbic acid) biological replicates with three technical replicates each.
Using chromatin immunoprecipitation (ChIP) with antibodies against total histone H3, H3K9me2, and H3K9me3, we extended the inhibitor studies by confirming the loss of H3K9me2, but not H3K9me3 marks or total H3 histone molecules, at the promoter region of Cd8a in cultures containing BIX01294 (Fig. 10B). This result is consistent with the specificity of BIX01294 for KMT1C/D, which mediates heterochromatin formation and silencing of target genes by writing H3K9me2 marks that prevent acetylation of H3K9 and recruit heterochromatin protein 1 and DNMT1 to result in stable silencing (16,22). In a second ChIP experiment, we evaluated the effect of ascorbic acid on histone modifications at the Cd8a promoter using 21-day cultures treated with ascorbic acid for 72 h. This experiment confirmed H3K9me2 marks in the absence of ascorbic acid (data not shown), and showed eviction of histone H3 from the promoter in the presence of ascorbic acid (Fig. 10C). Collectively, these data support three conclusions. First, Cd8a is silenced in part by H3K9me2 marks; second, loss of H3K9me2 marks is not sufficient to activate transcription; and third, ascorbic acid is necessary and sufficient for transcriptional activation of Cd8a through a process that involves demethylation of DNA.
Discussion
Ascorbic acid is widely regarded as a positive regulator of immune function; however, definitive evidence of such activity at a mechanistic level is largely lacking in the scientific literature. Our data demonstrate an active role for ascorbic acid in the development of T-cells, likely through epigenetic modulation of gene expression (Figs. 8 and 10) that leads to selective expansion of TCRαβ+ DP cells (Figs. 2, 3, and 5). We have identified a number of components of commercial cell culture medium formulations that influence T-cell differentiation in vitro, including nucleotides (Fig. 1) and ascorbic acid (Fig. 2). Additional components of the αMEM formulation, which we have not identified, contribute to a growth arrest at the time of lymphoid lineage commitment compared to MEM and DME (Fig. 1). Of all commercially available medium formulations we have tested, only αMEM contains ascorbic acid. However, minimal concentrations of ascorbate are detectable in liquid formulations of αMEM due to rapid spontaneous oxidation upon exposure to light, air, and metals. Supplementation of a basal MEM with ascorbic acid or with the stable derivative pAsc is necessary to promote progression to the stage of development where cell surface expression of TCRαβ in association with the coreceptors CD4 and CD8 allows positive and negative selection events to occur. The role of ascorbate in this process is at least partially due to regulation of Cd8a gene expression (Figs. 3, 8, and 10). If the mechanistic basis of the effect of ascorbate on T-cell maturation also includes modulation of signal transduction downstream of the TCR–coreceptor complex, it is likely that ascorbic acid will also function to regulate the activity of mature T cells.
Our modifications of the OP9-DL1 culture system establish a new model of T-cell maturation that efficiently initiates from bone marrow-derived hematopoietic progenitor cells. Over the course of several weeks, these cells commit to the T-cell lineage and progress to the third double-negative (DN3) stage of development. Progression of T-cell maturation is arrested at this developmental stage, and cells expressing the mature form of the TCR (TCRαβ) accumulate, but do not move to the DP stage (Figs. 2 and 6). Remarkably, the developmental block is not accompanied by a proliferative arrest, and large numbers of DN3 cells (>108) can be expanded from small numbers (∼103) of progenitor cells. We discovered that the developmental arrest is released by adding ascorbic acid to the cultures. Supplementation with ascorbic acid results in a progressive transit of the DN population to the DP stage of development where selection of functional TCRαβ occurs (Fig. 7). Epigenetic regulation of Cd4, Cd8, and loci encoding cytokines and transcription factors plays a major role during T-cell maturation and the subsequent programming of functional T-helper cell subsets (39). The mechanism of epigenetic regulation involves methylation and demethylation of both histone proteins and of CpG motifs in regulatory regions of DNA. Enzymatic removal of methyl marks is mediated in part by a superfamily of Fe2+- and 2-OG-dependent dioxygenases, including members of the Jumonji C histone–lysine demethylase family. Because many of these dioxygenases require ascorbic acid as a cofactor, we hypothesize that enhancement of the activity of these enzymes is the primary proximal mechanism by which ascorbic acid promotes T-cell maturation.
Our studies establish a new restriction point in T-cell maturation that is defined by a requirement for ascorbic acid. We propose two parallel pathways of developmental events that are normally coordinated to result in TCRα rearrangement and TCRαβ expression at the same time as the coreceptors CD4 and CD8. Together, these components associate with the CD3 complex to activate a signal transduction cascade that depends on the transcription factor AP-1 and a constellation of additional molecular signals that collectively result in selection of functional T-cells. Earlier in development, TCRβ rearranges and is expressed in complex with CD3 and pre-Tα in the absence of CD4 and CD8. Our data demonstrate that selection of TCRβ rearrangements does not require ascorbic acid supplementation, but that selection of mature TCRαβ is inefficient unless ascorbic acid is available (Fig. 7). We show that induction and maintenance of Cd8 expression are dependent on ascorbic acid (Figs. 3 and 8), consistent with a model in which Cd8 expression is regulated by histone methylation and demethylation (14). Epigenetic regulation of gene expression meets the need to reprogram Cd4 and Cd8 during T-cell maturation, since both genes are initially expressed in association with TCRαβ and CD3 at the DP stage of development. The DP stage is followed by permanent silencing of either Cd4 or Cd8. This stable memory state is maintained until eventual stimulation via TCRαβ leads to new memory states involving cytokine gene clusters that define T-helper cell subsets and that are also maintained by epigenetic modifications.
As originally described, the OP9-DL1 culture model for T-cell development routinely reproduces the DN-to-DP transition during T-cell maturation only when fetal liver-derived progenitor cells are cultured (17). Our modifications of culture conditions provide a window into T-cell development at a point when many critical gene regulatory events occur. This advance is significant for several reasons. First, the ability to synchronize lymphocytes at the DN-to-DP transition provides a unique opportunity to directly observe a sequence of gene regulatory events that are normally masked by the heterogeneity of the DN populations. Second, population synchronization at a developmental stage that requires ascorbic acid defines a new restriction point in T-cell maturation. Third, understanding how the immune system utilizes epigenetic gene regulation to establish persistent T-helper (Th) cell lineages with distinct functions (Th1, Th2, Th17, and Treg) will provide us with insight into establishing populations of T cells de novo that are characterized by defined antigenic specificity, migratory potential, and cytokine secretion programs.
In most animals, ascorbic acid is synthesized from glucose in the liver and circulates at concentrations ranging from 15 to 60 μM (34). Dietary sources of ascorbic acid are critical for species lacking the biosynthetic pathway, including primates, guinea pigs, and fruit bats. Two high-affinity sodium-dependent vitamin C transporters, Svct1 and Svct2, have been described (18). Svct1 is mainly expressed in gastrointestinal epithelium and in the tubules of the kidney, where it functions in dietary capture and recycling of ascorbate excreted in the urine. Svct2 is expressed in most other tissues. Due to the activity of these transporters, the ascorbate concentrations in most tissues, including the spleen and thymus is in the millimolar range (18). Circulating leukocytes contain 80-fold more ascorbate than does the plasma (12). These observations most likely account for the saturation of the effect of pAsc on T-cell maturation in our model system (Fig. 2B), since the maximal effect was seen at a dose (30 μM) that is physiologically relevant and within the range of the binding affinity of the transporters (10–100 μM) (35). Using mice deleted for the Svct2 transporter (Slc23a2−/−), we also demonstrate that ascorbic acid is also required for normal T-cell maturation in the thymus in vivo (Fig. 4).
Our culture model introduces a new and innovative platform that will be valuable in establishing a temporal sequence of events leading to immune cell maturation by deciphering mechanisms controlling the DN-to-DP conversion. The ascorbate-dependent culture model allows us to study stages of normal maturation that have not previously been possible to separate using existing targeted mutations (e.g., RAG enzymes, MHC molecules, or signal transduction components). Future studies will utilize an existing set of reagents and targeted mutations that modulate ascorbic acid synthesis (Gulo−/−), transport (Slc23a2−/−), and DNA and histone methylation. These will link ascorbic acid with enzymatic regulation of the chromatin code and will provide new insights into the epigenetic mechanisms by which ascorbic acid regulates stem cell maturation and differentiation, as recently established in the case of induced pluripotent stem cells (33).
The generation of stable gene expression states by the memory-establishing characteristics afforded by epigenetic mechanisms of gene regulation is well suited to function in the context of adaptive immunity. A model of ascorbate-dependent epigenetic gene regulation suggests a system that can integrate environmental sampling of dietary nutrients with a transcriptional switch that introduces memory in the form of methylation states. Such a system could regulate networks of interacting biological elements and optimize adaptation. This model represents an important intellectual advance in our understanding of gene regulation during T-cell development.
Materials and Methods
Isolation of lymphoid progenitor cells
Tibia and femora were removed from young adult (6–18 weeks old) C57BL/Ka-Thy-1.1 mice, dissected free of muscle tissue, and gently crushed using a mortar and pestle. Cells were sorted using a fluorescence-activated cell sorting (FACS) Aria instrument (Becton Dickinson Immunocytometry Systems) to isolate LinnegSca-1+c-kit+Thy-1-1neg (LSK-Thy-neg) bone marrow cells, which we have previously demonstrated that are enriched for lymphoid progenitor cells and lack primitive hematopoietic stem cells (21). An aliquot of each sorted cell population was taken for reanalysis to evaluate purity and cell count.
Lymphocyte differentiation cultures
Stromal cells (OP9 and OP9-DL1, originally obtained from Juan-Carlos Zúñiga-Pflücker, University of Toronto) were maintained in an αMEM supplemented with 20% fetal bovine serum (FBS), 2 mM l-glutamine, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.2), 50 U/ml penicillin, and 50 μg/ml streptomycin (all cell culture reagents were obtained from Invitrogen, unless otherwise indicated). For lymphocyte differentiation cultures, stromal cells were plated in 24-well tissue culture plates at a density of 4×104 cells/ml in 1 ml of medium and allowed to adhere for ∼1 h. The culture medium was then replaced with 1 ml lymphocyte differentiation medium, which consisted of one of the indicated basal medium formulations supplemented with 10% FBS, 2 mM l-alanyl-l-glutamine (GlutaMAX), 10 mM HEPES, 25 U/ml penicillin, 25 mg/ml streptomycin, 50 μM β-mercaptoethanol, 1 mM sodium pyruvate, 1% MEM vitamin solution, and 2% MEM nonessential amino acid solution. The MEM 10× concentrate medium (Invitrogen catalog No. 11430-030) was prepared by a 1:10 dilution into distilled, deionized water containing 2.2 g/L sodium bicarbonate and supplemented as described above. Each 1 ml culture was seeded with ∼2×103 LSK-Thy-neg bone marrow cells and supplemented with IL-7 and fms-like tyrosine kinase-3 ligand (both obtained from R&D Systems and used at a concentration of 5 ng/ml unless otherwise indicated). Cultures were harvested every 3–4 days by forceful pipetting, filtered through 70-μm nylon mesh (Small Parts, Inc.), centrifuged at 200 g for 5 min, and resuspended in 0.2 ml lymphocyte differentiation medium. Cells were counted at each cell passage using an Accuri C6 flow cytometer (BD Accuri Cytometers). Cultures were reseeded with a maximum of 1×105 cells per ml onto freshly plated stromal cells. Ascorbic acid was provided in cultures as sodium salt or as a stable phosphorylated derivative (l-ascorbic acid-2-phosphate sesquimagnesium salt, pAsc), both obtained from Sigma-Aldrich.
Flow cytometry analysis
Approximately 105 cells obtained from each passage were resuspended in 40 μl Hank's balanced salt solution (HBSS) supplemented with 5% newborn calf serum and 10 mM sodium azide and containing saturating amounts of monoclonal antibodies specific for lymphocyte differentiation antigens (B220, CD25, CD3, CD4, CD8, CD44, TCRβ, and TCRγδ). Biotinylated antibodies were detected with avidin allophycocyanin–AlexaFluor-750, and dead cells were detected using 4′,6-diamidino-2-phenylindole. All reagents were prepared in our laboratory or obtained from eBioscience and tested for staining specificity and compensation on mouse spleen and thymic cells. Flow cytometry data were collected using an FACS Canto II (Becton Dickinson Immunocytometry Systems) and analyzed using FlowJo software (Tree Star, Inc.).
Fetal liver transplantation and analysis
To evaluate the role of ascorbic acid in T-cell maturation in vivo, Svct2−/− fetal liver cells were obtained from individual fetal donors and cryopreserved as previously described (2). Fetal liver cells (Ly-5.2) were recovered from cryopreservation, washed, counted, and mixed with an equal number bone marrow cells obtained from B6×B6.SJL-Ptprca F1 mice (Ly-5.1/Ly-5.2). Transplant recipients (B6.SJL-Ptprca, Ly-5.1) were conditioned using gamma-radiation (two doses of 650 cGy delivered at a rate of 75 cGy/min with a 3-h rest between doses) as previously described (7). Cells (2×106 total per recipient) were transplanted into irradiated recipients by the retro-orbital route. Peripheral blood cells were collected beginning at 5 week post-transplantation and evaluated for contributions of each transplanted population to the nucleated blood lineages by flow cytometry as previously described (7), using Ly-5.1, Ly-5.2, CD4, CD8, B220, Gr-1, and CD11b antibodies.
Microarray analysis
Stromal cell or lymphocyte differentiation cultures were maintained for 14 days as described above, and then reseeded with or without ascorbic acid (50 μM each of ascorbic acid and pAsc). Cultures were harvested at the indicated times, and stromal cells were depleted from lymphoid cells by plating the cell suspension onto a 100-mm cell culture dish for 30 min, followed by gentle washing to recover the nonadherent lymphoid cells. Since the frequency of stromal cells remaining after this adherence depletion was usually 0.5%, we included OP9-DL1 cells in the microarray experiment to identify stromal cell genes regulated by ascorbate. Approximately 5×106 cells from each condition were harvested, and total RNA was subsequently purified using RNeasy Minicolumns (Qiagen). Purity and quality of RNA were evaluated using a 2100 Bioanalyzer (Agilent). The RNA was then labeled and hybridized as paired samples +/− ascorbate to Agilent 44K microarrays. TIF files generated from the scanned microarray images were analyzed using Agilent Feature Extraction Protocol 9.5.1, and spot intensities were normalized between arrays. Normalized data were analyzed using GeneSifter (Geospiza). Microarray data have been deposited in the gene expression omnibus (GEO) database under the accession number GSE31596 (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse31596).
Genomic PCR
Total DNA was isolated from cultured cells using a Qiagen DNeasy® Blood and Tissue Kit. An Invitrogen Platinum® Taq PCR kit using 50 ng of DNA template, 1.75 μM diversity and joining region (DJ) primers, 0.4 μM GADPH primers, and 2.5 U Taq polymerase were used for PCR in a 25-μl total volume. DJ primer-recognizing sequences 5′ of Dβ2.1 and 3′ of Jβ2.7 were those described by Rodewald et al. (24). This primer set amplifies a germline DNA fragment of 1858 bp and several shorter DNA products from DJβ rearranged loci ranging from 1279 to 224 bp. PCR samples were denatured (94°C, 1 min), annealed (63°C, 2 min), and extended (72°C, 2 min) for 29 cycles. Aliquots for each sample were fractionated on a 1.5% Tris-Borate-EDTA agarose gel. The gel was then stained with SYBR® Gold (Invitrogen) for 6 h and imaged using a Foto/Spectrum (Fotodyne®) dual transilluminator with Kodak Electrophoresis Documentation and Analysis system 120. Gel bands were quantitated using ImageJ software.
Real-time polymerase chain reaction
Total RNA was isolated from cultured cells using a Qiagen RNeasy® Mini Kit. cDNA was reverse transcribed using the Invitrogen SuperScript® First Strand Synthesis System with oligo(dT) primers. RT-PCR was performed with the Sigma SYBR Green Quantitative RT-PCR kit following protocol recommendations. All amplifications were standardized to β-actin. PCR was performed on a Roche Lightcycler II for 40 cycles. TCRα- and TCRβ-variable primers and nested constant primers were those described by Wettstein et al. (37,38). Additional primer sequences for RT-PCR and immunoscope analysis are available on request. Quantitative mRNA expression comparisons between ascorbate and nonascorbate culture conditions were evaluated for Cd8α, Cd8β, Vβ1, Vβ4, Vβ8.2, Vβ13, Vα2, Vα8, Patx1, and Zap70.
Immunoscope analysis
Immunoscope analysis was performed for the following variable regions: Vβ1, Vβ4, Vβ8.2, Vβ13, Vα1, Vα2, Vα5, Vα10, Vα13, Vα16, Vα18, and Vα19. A Roche Lightcycler II using the Sigma SYBR Green Quantitative RT-PCR kit was used for RT-PCR, following the protocol recommendations. Initially, a primary amplification of 30 cycles of template cDNA was performed with non-nested constant and variable primers. This product was then diluted to 1:200, and 1.5 μl of the dilution was used for a second amplification of 30 cycles with the same variable primers, but nested fluorochrome-labeled constant primers. Samples were then submitted to the University of Utah core DNA sequencing lab for analysis by capillary electrophoresis. To confirm the immunoscope analysis, we sequenced ∼25 cloned products each of Vα1, Vα5, and Vα19.
ChIP assay
Lymphoid cells isolated from OP9-DL1 cultures were fixed, sheared, and reacted with antibodies specific for histone proteins H3, H3K9me3, and H3K9me2 as previously described (29). Immunoprecipitates were collected using protein G-conjugated magnetic beads (Active Motif ). DNA purified from immunoprecipitates was amplified using primers specific for the promoter region of Cd8a (forward, 5′-AAT TCC TCCCAC TTG CTC TGC-3′; reverse, 5′-CTA GCT TGA CCTAAG CTG CTTG-3′) and for β-actin as a normalization control. Data were processed as previously described (29) and are represented as fold enrichment of Cd8a relative to β-actin.
Supplementary Material
Abbreviations Used
- αMEM
alpha modified minimal essential medium
- 2-OG
2-oxoglutarate
- CD4SP
CD4 single-positive
- CD8SP
CD8 single-positive
- ChIP
chromatin immunoprecipitation
- DJ
diversity and joining regions
- DME
Dulbecco's modified Eagle's medium
- DN
double-negative CD4−CD8−
- DNMT
DNA methyltransferase
- DP
double-positive CD4+CD8+
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- Flt3L
fms-like tyrosine kinase-3 ligand
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IL-7
Interleukin-7
- JmjC
Jumonji C domain
- KMT
histone lysine methyltransferase
- LSK-Thy-neg
LinnegSca-1+c-kit+Thy-1-1neg bone marrow cells
- pAsc
l-ascorbic acid 2-phosphate
- PCR
polymerase chain reaction
- PLC
phospholipase C
- RAG
recombination-activating gene
- RT-PCR
real-time polymerase chain reaction
- Svct
sodium-dependent vitamin C transporter
- TCR
T-cell receptor
Acknowledgments
This project was supported by funding from an institutional training grant (T32DK007115) and by individual research grants awarded to G.J.S. (R01DK057899 and RC1AI086238, cofunded by National Institutes of Allergy and Infectious Diseases and Office of the Director) and to P.C.S. (R01AI055599). Institutional flow cytometry, microarray, DNA sequencing, cell imaging, and primer synthesis core facilities were funded in part by P30CA042014 awarded to the Huntsman Cancer Institute. Additional funding was provided by the Brian Rooney Fund of the Lymphoma Foundation. J.M.M. is the guest editor of this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Arkan MC, Leonarduzzi G, Biasi F, Basaga H, and Poli G. Physiological amounts of ascorbate potentiate phorbol ester-induced nuclear-binding of AP-1 transcription factor in cells of macrophagic lineage. Free Radic Biol Med 31: 374–382, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Babaev VR, Whitesell RR, Li L, Linton MF, Fazio S, and May JM. Selective macrophage ascorbate deficiency suppresses early atherosclerosis. Free Radic Biol Med 50: 27–36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergeron S, Anderson DK, and Swanson PC. RAG and HMGB1 proteins: purification and biochemical analysis of recombination signal complexes. Methods Enzymol 408: 511–528, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Buer J, Aifantis I, DiSanto JP, Fehling HJ, and von Boehmer H. Role of different T cell receptors in the development of pre-T cells. J Exp Med 185: 1541–1547, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, and Frauwirth KA. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol 185: 1037–1044, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellani P, Angelini G, Delfino L, Matucci A, and Rubartelli A. The thiol redox state of lymphoid organs is modified by immunization: role of different immune cell populations. Eur J Immunol 38: 2419–2425, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Cho S, and Spangrude GJ. Enrichment of functionally distinct mouse hematopoietic progenitor cell populations using CD62L. J Immunol 187: 5203–5210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung TL, Brena RM, Kolle G, Grimmond SM, Berman BP, Laird PW, Pera MF, and Wolvetang EJ. Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells 28: 1848–1855, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Chung TL, Turner JP, Thaker NY, Kolle G, Cooper-White JJ, Grimmond SM, Pera MF, and Wolvetang EJ. Ascorbate promotes epigenetic activation of CD30 in human embryonic stem cells. Stem Cells 28: 1782–1793, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Doniger SW, Salomonis N, Dahlquist KD, Vranizan K, Lawlor SC, and Conklin BR. MAPPFinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol 4: R7, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte TL, Cooke MS, and Jones GD. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic Biol Med 46: 78–87, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Evans RM, Currie L, and Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br J Nutr 47: 473–482, 1982 [DOI] [PubMed] [Google Scholar]
- 13.Frikke-Schmidt H, and Lykkesfeldt J. Keeping the intracellular vitamin C at a physiologically relevant level in endothelial cell culture. Anal Biochem 397: 135–137, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Harker N, Garefalaki A, Menzel U, Ktistaki E, Naito T, Georgopoulos K, and Kioussis D. Pre-TCR signaling and CD8 gene bivalent chromatin resolution during thymocyte development. J Immunol 186: 6368–6377, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Hogquist KA. Signal strength in thymic selection and lineage commitment. Curr Opin Immunol 13: 225–231, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Honda S, Lewis ZA, Shimada K, Fischle W, Sack R, and Selker EU. Heterochromatin protein 1 forms distinct complexes to direct histone deacetylation and DNA methylation. Nat Struct Mol Biol 19: 471–477, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Garrett KP, Pelayo R, Zuniga-Pflucker JC, Petrie HT, and Kincade PW. Propensity of adult lymphoid progenitors to progress to DN2/3 stage thymocytes with Notch receptor ligation. J Immunol 175: 4858–4865, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May JM. The SLC23 family of ascorbate transporters: ensuring that you get and keep your daily dose of vitamin C. Br J Pharmacol 164: 1793–1801, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newsholme EA, Crabtree B, and Ardawi MS. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J Exp Physiol 70: 473–489, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Newsholme P, Curi R, Pithon Curi TC, Murphy CJ, Garcia C, and Pires de Melo M. Glutamine metabolism by lymphocytes, macrophages, and neutrophils: its importance in health and disease. J Nutr Biochem 10: 316–324, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Perry SS, Pierce LJ, Slayton WB, and Spangrude GJ. Characterization of thymic progenitors in adult mouse bone marrow. J Immunol 170: 1877–1886, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Rai K, Jafri IF, Chidester S, James SR, Karpf AR, Cairns BR, and Jones DA. Dnmt3 and G9a cooperate for tissue-specific development in zebrafish. J Biol Chem 285: 4110–4121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ria F, van den Elzen P, Madakamutil LT, Miller JE, Maverakis E, and Sercarz EE. Molecular characterization of the T cell repertoire using immunoscope analysis and its possible implementation in clinical practice. Curr Mol Med 1: 297–304, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Rodewald HR, Kretzschmar K, Takeda S, Hohl C, and Dessing M. Identification of pro-thymocytes in murine fetal blood: T lineage commitment can precede thymus colonization. EMBO J 13: 4229–4240, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Hombauer M, Bilic I, Naoe Y, Schebesta A, Taniuchi I, and Ellmeier W. The zinc-finger protein MAZR is part of the transcription factor network that controls the CD4 versus CD8 lineage fate of double-positive thymocytes. Nat Immunol 11: 442–448, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savini I, Catani MV, Rossi A, Duranti G, Melino G, and Avigliano L. Characterization of keratinocyte differentiation induced by ascorbic acid: protein kinase C involvement and vitamin C homeostasis. J Invest Dermatol 118: 372–379, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Schmitt TM, and Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17: 749–756, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Schmitt TM, and Zuniga-Pflucker JC. T-cell development, doing it in a dish. Immunol Rev 209: 95–102, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Shakya A, Kang J, Chumley J, Williams MA, and Tantin D. Oct1 is a switchable, bipotential stabilizer of repressed and inducible transcriptional states. J Biol Chem 286: 450–459, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons JM, Muller TA, and Hausinger RP. Fe(II)/alpha-ketoglutarate hydroxylases involved in nucleobase, nucleoside, nucleotide, and chromatin metabolism. Dalton Trans 5132–5142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommer D, Fakata KL, Swanson SA, and Stemmer PM. Modulation of the phosphatase activity of calcineurin by oxidants and antioxidants in vitro. Eur J Biochem 267: 2312–2322, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, and Nussbaum RL. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med 8: 514–517, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Stadtfeld M, Apostolou E, Ferrari F, Choi J, Walsh RM, Chen T, Ooi SS, Kim SY, Bestor TH, Shioda T, Park PJ, and Hochedlinger K. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat Genet 44: 398–405, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todhunter EN, and Mc MT. The ascorbic acid content of whole blood plasma of normal rats with evidence of a sex difference. J Nutr 31: 573–580, 1946 [DOI] [PubMed] [Google Scholar]
- 35.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen X-Z, Wang Y, Brubaker RF, and Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 399: 70–75, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Pierce LJ, and Spangrude GJ. Distinct roles of IL-7 and stem cell factor in the OP9-DL1 T-cell differentiation culture system. Exp Hematol 34: 1730–1740, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wettstein P, Strausbauch M, Therneau T, and Borson N. The application of real-time PCR to the analysis of T cell repertoires. Nucleic Acids Res 36: e140, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wettstein PJ, Strausbauch M, and Borson N. Repertoires of T cell receptors expressed by graft-infiltrating T cells evolve during long-term recall responses to single minor histocompatibility antigens. Int Immunol 19: 523–534, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Wilson CB, Rowell E, and Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol 9: 91–105, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Yan Z, Garg SK, and Banerjee R. Regulatory T cells interfere with glutathione metabolism in dendritic cells and T cells. J Biol Chem 285: 41525–41532, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu JS. Activation of protein phosphatase 2A by the Fe2+/ascorbate system. J Biochem 124: 225–230, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.