Abstract
Given their capacity to modulate the immune response, adipose mesenchymal stem or stromal cells (ASCs) have been used as therapeutic tools to treat chronic inflammatory and autoimmune diseases both in preclinical and clinical studies. Patients enrolled in such clinical trials are often concomitantly treated with immunomodulatory drugs such as methotrexate (MTX) or azathioprine (AZA). Therefore it is necessary to investigate the possible impact of these drugs on ASC function to learn if there are any interactions that would affect the therapeutic effects of either component and thus the clinical outcome of the trials. ASCs were cultured in the absence or presence of MTX or AZA and the effects on viability, proliferation, immunomodulatory properties, and immunogenic features were studied in vitro. The drugs did not affect the viability and proliferative capacity of ASCs. When the drugs and the ASCs were concomitantly used to inhibit lymphocyte proliferation, no synergistic or antagonizing inhibitory effects were found. MTX and AZA did not impair the capacity of ASCs to induce the generation of regulatory T cells in vitro. These data confirm that the immunomodulating features of ASCs are fully functional after exposure to these drugs. Interestingly, whereas MTX did not affect the capacity of natural killer (NK) cells to lyse allogeneic ASCs in vitro, AZA protected allogeneic ASCs from NK cell lysis.
Key words: : azathioprine, cell therapy, mesenchymal stem cells, methotrexate
Introduction
Adult mesenchymal stem or stromal cells (MSCs) can be found in several adult tissues including bone marrow (BM-MSCs) and adipose tissue (ASCs).1–6 In recent years, MSCs have been used as therapeutic tools for the treatment of chronic inflammatory and autoimmune diseases (including rheumatoid arthritis and inflammatory bowel disease) at preclinical and clinical levels7–15 (for an extensive review, Singer and Caplan15). This value of MSCs is based on the capacity to modulate the function of immune cells, where paracrine effects, via a broad panel of soluble factors (with special relevance for tryptophan metabolism through indoleamine 2,3-dioxygenase [IDO] enzymatic activity), and enhanced activity of regulatory T (Treg) cells, have been described as important players.16–19 IDO is a cytoplasmic enzyme that participates in the catabolic metabolism of tryptophan and is known to modulate the immune system and to induce tolerogenic responses.20 IDO is not expressed in resting ASCs, but it is induced by activation through pro-inflammatory mediators and cytokines, mainly interferon (IFN)-γ. In response to IFN-γ, ASCs express IDO and the tryptophan concentration consequently declines rapidly in the surrounding environment with a concomitant accumulation of kynurenine. As a consequence of this modulation of tryptophan metabolism, lymphocyte proliferation is inhibited.17,18 In addition to this immunomodulatory capacity, an additional potential advantage of the clinical use of MSCs is that the immunogenicity of these cells is considered to be low. This is due to the fact that in unstimulated MSCs, the expression of HLA class I is very low and HLA class II and the classic co-stimulatory molecules CD40, CD80, and CD86 are not detectable. Stimulation with IFN-γ has been shown to increase both class I and class II molecules, without affecting the expression of CD40, CD80, and CD86.21–24 It has been proposed that these features may allow MSCs to avoid immune allo-recognition, which is currently under debate.25
The clinical use of MSCs for the treatment of chronic inflammatory and autoimmune diseases is currently being evaluated in patients that are concomitantly treated with other immunomodulatory drugs. This implies that MSCs could possibly interact with these drugs, which may alter their respective therapeutic properties and affect the clinical outcome. Methotrexate (MTX) and azathioprine (AZA) are among the most commonly used anti-inflammatory drugs to treat immune-mediated chronic inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease.26–28 MTX is a folate analogue with anti-inflammatory effects due to the blockage of the synthesis of purine and pyrimidine precursors of DNA and RNA, leading to inhibition of cell proliferation and induction of cell death.29 Likewise, AZA, which is a purine analogue, shows immunosuppressive properties based on the blockage of the synthesis of purines (leading to inhibition of cell proliferation) and the inhibition of CD28 pathway in T lymphocytes by targeting Rac1 activation, leading to increased apoptosis.30,31
So far, little is known about the effects of MTX and AZA on BM-MSC function.32 Moreover, our understanding of what these drugs can do to ASCs is fully missing. We are currently conducting clinical trials using allogeneic ASCs, obtained from liposuction of healthy donors, to treat patients suffering from rheumatoid arthritis (phase I–IIb) or complex perianal fistulae in Crohn's disease (phase III), who were previously or may eventually be treated with MTX or AZA. These drugs may interact with ASCs in the patients and potentially alter their therapeutic properties, while ASCs could in turn alter the AZA or MTX immunosuppressive capacity. Therefore, it is important to investigate the interaction between these drugs and ASCs to learn if their concomitant use might affect each other's therapeutic effects and impact the clinical outcome of the trials.
Here, we report that MTX and AZA do not affect the functionality of ASCs (including viability, IDO activity, capacity to inhibit T lymphocyte proliferation, and capacity to induce Treg cells in vitro). Interestingly AZA, but not MTX, reduced the capacity of natural killer (NK) cells to lyse allogeneic ASCs.
Materials and Methods
Isolation and cell culture
Human adipose tissue aspirates from healthy donors were washed twice with phosphate-buffered saline (PBS) and digested with 0.075% collagenase (Type I, Invitrogen, Carlsbad, CA). The digested sample was washed with 10% fetal bovine serum (FBS), treated with 160 mM NH4Cl to eliminate remaining erythrocytes and suspended in culture medium (Dulbecco's modified Eagle's medium [DMEM] with 10% FBS). Cells were seeded ([2–3]×104 cells/cm2) in tissue culture flasks and expanded (37°C, 5% CO2) with change of culture medium every 3–4 days. Cells were transferred to a new flask (103 cells/cm2) when they reached 90% confluence. Experiments were performed with two male and two female adult donors. Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats using Ficoll-paque Plus (GE Healthcare Biosciences AB, Uppsala, Sweden) following the supplier's protocol. Briefly, blood samples were diluted with balanced salt solution, and Ficoll was added to create a density gradient. After centrifugation of the samples, we gently collected the interface containing mononuclear cells. Purity was verified by flow cytometry. Buffy coats were provided by the National Transfusion Centre of the Comunidad Autónoma of Madrid, Spain.
Reagents and antibodies
Antibodies against HLA-I, HLA-II, CD80, and CD86 were from BD Bioscience (San Jose, CA). 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester (CFSE) and azathioprine (AZA) were from Sigma-Aldrich (St. Louis, MO). Methotrexate (MTX) was from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant human interleukin (IL)-2 and IFN-γ were from Prepotech (London, UK). Anti-CD25 antibody and Pan T cell Activation kit (anti-CD3-, anti-CD2-, and anti-CD28-coated beads) were from Milteny Biotech (Bergisch Gladbach, Germany). Anti-CD3, anti-CD4, and anti-CD8 antibodies and 7-amino-actinomycin D (7-AAD) were from Becton Dickinson (San Diego, CA). Anti-FoxP3 antibody was from eBioscience (San Diego, CA).
Proliferation assays
For the MTT assay, ASC growth was assessed by the CellTiter AQueous One Solution Cell Proliferation Assay (Promega, Madison, WA). Briefly, ASCs were seeded in a 96-well plate (3×103 cells/well) and cultured for 7 days, alone or with MTX (10, 100, 1000 μg/mL) or AZA (0.1, 1, 10, 100, 1000 μM) and absorbance (OD 492 nm) measured in an enzyme-linked immunosorbent assay reader at different time points. Drugs were added to the culture on days 0 and 3. Six independent experiments with four different donors of ASCs were performed and triplicates were included for each condition.
ASC immunogenic phenotype
To determine the effect of the drugs on the immunogenic phenotype of ASCs, cells were either left unstimulated or stimulated with IFN-γ (3 ng/mL) in the absence or presence of MTX (100 μg/mL) or AZA (1 μM) and the expression of HLA-I, HLA-II, CD80, and CD86 was analyzed by flow cytometry after 72 h. A total of 10×103 events were acquired using a FACScalibur (BD Bioscience). CellQuest-pro software was used for acquisition and analysis. CALIBRITE beads (BD Bioscience) were used prior to each assay to calibrate the cytometer.
IDO activity
ASCs seeded in a 24-well plate (6×104 cells/well) were either left untreated or stimulated with IFN-γ (3 ng/mL) in the presence or absence of MTX (100 or 1000 μg/mL) and AZA (1 or 10 μM). IDO activity was measured by determining both tryptophan and kynurenine concentrations on conditioned supernatants at different time points. A total of 100 μL of conditioned supernatants were mixed with 100 μL of buffer phosphate 50 mM and 25 μL of 2 M trichloroacetic acid. After centrifugation for 10 min at 15,600 g, 100 μL of supernatant was analyzed by HPLC (Waters 717plus Autosampler, Milford, MA).
Immunosuppression assay
For CFSE labeling, PBMCs were washed extensively to remove FBS, resuspended in a 10 μM CFSE solution (107 PBMCs per 200 μL of solution), and incubated with constant shaking (37°C, 10 min). Cells were washed and cultured overnight, and one aliquot was used to set up and control FL-1 voltage for CFSE in the cytometer. After overnight resting, CFSE-labeled PBMCs were activated with the Pan T Cell Activation Kit (microbeads coated with anti-CD3, anti-CD2, and anti-CD28, Miltenyi Biotech) following manufacturer's instructions, or were left unstimulated. Two days before PBMC activation, ASCs were seeded in a 24-well plate (4×104 cells/well). PBMCs (106 cells/mL) were cultured alone or with ASCs, with or without MTX (0.01 or 100 μg/mL) or AZA (1 or 10 μM). At day 5, PBMCs were harvested, labeled with 7-AAD and anti-CD3 antibody, and cell proliferation of the CD3+/7-AAD− population (viable CD3 T lymphocytes) was determined according to loss of CFSE signal. For pretreatment experiments, ASCs were cultured 7 days alone or with MTX (0.01 or 100 μg/mL) or AZA (1 or 10 μM). Culture media were refreshed at day 3. Then, ASCs were washed twice with PBS, CFSE-labeled PBMCs (106 cells/mL) were cultured with pretreated ASCs, and proliferation was determined as indicated before. Data were analyzed using FCSExpress 4 software. CaliBRITE beads (BD Bioscience) were used prior to each assay to calibrate the cytometer.
Generation of Treg cells
PBMCs (2×106 cells/well) were cultured with IL-2 (100 U/mL) in the presence or absence of MTX (0.01 or 100 μg/mL) or AZA (1 or 10μM), either alone or with ASCs (ratio 1:50, ASC:PBMC). After 7 days in culture, PBMCs were harvested and stained with specific antibodies against CD3, CD4, CD25, and Foxp3. The Treg cell population was defined as CD4+CD25highFoxp3+. Results were expressed as the ratio of Treg cells of PBMCs+ASCs relative to PBMCs alone for the corresponding condition (PBMCs+ASCs/PBMCs). Experiments were done with three different buffy coats and four different ASC donors.
Cell-mediated lympholysis assay
Cytotoxicity-mediated lysis of ASCs was determined by europium release assay as described previously.33 In brief, ASCs were used as target cells and were labeled with europium–diethylenetriaminepentaacetate (Sigma-Aldrich). Effector cells (cell-sorted NK or CD8 cells) were incubated with 5000 target cells at effector-to-target (E:T) ratios of 40:1 to 0.6:1 in round-bottom 96-well plates (Nunc, Roskilde, Denmark) for 4 h at 37°C in the presence of AZA (1 μM), MTX (100 μg/mL), or regular medium. The plates were then centrifuged and 20 μL of the supernatant was transferred to 96-well plates with low background fluorescence (fluoroimmunoplates [FluoroNunc plates]; Nunc). Subsequently, 100 μL of enhancement solution (PerkinElmer, Groningen, The Netherlands) was added to each well. Released europium fluorescence was measured using a fluorometer Victor2 1420 multilabel counter (Wallac, MA). Maximal release of europium by target cells was measured by incubation of 5000 labeled target cells with 1% Triton (Sigma-Aldrich, Zwijndrecht, The Netherlands) for 4 h. Spontaneous release of europium was measured by incubation of labeled target cells without effector cells for 4 h. Percentage leakage was then calculated as: (spontaneous release/maximal release)×100%. The percentage cytotoxicity-mediated lysis was calculated as follows: percent lysis=(measured lysis − spontaneous release)/(maximal release − spontaneous release)×100%.
NK cell apoptosis assay
NK cells were isolated from PBMCs by cell sorting (MACS, Miltenyi Biotech) and cultured without or with MSCs at different ratios (40:1, 20:1, 10:1) and without or with AZA (1 μM) or MTX (100 μg/mL) in the same way it was done for the cell-mediated lympholysis (CML) assay. After 4 h of co-incubation, NK cells were recovered from the cultures, washed, and stained for Annexin V-PE and 7AAD for apoptosis evaluation by flow cytometry (Facs Canto, BD Biosciences). Alive (Annexin V− 7AAD−), early apoptosis (Annexin V+ 7AAD−), and late apoptosis (Annexin V+ 7AAD+) cells were evaluated.
Results
MTX and AZA do not affect ASC viability and proliferation
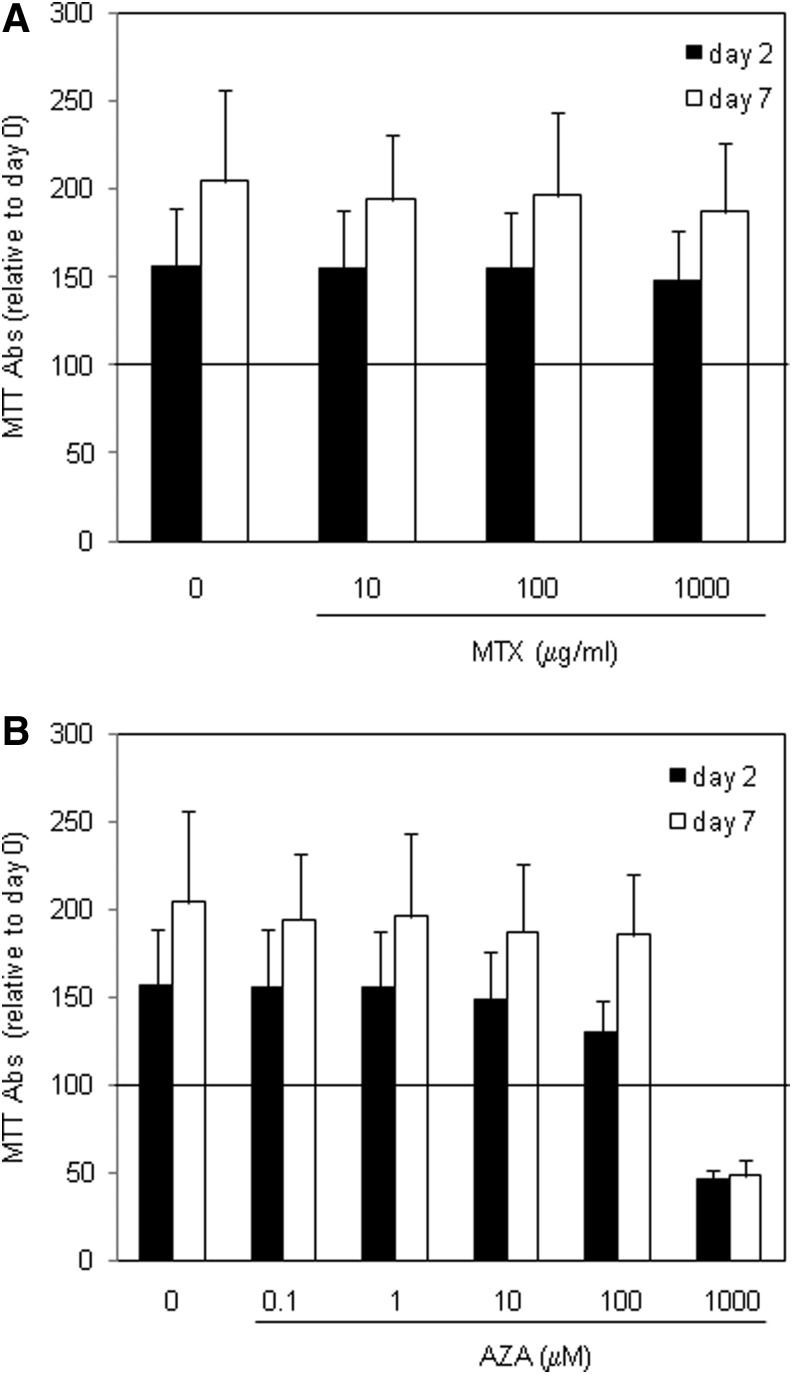
MTX and AZA are immunosuppressors with a cytotoxic capacity that rely on their capability to inhibit cell proliferation and viability, which may also potentially affect ASCs. Therefore, the effect of MTX and AZA on viability and proliferation of ASCs was studied. ASCs were cultured in the absence or presence of clinically relevant concentrations of MTX or AZA,32 and viability and proliferation were evaluated at different time points by MTT assay. In the absence of any drug, ASCs were viable and proliferated during the 7 days of follow-up, as indicated by increased MTT conversion after 2 and 7 days compared to day 0 (Fig. 1). Incubation with MTX did not significantly affect the viability and proliferation of ASCs at the concentrations employed (10, 100, 1000 μg/mL) because MTT activity was similar at days 2 and 7 to that of the ASCs alone (Fig. 1A). Incubation with AZA did not affect viability and proliferation between 0.1 and 10 μM (considered clinically relevant), but was toxic at the high concentration of 1000 μM, which resulted in a drastic reduction in viability (MTT measurements at day 2 below 50% of those at day 0) and proliferation (MTT levels at day 7 similar to those at day 2; Fig. 1B). These results demonstrate that the viability of ASCs and their capacity to proliferate are not reduced by relevant concentrations of MTX and AZA.
FIG. 1.
Viability of ASCs after treatment with the drugs. Cells were cultured in the absence or presence of increasing concentrations of MTX (A) or AZA (B) and viability and proliferation was monitored by MTT assay at day 2 (white bars) and day 7 (black bars). Mean and standard deviation relative to day 0 are represented. N=6. Abs, absorbance; ASC, adipose mesenchymal stem or stromal cells; MTX, methotrexate; AZA, azathioprine.
MTX and AZA do not affect the induction of IDO by ASCs
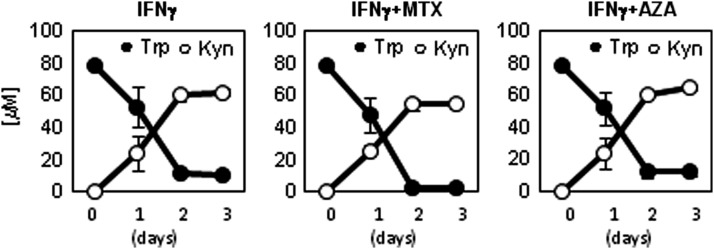
We studied the effect of MTX and AZA on the expression of IDO in ASCs because any interference in this enzymatic activity may potentially impair ASC-mediated immunomodulation and therapeutic effects. Therefore, we stimulated ASCs with IFN-γ (3 ng/mL) in the absence or presence of MTX (100 or 1000 μg/mL) or AZA (1 or 10 μM), and concentrations of tryptophan and kynurenine (indicative of IDO activity) were determined by HPLC in conditioned supernatants harvested at different time points. As expected, unstimulated ASCs did not metabolize tryptophan, indicating that IDO is not expressed in resting conditions (data not shown). However, when ASCs were stimulated with IFN-γ, IDO activity was rapidly induced as demonstrated by the quick reduction of tryptophan concentration and the concomitant accumulation of kynurenine (Fig. 2). Incubation of ASCs with MTX or AZA alone did not induce IDO activity (data not shown). Presence of MTX or AZA at the selected concentrations did not significantly alter IFN-γ–mediated IDO induction (Fig. 2).
FIG. 2.
Effect of MTX and AZA on induction of IDO activity by ASCs. ASCs were stimulated with IFN-γ (3 ng/mL) in the absence or presence of MTX (100 μg/mL) or AZA (1 μM), and concentration of tryptophan and kynurenine was determined by HPLC at different time points. Mean and standard deviation of four experiments are shown. Similar results were observed with MTX (1000 μg/mL) and AZA (10 μM). IDO, indoleamine 2,3-dioxygenase; IFN, interferon.
Effect of MTX and AZA on the capacity of ASCs to inhibit lymphocyte proliferation
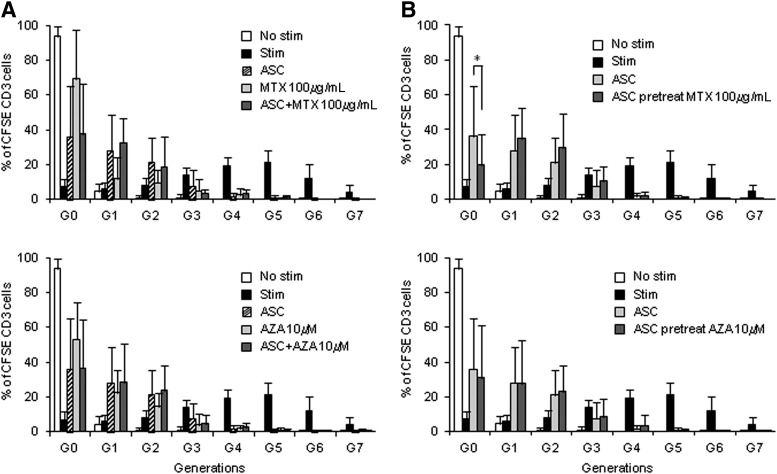
MTX and AZA, like ASCs, have immunomodulatory properties, which may directly affect the function of ASCs or could indirectly affect the mode of action of ASCs by their interaction with the same immune cell types. This may lead to synergistic or antagonizing effects when used concomitantly. Therefore, we investigated the effect of the concomitant use of the drugs on the immunomodulatory capacity of ASCs in vitro. PBMCs were stimulated alone or in the presence of ASCs (ratio 1:25 ASC:PBMC), MTX (0.01 μg/mL, 100 μg/mL), AZA (1 μM, 10 μM), or a combination of ASCs and the drugs at the indicated concentrations. Proliferation of viable CD3 T cells was determined after 120 h by flow cytometry. T lymphocytes proliferated up to seven generations (G7) upon stimulation (Fig. 3). ASCs, MTX, and AZA shared a strong inhibitory effect because T lymphocytes were not able to appreciably proliferate beyond the third generation (G3) in their presence. The inhibitory effect seemed to differ between treatments because MTX (at both concentrations) and AZA (10 μM) inhibited proliferation mostly at G0 (percentage of T cells at G0 with MTX 100 μg/mL: 69.22%±27.92%; MTX 0.01 μg/mL: 61.22%±26.36%; AZA 10 μM: 52.96%±21.15%), while AZA (1 μM) and ASCs did not (AZA 1 μM: 25.86%±14.24%; ASCs: 36.02%±28.83%; Fig. 3A and data not shown). When ASCs were combined with MTX or AZA, the inhibitory effect was similar to that found with ASCs alone (Fig. 3A and data not shown). We could not find synergism between the drugs and ASCs when combined together, but rather a predominant effect of ASCs over the drugs as the percentage of T cells retained in G0 was similar to that of ASCs alone (ASCs alone: 36.02%±28.83%; ASCs+MTX 0.01 μg/mL: 38.59%±29.89%; ASCs+MTX 100 μg/mL: 37.49%±29.89%; ASC+AZA 10 μM: 36.51%±28.22%; Fig. 3A and data not shown).
FIG. 3.
Effect of MTX and AZA on ASC-mediated inhibition of T-lymphocyte proliferation. (A) CFSE-labeled PBMCs were stimulated with anti-CD3/CD2/CD28-coated beads in the absence or presence of ASCs (ratio 1:25 ASC:PBMC), MTX (100 μg/mL), AZA (10μM), or a combination of ASCs and drugs. Proliferation of the viable population of CD3+ cells (CD3+/7AAD− cells) was monitored after 120 h by flow cytometry, and the percentage of cells per generation was determined using FCSExpress software. Mean and standard deviation of the percentage of cells accumulated on each generation is shown. N=8. Similar results were obtained when combination of ASCs with MTX (0.01 μg/mL) and AZA (1 μM) were used. (B) The immunosuppressive capacity of ASCs pretreated with the drugs was studied. ASCs were cultured for 7 days in medium alone or with MTX (100 μg/mL) or AZA (10 μM). Then, PBMCs were stimulated in the presence of control or MTX- or AZA-pretreated ASCs (ratio 1:25 ASCs:PBMCs) and proliferation of the viable population of CD3+ cells was monitored after 120 h by flow cytometry. Mean and standard deviation of the percentage of cells accumulated in each generation is shown. N=8. Similar results were obtained when ASCs were pretreated with MTX (0.01 μg/mL) and AZA (1 μM). *p=0.043. CFSE, carboxyfluorescein diacetate N-succinimidyl ester; PBMCs, peripheral blood mononuclear cells.
Furthermore, we tested the immunosuppressive capacity of ASCs pretreated with the drugs. To do so, ASCs were cultured for 7 days in medium alone or with MTX (0.01 or 100 μg/mL) or AZA (1 or 10 μM). Then, PBMCs were stimulated in the presence of control ASCs (pretreated with medium alone) or ASCs pretreated with MTX or AZA and proliferation was monitored after 120 h. Our results show that, overall, ASCs pretreated with the drugs maintained a strong immunomodulatory capacity, similar to control ASCs because no significant proliferation of T lymphocytes beyond third generation (G3) was observed (Fig. 3B). However, while pretreatment with AZA or MTX at 0.01 μg/mL did not affect ASC inhibitory effect between G0 and G3, pretreatment with MTX at the higher concentration (100 μg/mL) reduced the percentage of T cells in G0 compared to control ASCs (ASCs: 36.02%±28.83% vs. ASCs pretreated with MTX: 19.79%±17.78%, in G0). This suggests that pretreatment of ASCs with high doses of MTX might slightly impact the capacity of ASCs to inhibit T-cell proliferation (Fig. 3B).
MTX and AZA do not affect the capacity of ASCs to induce Treg cells in vitro
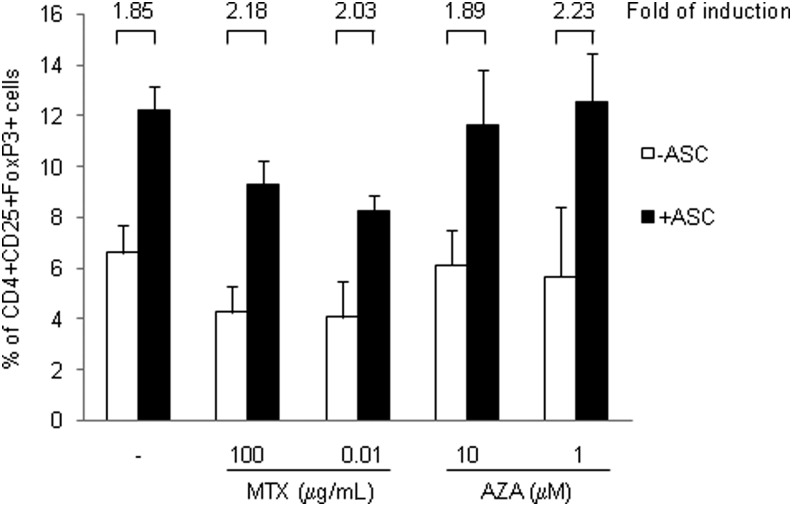
ASCs have been described to induce the generation of Treg cells both in vitro and in vivo, which is believed to have an important role in their immunomodulating properties.7–10,19 To determine whether MTX or AZA may influence this capacity of ASCs, PBMCs were activated with IL-2 (100 U/mL) and cultured in the following conditions: alone, with MTX (0.01 or 100 μg/mL), with AZA (1 or 10 μM), with ASCs, with ASC+MTX (both concentrations) or ASC+AZA (both concentrations). After 7 days in culture, the percentage of Treg population (CD4+CD25highFoxP3+ cells) was determined by flow cytometry. Culture of PBMCs with AZA did not affect the percentage of the Treg population, but when PBMCs were cultured with MTX, a reduced percentage of Treg cells was found (PBMCs: 6.6%±1.08%; PBMC+MTX 100 μg/mL: 4.26%±1.01%; PBMCs+MTX 0.01 μg/mL: 4.06%±1.4%; Fig. 4). As expected, culture of PBMCs with ASCs increased the percentage of Treg population (PBMCs: 6.6%±1.08%; PBMCs+ASCs: 12.23%±0.94%). Importantly, when PBMCs were cultured concomitantly with ASCs and MTX or AZA, the relative induction of the Treg population by ASCs was not affected (fold of induction range 1.85 to 2.23). These results indicate that MTX and AZA do not interfere with the mechanism of induction of Treg cells by ASCs.
FIG. 4.
MTX and AZA did not affect the generation of Treg cells by ASCs. PBMCs were cultured alone or with ASCs and stimulated with IL-2 (100 U/mL) in the absence or presence of MTX (0.01 or 100 μg/mL) and AZA (1 or 10 μM). The percentage of Treg population (defined as CD4+CD25+Foxp3+ cells) was measured at 7 days. White bars: PBMCs without ASCs. Black bars: PBMCs with ASCs. Fold of induction for each condition relative to the corresponding control without ASCs is also represented. n=3. Treg cells, regulatory T cells; IL, interleukin.
MTX and AZA do not affect the immunogenic phenotype of ASCs
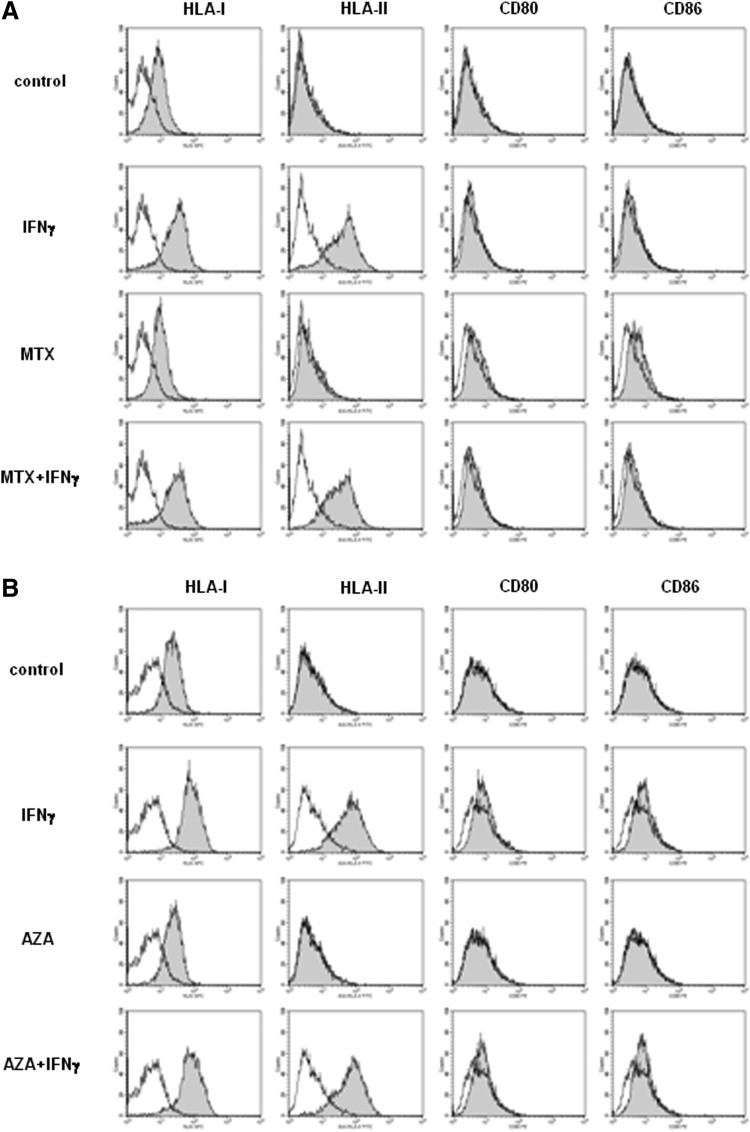
From an immunogenic point of view, MSCs are characterized by low expression of HLA-I and absence of expression of HLA-II, CD80, and CD86. To determine the effect of MTX and AZA on the immunogenicity of ASCs, we studied the effect of the drugs on the expression of HLA-I, HLA-II, CD80, and CD86 in resting conditions or upon stimulation with IFN-γ, known to up-regulate the expression of HLA-I and to induce the expression of HLA-II, without affecting the expression of CD80 and CD86. Therefore, ASCs were either left unstimulated or stimulated with IFN-γ (3 ng/mL) in the absence or presence of MTX (100 μg/mL) or AZA (1 μM) and the expression of these markers was analyzed by flow cytometry after 72 h. As expected, stimulation with IFN-γ increased the expression of HLA-I and induced the expression of HLA-II (Fig. 5). Expression of CD80 and CD86 was considered negative, although in our hands a slight shift of the peak could be observed in some experiments when these antibodies were used; this is believed to be part of the typical variability between experiments. Incubation of ASCs with MTX or AZA did not alter the expression of these markers compared to control resting or IFN-γ–stimulated ASCs. These results indicate that these drugs do not increase immunogenicity of ASCs (as measured by cell surface marker profile).
FIG. 5.
MTX and AZA did not alter the expression of immunogenic markers on ASCs. ASCs were stimulated or not with IFN-γ (3 ng/mL) in the absence or presence of 100 μg/mL of MTX (A) or 1 μM of AZA (B) and the expression of HLA-I, HLA-II, CD80, and CD86 was determined by flow cytometry after 72 h. Specific antibodies (gray peak) and isotype controls (white peak). One representative experiment is shown (n=6).
AZA impairs NK cell-mediated lysis of ASCs
Although allogeneic ASCs are considered to be immunoprivileged, it has been previously demonstrated that activated NK cells and, to a minor extent, CD8 T cells are capable of lysing allogeneic ASCs.33 In order to better understand how MTX and AZA may influence the immune reactions against allogeneic ASCs, we evaluated the effect of the drugs on the capacity of NK and CD8 T cells to lyse allogeneic ASCs.
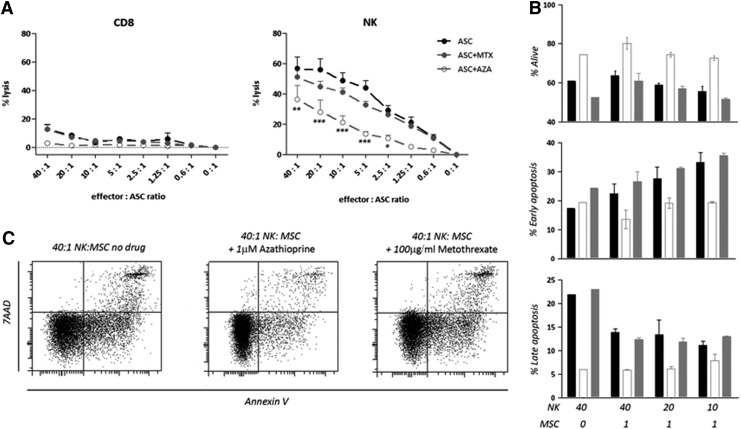
ASCs were labeled with europium and exposed to NK or CD8 T cells in the presence of MTX (100 μg/mL), AZA (1 μM), or medium without drug. NK cells were able to lyse ASCs, whereas lysis by CD8 T cells was hardly detectable. The presence of MTX did not affect the lysis of ASCs by NK cells. However, in the presence of AZA there was a statistically significant reduction in NK cell–mediated lysis compared to the lysis in the absence of drug (Fig. 6A).
FIG. 6.
Effect of MTX and AZA on the capacity of NK and CD8 T cells to lyse allogeneic ASCs. (A) Pre-activated and sorted CD8 T and NK cells lyse ASCs in a ratio-dependent manner (●). The presence of MTX (100 μg/mL;  ) did not affect the lysis of the ASCs while AZA (1 μM; ○) reduced the lysis mediated by NK cells even at low ratios of 2.5:1. Mean and standard deviations of four experiments are shown. *p<0.05, **p<0.01, ***p<0.001. (B) Apoptosis was tested on NK cells with ASCs at different ratios (40:1 to 10:1) or without ASCs (40:0). Black bars represent no drug, white bars represent AZA (1 μM) and gray bars represent MTX (100 μg/mL). (C) Representative plots of the apoptosis on NK cells in the presence of ASCs at a 40:1 ratio with or without AZA or MTX.
) did not affect the lysis of the ASCs while AZA (1 μM; ○) reduced the lysis mediated by NK cells even at low ratios of 2.5:1. Mean and standard deviations of four experiments are shown. *p<0.05, **p<0.01, ***p<0.001. (B) Apoptosis was tested on NK cells with ASCs at different ratios (40:1 to 10:1) or without ASCs (40:0). Black bars represent no drug, white bars represent AZA (1 μM) and gray bars represent MTX (100 μg/mL). (C) Representative plots of the apoptosis on NK cells in the presence of ASCs at a 40:1 ratio with or without AZA or MTX.
To test whether the effect of reduced lysis of target ASCs by the effector NK cells in the presence of AZA was due to increased NK cell death and therefore to the presence of fewer effectors, we evaluated the apoptosis on NK cells in this setting. Apoptosis was evaluated by Annexin V and 7AAD staining classifying three populations: alive (Annexin V− 7AAD−), early apoptosis (Annexin V+ 7AAD−), and late apoptosis (Annexin V+ 7AAD+; Fig. 6B and 6C). We observed that NK cell death was not increased by AZA but rather reduced when the drug was added to the cultures, while MTX had no effect on activation-induced NK cell death with levels comparable to the culture in the absence of drug. Thus, the observed decreased killing of ASCs by NK cells in the presence of AZA is not due to AZA toxicity on NK cells.
Discussion
Cell therapy using MSCs to treat chronic inflammatory and autoimmune disorders (e.g., rheumatoid arthritis or inflammatory bowel disease) is currently under evaluation at the clinical level. Patients enrolled in these trials are likely to be or to have been under treatment with immunosuppressive drugs such as MTX and AZA. Therefore, it is important to understand the interactions between these drugs and the MSCs because these may impact their therapeutic effects and clinical outcome. Interaction between these drugs and MSCs has been poorly studied so far. Recently, Duijvestein et al.32 reported that drugs used in the treatment of inflammatory bowel disease, including MTX and AZA, do not affect BM-MSC phenotype, viability, differentiation, or immunosuppressive capacity. Along the same line, our results demonstrate that exposure of ASCs to MTX and AZA does not affect their viability, phenotype, or immunomodulating properties in vitro. Moreover we investigated, for the first time to our knowledge, the effects of these drugs on other important functions of MSCs, such as the capacity to induce the generation of Treg cells and the allo-recognition of ASCs by cytolytic immune cells.
Exposure to MTX and AZA did not affect viability and proliferation of ASCs. Taking into account that MTX and AZA affect the synthesis of DNA, a basic step in the biology of any cell type including MSCs, one explanation for the absence of effect on MSC proliferation might be the low duplication rates of MSCs in vitro compared with activated lymphocytes. Alternatively, ASCs might have some still unknown mechanism of drug resistance.
In the clinical application of ASC for inflammatory and autoimmune diseases like rheumatoid arthritis or inflammatory bowel disease, it is highly probable that these cells will encounter different levels of MTX and AZA, which may imply synergistic or antagonizing effects to each other. We tried to titer MTX and AZA immunosuppressive effects in vitro, in order to find concentrations of the drugs with different levels of inhibition on T-lymphocyte proliferation, which would have allowed us to better study synergistic or antagonizing effects between the drugs and ASCs. That was feasible for AZA, but, in our hands, MTX turned out to be a very potent inhibitor, and we could not find concentrations with weak or intermediate inhibitory effects: MTX either mainly inhibited T-lymphocyte proliferation at G0 (0.01–1000 μg/mL) or had no inhibitory effect (0.001–0.005 μg/mL). Nevertheless, in all the tested conditions, ASCs and both drugs highly impaired proliferation beyond the G3 (Fig. 3A). We could not find synergism between the drugs and ASCs when combined together, but rather a predominant effect of ASCs over the drugs, with the percentage of cells in G0 being similar to that of ASCs alone. Pretreatment of ASCs with the drugs did not significantly affect their immunosuppressive effect, although some reduction of the percentage of T cells in G0 was observed in MTX-pretreated ASCs compared with ASCs alone, suggesting some effect of the drug. However, this effect was relatively minor because it did not affect the complete inhibition of lymphocyte proliferation beyond G3 (Fig. 3B). Furthermore, despite the effects that MTX and AZA exert on T lymphocytes, they did not impair the capacity of ASCs to induce the generation of Treg cells in vitro (Fig. 4), confirming that the immunomodulating features of ASCs are fully functional after exposure to these drugs.
Importantly, a differential effect of MTX and AZA regarding the allo-recognition of ASCs was observed. It has been previously shown that NK cells but not CD8 can lyse nonactivated ASCs in a dose-dependent manner due to the low expression of HLA-I on their surface,33 so we aimed to analyze the effect of AZA and MTX on ASC cell-mediated lysis by NK or CD8 cells. Whereas MTX did not affect the capacity of NK or CD8 T cells to lyse allogeneic ASCs in vitro, AZA significantly affected the cytolytic activity of NK cells, but not CD8 T cells. These results indicate that the reduced cell-mediated lysis observed in the presence of AZA is not due to toxicity and increased death of NK cells but rather to a reduced activation and degranulation of NK cells, as reflected by lower activation-induced cell death.34 Based on these results, it might be speculated that patients could benefit from concomitant treatment with AZA because the clearance of allogeneic ASCs might be delayed, providing allogeneic ASCs with an extended time frame to carry out their therapeutic effect.
Overall, our results show that the main functional properties of ASCs considered to be of therapeutic relevance in controlling inflammation are not impaired by the interaction with MTX and AZA.
Abbreviations
- 7-AAD
7-amino-actinomycin D
- ASCs
adipose mesenchymal stem or stromal cells
- AZA
azathioprine
- BM
bone marrow
- CFSE
carboxyfluorescein diacetate N-succinimidyl ester
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- IDO
indoleamine 2,3-dioxygenase
- IFN
interferon
- IL
interleukin
- MSCs
mesenchymal stem or stromal cells
- MTX
methotrexate
- NK
natural killer
- PBMCs
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- Treg cells
regulatory T cells
Acknowledgments
We thank Debjani Roy (TiGenix NV) for critical reading of the manuscript. The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under the grant agreement no. 279174 to TiGenix and Erasmus. This work was supported by funding from the Ministry of Science and Innovation of Spain (INNPACTO IPT-010000-2010-40) to TiGenix.
Author Disclosure Statement
P.M.-C., O.R., C.R., L.G.-B., V.F., R.M., A.B., W.D., and E.L. are full-time employees of TiGenix. M.F. and M.J.H. report no competing financial interests.
References
- 1.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274 [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuchi Y, Nakajima H, Sugiyama D, et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658 [DOI] [PubMed] [Google Scholar]
- 4.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110 [DOI] [PubMed] [Google Scholar]
- 5.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bari C, Dell'Accio F, Tylzanowski P, et al. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942 [DOI] [PubMed] [Google Scholar]
- 7.González MA, Gonzalez-Rey E, Rico L, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989 [DOI] [PubMed] [Google Scholar]
- 8.González MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019 [DOI] [PubMed] [Google Scholar]
- 9.González-Rey E, Anderson P, González MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939 [DOI] [PubMed] [Google Scholar]
- 10.González-Rey E, Gonzalez MA, Varela N, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248 [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86 [DOI] [PubMed] [Google Scholar]
- 12.Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662–1669 [DOI] [PubMed] [Google Scholar]
- 13.Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798 [DOI] [PubMed] [Google Scholar]
- 14.de la Portilla F, Alba F, García-Olmo D, et al. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn's disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323 [DOI] [PubMed] [Google Scholar]
- 15.Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol Mech Dis. 2011;6:457–478 [DOI] [PubMed] [Google Scholar]
- 16.Doorn J, Moll G, Le Blanc K, et al. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev. 201218:101–115 [DOI] [PubMed] [Google Scholar]
- 17.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621 [DOI] [PubMed] [Google Scholar]
- 18.DelaRosa O, Lombardo E, Beraza A, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795–2806 [DOI] [PubMed] [Google Scholar]
- 19.Maccario R, Podestà M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525 [PubMed] [Google Scholar]
- 20.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896 [DOI] [PubMed] [Google Scholar]
- 22.Majumdar MK, Keane-Moore M, Buyaner D, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241 [DOI] [PubMed] [Google Scholar]
- 23.McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253 [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749 [DOI] [PubMed] [Google Scholar]
- 25.Griffin MD, Ryan AE, Alagesan S, et al. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013;91:40–51 [DOI] [PubMed] [Google Scholar]
- 26.Kay J, Westhovens R. Methotrexate: the gold standard without standardisation. Ann Rheum Dis. 2009;68:1081–1082 [DOI] [PubMed] [Google Scholar]
- 27.Preiss JC, Zeitz M. Use of methotrexate in patients with inflammatory bowel diseases. Clin Exp Rheumatol. 2010;28:S151–S155 [PubMed] [Google Scholar]
- 28.Blonski W, Buchner AM, Lichtenstein GR. Inflammatory bowel disease therapy: current state-of-the-art. Curr Opin Gastroenterol. 2011;27:346–357 [DOI] [PubMed] [Google Scholar]
- 29.Cutolo M, Sulli A, Pizzorni C, et al. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2001;60:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maltzman JS, Koretzky GA. Azathioprine: old drug, new actions. J Clin Invest. 2003;111:1122–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duijvestein M, Molendijk I, Roelofs H, et al. Mesenchymal stromal cell function is not affected by drugs used in the treatment of inflammatory bowel disease. Cytotherapy. 2011;13:1066–1073 [DOI] [PubMed] [Google Scholar]
- 33.Crop MJ, Korevaar SS, de Kuiper R, et al. Human mesenchymal stem cells are susceptible to lysis by CD8(+) T cells and NK cells. Cell Transplant. 2011;20:1547–1559 [DOI] [PubMed] [Google Scholar]
- 34.Cseuz R, Panayi GS. The inhibition of NK cell function by azathioprine during the treatment of patients with rheumatoid arthritis. Br J Rheumatol. 1990;29:358–362 [DOI] [PubMed] [Google Scholar]