Abstract
Aims: Hemoglobin-based oxygen carriers (HBOC) provide a potential alternative to red blood cell (RBC) transfusion. Their clinical application has been limited by adverse effects, in large part thought to be mediated by the intravascular scavenging of the vasodilator nitric oxide (NO) by cell-free plasma oxy-hemoglobin. Free hemoglobin may also cause endothelial dysfunction and platelet activation in hemolytic diseases and after transfusion of aged stored RBCs. The new soluble guanylate cyclase (sGC) stimulator Bay 41-8543 and sGC activator Bay 60-2770 directly modulate sGC, independent of NO bioavailability, providing a potential therapeutic mechanism to bypass hemoglobin-mediated NO inactivation. Results: Infusions of human hemoglobin solutions and the HBOC Oxyglobin into rats produced a severe hypertensive response, even at low plasma heme concentrations approaching 10 μM. These reactions were only observed for ferrous oxy-hemoglobin and not analogs that do not rapidly scavenge NO. Infusions of L-NG-Nitroarginine methyl ester (L-NAME), a competitive NO synthase inhibitor, after hemoglobin infusion did not produce additive vasoconstriction, suggesting that vasoconstriction is related to scavenging of vascular NO. Open-chest hemodynamic studies confirmed that hypertension occurred secondary to direct effects on increasing vascular resistance, with limited negative cardiac inotropic effects. Intravascular hemoglobin reduced the vasodilatory potency of sodium nitroprusside (SNP) and sildenafil, but had no effect on vasodilatation by direct NO-independent activation of sGC by BAY 41-8543 and BAY 60-2770. Innovation and Conclusion: These data suggest that both sGC stimulators and sGC activators could be used to restore cyclic guanosine monophosphate-dependent vasodilation in conditions where cell-free plasma hemoglobin is sufficient to inhibit endogenous NO signaling. Antioxid. Redox Signal. 19, 2232–2243.
Introduction
In several clinically relevant conditions, such as hemolytic diseases (e.g., sickle cell disease [SCD]) (25), during the infusion of hemoglobin-based oxygen carriers (HBOCs) and after blood transfusion (2, 5), plasma levels of free hemoglobin are increased. Hemoglobin not only binds and transports oxygen in the circulation, but is also a potent scavenger of nitric oxide (NO) (6). Besides being a potent vasodilator (12, 22), NO also inhibits platelet aggregation, plays a role in neurotransmission, and acts as an antioxidant and host defense molecule (18). The signaling functions of NO within the vessel wall are maintained in the presence of large concentrations of intravascular hemoglobin, because the compartmentalization of hemoglobin in red blood cells (RBCs) greatly limits the rate of NO-scavenging reactions. The NO-scavenging rate of red cell hemoglobin is reduced by a red cell free zone along the endothelium in laminar flowing blood, extracellular diffusion of NO to the RBC, and reduced NO diffusion over the RBC membrane (13, 15). However, all three of these mechanisms that limit NO scavenging by intra-erythrocytic hemoglobin are eliminated during red cell hemolysis or during the direct intravascular infusions of hemoglobin and HBOCs. The amount of bioavailable NO will, therefore, be lower in the presence of free plasma hemoglobin and cause vasoconstriction and hypertension, increased platelet aggregation, and other clinical side effects related to NO depletion (27).
Innovation.
Hemoglobin-based oxygen carriers (HBOC) provide a potential alternative to red blood cell transfusion. Their clinical application has been limited by adverse effects, largely thought to be mediated by the intra-vascular scavenging of the vasodilator nitric oxide (NO) by cell-free plasma oxy-hemoglobin.
We show that both the soluble guanylate cyclase (sGC) stimulator Bay 41-8543 and the sGC activator Bay 60-2770 restore cyclic guanosine monophosphate-dependent vasodilation when cell-free plasma hemoglobin is sufficient to inhibit endogenous NO signaling. These results imply that these drugs could be used to bypass hemoglobin-mediated NO inactivation and provide a potential therapy.
Until recently, the link between NO scavenging by cell-free plasma hemoglobin and clinical complications has been disregarded, as cell-free plasma hemoglobin levels in patients were very small (25). However, changes in vascular function have been demonstrated in SCD patients with plasma heme concentrations as low as 6 μM (25). NO scavenging by hemoglobin inhibits NO signaling, leading to acute endothelial cell dysfunction and NO resistance, and with lifelong disease a proliferative vasculopathy evolves, which is characterized by both systemic and pulmonary hypertension (7, 10, 25, 28, 41).
Acute systemic hypertension during infusion of HBOCs has been appreciated for more than a decade, while a recent meta-analysis of clinical trials suggests a higher risk for myocardial infarction and death (20). The serious adverse event profiles among these products suggest a common underlying mechanism or mechanisms of toxicity, and one of the candidates is NO scavenging (31).
We have recently suggested that increases in cell-free plasma hemoglobin after the transfusion of stored RBCs could be a new mechanism for endothelial injury and impaired vascular function associated with the most fundamental of blood storage lesions, hemolysis (5).
Previous studies in SCD patients and patients receiving HBOCs have attempted to counteract the NO-scavenging effects using direct NO donor medications, such as sodium nitroprusside (SNP), and the endogenous NO-dependent phosphodiesterase-5 (PDE-5) inhibitors, such as sildenafil. However, the potencies of these agents are reduced due to NO reactions with high levels of intravascular hemoglobin (25). To overcome the reduced potency of NO donors, such therapies should be dosed in a higher concentration, which potentially results in additional adverse effects and off-target reactions that may drive nitration reactions and methemoglobin formation. Although a PDE-5 inhibitor such as sildenafil acts more downstream in the NO signaling pathway by preventing the breakdown of cyclic guanosine monophosphate (cGMP) formed after soluble guanylate cyclase (sGC) activation, sGC initially still needs to be activated by endogenous NO.
Since the emergence of sGC as a therapeutic target for cardiovascular disease, two classes of molecules have been developed: sGC stimulators and sGC activators. sGC stimulators, such as BAY 41-2272 and Riociguat (BAY 63-2521), act directly on native, ferrous sGC that sensitizes sGC to low levels of bioavailable NO by stabilizing the nitrosyl-heme complex, maintaining the enzyme in its active configuration (4, 34). In contrast, recently discovered sGC activators, such as BAY 60-2770 and BAY 58-2667 (cinaciguat), effectively activate sGC even when it is in an oxidized or heme-free state (16, 23, 29, 34, 37).
We hypothesized that a drug which directly activates sGC independent of the presence of NO, and is not scavenged by hemoglobin, would be advantageous in the presence of HBCOs, intravascular hemolysis, or during transfusion of aged blood. We, therefore, have tested whether both the sGC stimulator BAY 41-8543 and sGC activator BAY 60-2770 could be used to restore vasodilation in the presence of intravascular hemoglobin.
Results
Effect of human hemoglobin and HBOC on mean arterial pressure
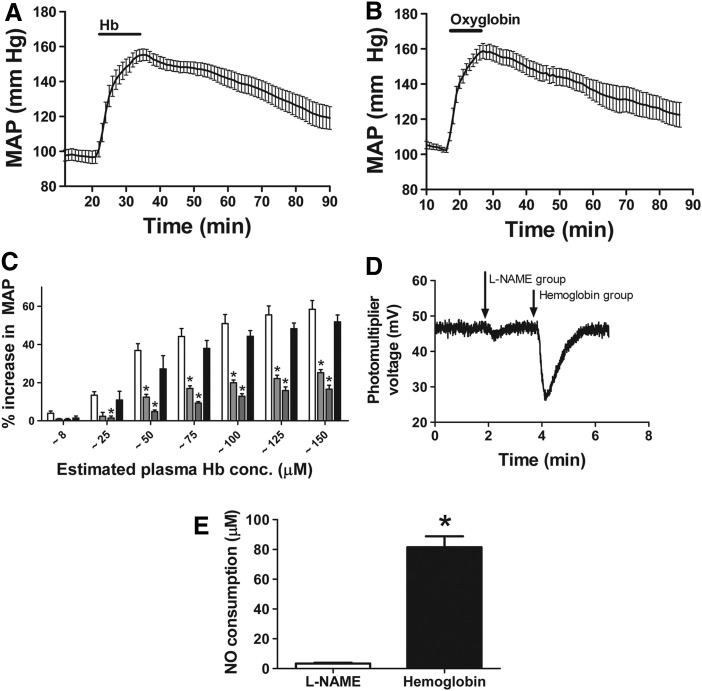
Intravenous infusion of 175 mg/kg purified human hemoglobin in anaesthetized rats immediately increased mean arterial pressure (MAP) from a baseline value of 97±4 mm Hg to 155±4 mg Hg (54% increase; paired t-test, p<0.0001, n=6) (Fig. 1A). After infusion had been discontinued, the MAP slowly dropped to 119±7 mm Hg (21% decrease) over an hour. To confirm that this effect was likely mediated by NO scavenging, and similar to effects of HBOCs, we compared the hypertensive effects in this top-load model to Oxyglobin, a HBOC with similar intrinsic NO-scavenging reaction rates as human hemoglobin. Oxyglobin elicited a similar effect on blood pressure (Fig. 1B), and both human hemoglobin and Oxyglobin produced an effect on blood pressure at concentrations as low as 8 μM (heme concentrations, Fig. 1C). Consistent with our earlier studies (5), conversion of hemoglobin to methemoglobin or cyano-methemoglobin inhibited the vasoconstriction effects and the ability of plasma to consume NO, suggesting that direct NO scavenging was responsible for the vasoconstriction observed in this model (Fig. 1C).
FIG. 1.
Effect of hemoglobin and Oxyglobin infusion on mean arterial pressure (MAP). (A) Average change in MAP over time after infusion of 175 mg/kg purified human hemoglobin (Mean±SEM, n=6). (B) Average change in MAP over time after infusion of 175 mg/kg Oxyglobin (Mean±SEM, n=5). (C) Percentage increase in MAP after infusion of 175 mg/kg hemoglobin analogs. Plasma concentration was estimated to be ∼8, 25, 50, 75, 100, 125, and 150 μM. Human hemoglobin (▭), methemoglobin ( ), cyano-methemoglobin (
), cyano-methemoglobin ( ), and Oxyglobin (
), and Oxyglobin ( ). (Mean±SEM, n=5). *Significantly different (p<0.05) from hemoglobin by two-way ANOVA with Bonferroni correction. (D) Raw data for plasma NO consumption after experiment as analyzed by triiodide chemiluminescence. L-NAME infusion and hemoglobin infusion were compared. (E) Plasma NO consumption determined at the end of experiments in which either L-NAME or hemoglobin was infused. Mean±SEM (n=5). *Significantly different (p<0.0001) from L-NAME group. L-NAME, L-NG-Nitroarginine methyl ester; NO, nitric oxide.
). (Mean±SEM, n=5). *Significantly different (p<0.05) from hemoglobin by two-way ANOVA with Bonferroni correction. (D) Raw data for plasma NO consumption after experiment as analyzed by triiodide chemiluminescence. L-NAME infusion and hemoglobin infusion were compared. (E) Plasma NO consumption determined at the end of experiments in which either L-NAME or hemoglobin was infused. Mean±SEM (n=5). *Significantly different (p<0.0001) from L-NAME group. L-NAME, L-NG-Nitroarginine methyl ester; NO, nitric oxide.
Plasma NO consumption was determined after the hemoglobin infusion experiments. A typical example of raw data is displayed in Figure 1D. L-NG-Nitroarginine methyl ester (L-NAME) infusion and hemoglobin infusion were compared. In Figure 1E, the plasma NO consumption was measured after either L-NAME or hemoglobin was infused.
Hypertensive effects of acute administration oxy-hemoglobin are mediated primarily by systemic vasoconstriction and not changes in cardiac output
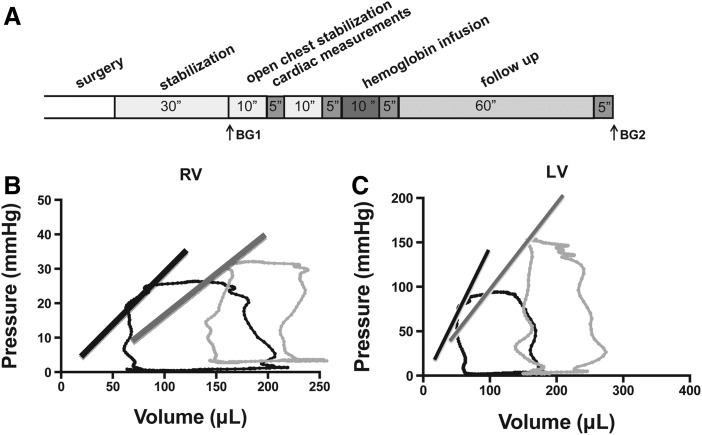
In order to determine the mechanism for increases in mean arterial blood pressure in the rat model after hemoglobin infusions, we performed open heart hemodynamic assessments (Fig. 2A). The right and left ventricle pressures and volumes were assessed during the cardiac cycle in situ, allowing for direct determination of systolic, diastolic, vascular, and ventricular-vascular performance. As shown in Figure 2B, the left ventricular pressure-volume relationship changes after the infusion of oxy-hemoglobin. The preload did not change, and the left ventricular systolic pressure increased significantly. In the left ventricle, contractility did not change as determined by three modalities: the slope of the end-systolic pressure-volume relation (Ees), preload recruitable stroke work, and the slope of the dP/dtmax-end diastolic volume (dP/dtmax-EDV). The lack of effect of hemoglobin infusion on cardiac contractility suggests that acute infusions of oxy-hemoglobin do not affect ventricular inotropy. A significant reduction was observed in left ventricular ejection fraction. However, ejection fraction is directly related to afterload, which was increased with the infusion. Measurements of left ventricular function and left ventricular-aortic vascular coupling efficiency at baseline and after oxy-hemoglobin infusion are summarized in Table 1.
FIG. 2.
Representative pressure-volume loops at baseline and after infusion of Oxyglobin. (A) Experimental timeline. Rats were stabilized for 30 min after surgery before cardiac performance was measured. Blood gasses were drawn as indicated (BG1 and BG2). Purified human hemoglobin (8.1 mM) was infused until a target dose of 175 mg/kg was attained. Rats were monitored for 1 h after hemoglobin infusion. Pressure-volume loops were measured in (B) right ventricles and (C) left ventricles in situ. Steady-state loops from a control (black) and with hemoglobin infusion (gray) are shown. The lines represent the ESPVR. RV, right ventricles; LV, left ventricles.
Table 1.
Hemodynamic Parameters and Indexes of Systolic and Diastolic Function Derived from Left Ventricular Pressure-Volume Relationships During Baseline (n=10), Oxy-Hemoglobin Infusion (n=6), and 1 h After Infusion (n=4) (Mean±SEM)
| Parameter | Baseline | Oxy-hemoglobin | Oxy-hemoglobin 1 h |
|---|---|---|---|
| Heart rate [beats/min] |
280.6±10.2 |
276.3±16.4 |
297±10 |
| LV- Systolic pressure [mmHg] |
80.5±5.7 |
105.7±6.8a |
95.0±8.1 |
| LV- Diastolic pressure [mmHg] |
4.8±0.6 |
8.14±1.8 |
7.0±0.33 |
| EDV [μl] |
216.6±20.9 |
212.0±31.2 |
160.0±38.1 |
| Contraction time [ms] |
20.9±0.5 |
21.0±0.3 |
22.0±0.6 |
| Relaxation time [ms] |
42.9±1.6 |
45.0±2.6 |
54.3±7.4 |
| Stroke work [mmHg μl] |
8967.5±975.4 |
8994.0±1722.4 |
6402.7±1636.4 |
| Cardiac output [μl/min] |
46594.5±5863.8 |
40137.9±7312.8 |
26368.7±6565.8 |
| Chamber compliance [μl/mmHg] |
2.34±0.3 |
1.55±0.3 |
1.16±0.3 |
| Systemic vascular resistance [mmHg/ml] |
1.17±0.1 |
1.58±0.2a |
1.64±0.2 |
| Pulmonary wedge pressure [mmHg] |
1.80±0.2 |
3.14±0.7 |
4.00±0.6b |
| Systolic indices | |||
| Ejection fraction [%] |
63.2±3.8 |
55.3±4.6a |
53.0±2.2a |
| dP/dtmax [mmHg/s] |
5051.1±288.0 |
5736.4±372.1a |
4610.3±308.8 |
| dP/dtmax- EDV [mmHg/s/μl] |
13.7±3.5 |
18.7±4.2 |
23.7±10.0 |
| Ventricular end-systolic elastance (Ees) [mmHg/μl] |
1.10±0.2 |
1.12±0.2 |
3.00±1.9 |
| Preload recruitable stroke work [mmHg] |
59.0±13.0 |
49.5±9.5 |
31.6±14.6 |
| Diastolic indices | |||
| dP/dtmin [mmHg/s)] |
−3939.9±243.1 |
−5141.4±609.9a |
−3528.7±241.8 |
| Relaxation factor tau (τ) [ms] |
5.8±0.3 |
6.3±0.6 |
8.7±1.8 |
| Aortic vascular indices | |||
| Effective arterial elastance (Ea) [mmHg/μl] |
0.8±0.6 |
1.4±0.8 |
2.7±0.96 |
| Coupling efficiency | |||
| Ees/Ea [-] | 1.38±0.2 | 0.79±0.3a | 1.11±1.1 |
Significantly different (p<0.05) from baseline by paired t-test.
Significantly different (p<0.05) from oxy-hemoglobin infusion by paired t-test.
EDV, end diastolic volume; LV, left ventricles.
A significant increase in systolic pressure in the right ventricle was observed (Table 2). In the right ventricle, there was a significant increase in the isovolumic relaxation time constant 1 h after infusion, suggesting the development of increased right ventricular chamber stiffness. In addition, there was a significant reduction in right ventricle ejection fraction at the 1-h time point. As with the left ventricle, ejection fraction is related to afterload. With these acute infusions, there was a trend toward decreased right ventricular function as noted by reductions in cardiac output, dP/dtmax, and a trend toward increased right ventricular and diastolic pressure. However, the dominant effect of acute infusion involves vasoconstriction in both the pulmonary vascular bed and the systemic vascular bed. The trends of these data for both systolic and diastolic function raise the question as to a potential direct effect of hemoglobin on myocardial performance with long-term exposure. This will be the subject of future studies.
Table 2.
Hemodynamic Parameters and Indexes of Systolic and Diastolic Function Derived from Right Ventricular Pressure-Volume Relationships During Baseline (n=10), Oxy-Hemoglobin Infusion (n=6), and 1 h After Infusion (n=4) (Mean±SEM)
| Parameter | Baseline | Oxy-hemoglobin | Oxy-hemoglobin 1 h |
|---|---|---|---|
| Heart rate [beats/min] |
290.6±12.7 |
307.2±17.4 |
277.3±11.8 |
| RV- Systolic pressure [mmHg] |
21.8±1.3 |
25.7±1.0a |
28.3±3.5 |
| RV- Diastolic pressure [mmHg] |
1.8±0.3 |
2.3±0.4 |
3.8±0.8 |
| EDV [μl] |
208.2±15.8 |
202.5±15.4 |
214.0±31.8 |
| Contraction time [ms] |
15.4±0.4 |
16.3±0.3 |
18.3±2.3 |
| Relaxation time [ms] |
34.3±1.4 |
36.5±1.3 |
47.8±7.4 |
| Stroke work [mmHg μl] |
2635.3±205.7 |
2543.2±192.5 |
1899.3±235.5 |
| Cardiac output [μl/min] |
51596.9±4070.1 |
46437.2±3952.2 |
35867±6216.3 |
| Chamber compliance [μl/mmHg] |
9.2±0.9 |
6.5±0.5 |
5.6±1.3 |
| Pulmonary vascular resistance [mmHg/ml] |
0.26±0.03 |
0.29±0.05 |
0.37±0.08 |
| LVEDP [mmHg] |
4.80±0.5 |
6.83±1.2 |
7.50±0.65a |
| Systolic indices | |||
| Ejection fraction [%] |
73.9±2.8 |
63.8±4.2 |
53.3±7.7a |
| dP/dtmax [mmHg/s] |
1585.0±75.2 |
1613.2±78.0 |
1251.0±184.0 |
| dP/dtmax- EDV [mmHg/s/μl] |
6.3±1.3 |
5.8±0.9 |
3.8±0.3 |
| Ventricular end-systolic elastance (Ees) [mmHg/μl] |
0.5±0.1 |
0.7±0.2 |
0.6±0.2 |
| Preload recruitable stroke work [mmHg] |
19.1±2.2 |
14.7±3.6 |
16.0±2.2 |
| Diastolic indices | |||
| dP/dtmin [mmHg/s)] |
−1231.7±100.3 |
−1288.3±82.3 |
−1116.3±256.0 |
| Relaxation factor tau (τ) [ms] | 3.8±0.4 | 4.8±0.4 | 7.8±2.1a |
Significantly different (p<0.05) from baseline by paired t-test.
RV, right ventricles; LVEDP, left ventricular end diastolic pressure.
Effect of NO synthase inhibition on changes in MAP during oxy-hemoglobin infusions
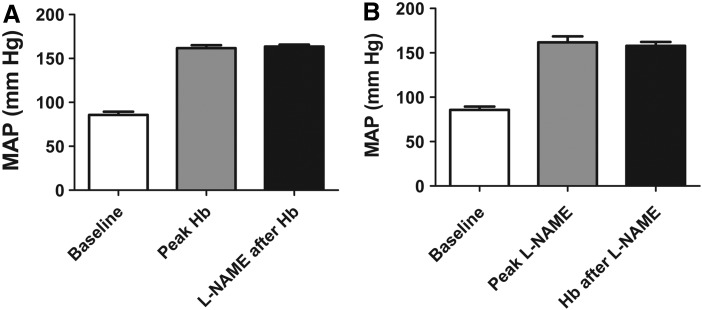
To investigate the role of scavenging of NO in the increase in MAP after hemoglobin infusion, we infused the non-specific nitric oxide synthase (NOS) inhibitor L-NAME. A dose of L-NAME (1 mg/kg) was chosen such that it resulted in comparable increases in MAP as with infusion of 175 mg/kg Hb. MAP increased to 162±8 mm Hg (n=3), and subsequent infusion of 175 mg/kg Hb did not change MAP (158±5 mmHg, paired t-test, p=0.42; n=3) (Fig. 3A). Conversely, after infusion of 175 mg/kg Hb, which increased MAP to 162±4 mm Hg (n=3), infusion of 24 mg/kg L-NAME did not significantly further increase blood pressure (164±3 mm Hg, paired t-test, p=0.36; n=3) (Fig. 3B). These data show that both L-NAME and hemoglobin produce significant hypertensive effects in the rat and do not produce additive vasoconstriction, thus supporting the hypothesis that vasoconstriction is related to a lowering of endothelial NO synthase (eNOS)-derived vascular NO.
FIG. 3.
Effect of hemoglobin and L-NAME infusion on MAP. (A) Baseline MAP (“baseline”), MAP after infusion of 175 mg/kg purified human hemoglobin (“peak Hb”), and MAP after infusion of 175 mg/kg purified human hemoglobin followed by infusion of 24 mg/kg L-NAME (“L-NAME after Hb”) (Mean±SEM, n=3). Paired t-test showed no difference between “peak Hb” and “L-NAME after Hb”(p=0.42; n=3) (B). Baseline MAP (“baseline”), MAP after infusion of 1 mg/kg L-NAME (“peak L-NAME”) and MAP after infusion of 1 mg/kg L-NA followed by infusion of 175 mg/kg purified human hemoglobin (Hb after L-NAME”) (Mean±SEM, n=3). Paired t-test showed no difference between “peak L-NAME” and “Hb after L-NAME” (p=0.36; n=3).
Effect of pre-infusion of vasodilators on Hb-induced vasoconstriction
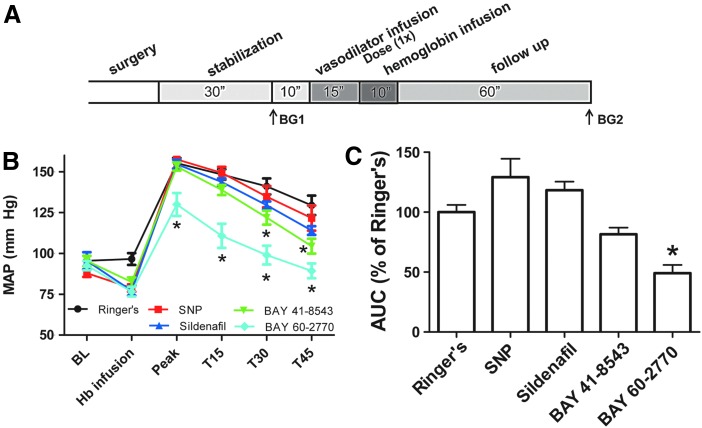
We determined whether inhibition of the NO signaling pathway due to NO depletion after Hb infusion could be counteracted by NO donors or sGC activators. The effect of four different NO pathway-activating drugs was studied. The drugs were infused for 15 min before Hb infusion. Pre-infusion with control Ringer's solution had no effect on baseline MAP (96±2 vs. 97±4 mm Hg paired t-test p=0.54, n=6). The doses of all four drugs were chosen such that they produced a comparable baseline vasodilation. Pre-infusion with SNP (0.4 μg/kg/min) or sildenafil (10 μg/kg/min) decreased baseline MAP by 10% and 19% (Fig. 4B), respectively. Bolus administration of BAY 41-8543 (10 μg/kg) and BAY 60-2770 (1 μg/kg) decreased baseline MAP by 13% and 17% (Fig. 4B), respectively.
FIG. 4.
Effect of pre-infusion of vasodilators on hemoglobin-induced vasoconstriction. (A) Experimental timeline. Rats were stabilized for 30 min after surgery, and blood gases were drawn as indicated (BG1 and BG2). After determining a baseline (BL), vasodilators were infused, followed by infusion of purified human hemoglobin (8.1 mM) until an end concentration of 175 mg/kg was reached. MAP at T=15, 30, and 45 min after hemoglobin infusion is displayed. (B) Effect of pre-infusion with Ringer's (n=6) (–), SNP (0.4 μg/kg/min, n=7) (–), or sildenafil (10 μg/kg/min, n=8) (–) and bolus administration of BAY 41-8543 (10 μg/kg, n=8) (–) and BAY 60-2770 (1 μg/kg, n=9) (–) on basal MAP and peak MAP after infusion of hemoglobin. *Significantly different (p<0.05) from Ringer's by two-way ANOVA with Bonferroni correction. (C) Area under the curve (AUC) values from the graphs in Fig. A expressed as percentage from Ringer's values. *Significantly different (p<0.001) from Ringer's by one-way ANOVA with Bonferroni correction. SNP, sodium nitroprusside. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Infusion of human hemoglobin after SNP, sildenafil, and BAY 41-8543 increased hemoglobin peak MAP to a similar extent as Ringer's control (Fig. 4B). Infusion of BAY 60-2770 before hemoglobin infusion reduced peak MAP by 16% (130±7 mm Hg vs. 155±4 mm Hg for Ringer's, p<0.05 two-way ANOVA) (Fig. 4B). As shown in Figure 4C, only preinfusion with BAY 60-2770 was able to significantly reduce the area under the curve for the rise in blood pressure after hemoglobin infusion. These studies indicate that SNP and sildenafil have limited vasodilatory activity in the presence of plasma hemoglobin, while direct sGC activation has the potential to maintain sGC/cGMP signaling and vasodilation in the presence of high concentrations of plasma hemoglobin. In these experiments, the BAY 60-2770 was the most potent in the presence of hemoglobin. While we observed clear vasodilation, there were no changes in in vivo cGMP levels in plasma or liver tissue (data not shown). As further explained in the discussion, this is likely based on primary cGMP formation in vascular smooth muscle.
Effect of vasodilator infusion during steady-state hemoglobin or L-NAME vasoconstriction
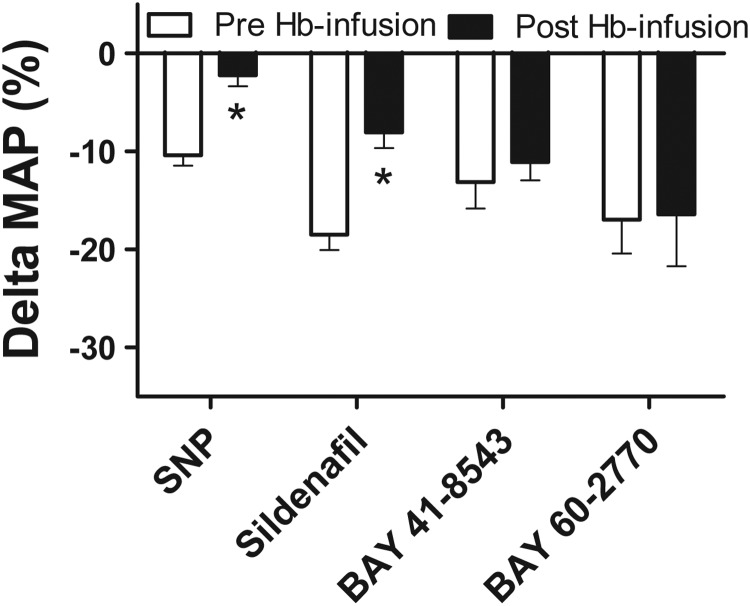
In separate experiments, the same doses of vasodilators (SNP, sildenafil, BAY 41-8543, and BAY 60-2770) infused before Hb infusion were also infused after reaching a peak in MAP after Hb infusion (post-infusion). No differences in peak MAP levels during hemoglobin infusions between groups before the infusions of vasodilators were present (data not shown). The vasodilatory efficacy of SNP and sildenafil was diminished in the presence of hemoglobin (175 mg/kg) compared with the vasodilatory effect in the absence of hemoglobin. The percentage decrease in MAP after SNP without Hb was 10.4%±1.1% versus 2.2%±1.2% in the presence of Hb (t-test; p=0.0002; n=7–8). The percentage decrease in MAP after sildenafil without Hb was 18.5%±1.7% versus 8.0%±1.7% in the presence of Hb (t-test; p=0.0003; n=9, Fig. 5). No significant decrease in vasodilatory efficacy in the presence of Hb was observed for BAY 41-8543 (t-test; p=0.55; n=9) and BAY 60-2770 (t-test; p=0.94; n=9 (Fig. 5).
FIG. 5.
Difference between pre-infusion and post-infusion of vasodilators on hemoglobin-induced vasoconstriction. Difference in percentage change in MAP between pre-infusion and post-infusion of SNP (0.4 μg/kg/min) or sildenafil (10 μg/kg/min) and bolus administration of BAY 41-8543 (10 μg/kg) and BAY 60-2770 (1 μg/kg) (Mean±SEM, n=7–9). *Significantly different (p<0.05) from pre-infusion values by t-test (without correction for multiple comparisons).
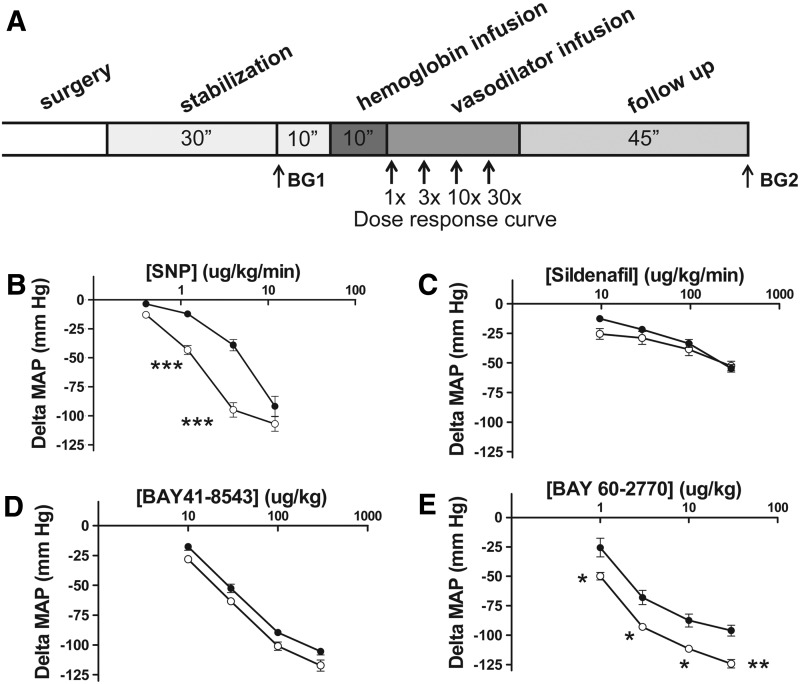
In order to more directly test the potency of these vasodilators in the presence and absence of hemoglobin while controlling for baseline vasoconstrictor state, we compared vasodilator potencies in the presence of steady-state vasoconstriction with hemoglobin or L-NAME (1 mg/kg). As displayed in Figure 6, the potency of SNP was significantly reduced in the presence of hemoglobin compared with L-NAME infusion, consistent with scavenging of the NO in the presence of hemoglobin. In these series of experiments, sildenafil exhibited equal potency in the presence of hemoglobin and L-NAME, although sildenafil exhibited reduced potency in another experimental model (9). This is likely attributable to the equivalent reduction of NO and sGC activation with L-NAME and hemoglobin, such that inhibition of PDE-5 has lesser effects on vasodilation. Both an sGC activator and an sGC stimulator exhibited more potent vasodilation overall in the presence of L-NAME and hemoglobin. Based on molecular weight and reduction in MAP, BAY 60-2770 was the vasodilator with the most potency in the presence of hemoglobin and L-NAME (Fig. 6).
FIG. 6.
Dose responses of vasodilators after steady-state hemoglobin versus L-NAME infusions. (A) Experimental timeline. Rats were stabilized for 30 min after surgery, and blood gases were drawn as indicated (BG1 and BG2). Subsequently, purified human hemoglobin (8.1 mM) was infused until an end concentration of 175 mg/kg was reached. After steady-state vasoconstriction, a dose response with vasodilator was performed. Rats were followed for approximately 45 mins after hemoglobin infusion. Effect of dose response of (B). SNP (0.4, 1.2, 4, and 12 μg/kg/min, n=6) or (C). sildenafil (10, 30, 100 and 300 μg/kg/min, n=5) and (D). bolus administration of BAY 41-8543 (10, 30, 100, and 300 μg/kg, n=5) and (E). BAY 60-2770 (1, 3, 10, and 30 μg/kg, n=5) on steady-state MAP after human hemoglobin (175 mg/kg) infusions (- ● -closed dots) compared with effects of the same vasodilator infusions after steady-state L-NAME (1 mg/kg) infusion (- ◯ -open dots). Asterisks indicate significant difference from hemoglobin at same dose (*p<0.05, **p<0.01, ***p<0.001) by two-way ANOVA with Bonferroni correction.
Discussion
In this article, we demonstrate that a top-load infusion of human hemoglobin as well as the HBOC Oxyglobin results in an immediate and significant increase in MAP. The dependency of the pressure increase on scavenging of endothelial NO was demonstrated in two ways: by either infusing hemoglobin analogs that do not react with NO (methemoglobin and cyano-methemoglobin) or inhibiting endothelial NO production with L-NAME. In addition, we used an open heart hemodynamic assessment to determine systolic, diastolic vascular, and ventricular-vascular performance. Under these experimental conditions, the dominant effect of acute infused hemoglobin involved vasoconstriction in both the pulmonary vascular bed and the systemic vascular bed. No significant effect on acute cardiac contractility was found, further enforcing our finding that the observed increase in MAP is primarily mediated by systemic vasoconstriction.
We were unable to show differences in plasma and liver cGMP levels between the four vasodilator groups. The levels of cGMP levels in plasma and tissue are known to offer only a limited amount of information. cGMP values in plasma are difficult to interpret, as the half-life is very long and, importantly, plasma levels of cGMP are more regulated by natriuretic peptides via particulate guanylate cyclase than by sGC stimulation (8, 33). However, the vasodilatory effects of BAY 41-8543 and BAY 60-2770 have been previously established to be mediated by the increase in cGMP in vascular smooth muscle cells. In separate studies, the in vitro efficacy of the sGC stimulator BAY 41-8543 and sGC activator BAY 60-2770 was demonstrated, which stimulates sGC directly to increase cGMP production and vasodilation (16, 17, 23, 30, 38). In addition, it was shown in other studies that this type of sGC stimulators (BAY 41-2272), indeed, directly stimulates sGC, hence enhancing renal cGMP production that results in an improved renal NO-cGMP signaling and limited progression in anti-Thy-1-induced chronic renal fibrosis (40). Therefore, our observed effects of the sGC stimulator and sGC activator on blood pressure in the present study are consistent with an increase in vascular cGMP.
Our experiments suggest that an sGC stimulator or sGC activator by directly stimulating sGC can bypass the effect of NO scavenging. These vasodilator agents remain potent even in the presence of high concentrations of plasma hemoglobin, while both sildenafil and SNP exhibit reduced vasodilatory activity. Thus, one could conclude from this study that an sGC stimulator or a sGC activator could potentially be applied to counteract hypertension, enhanced platelet aggregation, and other NO-scavenging-induced side effects encountered after HBOC administration. Two recent studies using either the sGC stimulator BAY 41-2272 (19) or Riociguat (BAY 63-2521) (21) in eNOS knockout mice demonstrated a decrease in blood pressure of these compounds. These results further indicate that both BAY 41-2272 and Riociguat can activate sGC independent of the presence of NO. Administration of BAY 41-8543 has been shown to have an antiplatelet effect activity in a low NO, high renin rat model of hypertension (32). BAY 58-2667, a close analog of BAY 60-2770, has been shown to be a relevant anti-aggregating agent when the sGC is oxidized (26). In addition, an sGC stimulator or an sGC activator could potentially be useful in other clinical conditions where plasma cell-free hemoglobin is elevated, such as hemolytic diseases (27), malaria, cardiopulmonary bypass (39), and transfusion of aged-stored RBCs (2, 5).
In contrast to SNP, sildenafil, and the sGC stimulator BAY 41-8543, the sGC activator BAY 60-2770 was the only compound that was able to reduce peak pressure as well as the total area under the curve in the presence of hemoglobin measured for approximately 1 h after infusion. The NO generated by SNP infusion is immediately scavenged by the infused hemoglobin as is also shown by the 4-fold reduction in vasodilatory potency when given after the hemoglobin infusion. Although a PDE-5 inhibitor such as sildenafil acts further downstream in the NO signaling pathway by preventing the breakdown of cGMP formed after sGC activation, sGC initially still needs to be activated by endogenous NO to form cGMP. Therefore, scavenging of NO by hemoglobin still decreased vasodilatory potency by a factor of two, explaining why peak pressure was not reduced with sildenafil. We currently cannot explain why pre-infusion with BAY 41-8543 did not lower the peak pressure after hemoglobin infusion, as the presence of hemoglobin did not have an inhibitory effect on vasodilation as was shown in Figure 5. BAY 41-8543 has been shown to activate sGC independent of NO (35). A study by Badejo et al., however, has shown an effect of lowering endogenous NO on the potency of BAY 41-8543 when L-NAME was administered (1). In our experiments, we saw no significant effect of L-NAME on the potency of BAY 41-8543. This could be due to the 25-fold lower dose of 1 mg/kg L-NAME that we used to obtain a comparable vasoconstriction as during hemoglobin infusion. Furthermore, NOS inhibition is known to increase the potency of NO through a mechanism that is still not completely understood. A recent in vivo study suggests that the increased vasoconstriction after L-NAME is related to a shift in the balance of endothelin-1 and NO levels (3). Depending on the exact in vivo conditions, L-NAME could have opposing results on the vasodilatory effects of BAY 41-8543.
The vasodilatory effect of BAY 60-2770 on peak pressure may be due to the presence of hemoglobin that has oxidized a part of the total sGC present which is known to potentiate the vasodilatory effect of BAY 60-2770 (16). Activators of sGC, such as BAY 60-2770 and the structurally closely related Cinaciguat, are the first tools to functionally analyze the oxidation state of sGC in vivo under physiological and pathophysiological conditions. It would clearly be desirable to directly measure the intracellular molar ratio of reduced, oxidized, and heme-free sGC. However, even under well-defined and controlled experimental settings, it is currently technically impossible to determine this ratio given the small amounts of oxidized/heme-free sGC and the major population of reduced sGC within a given enzyme preparation (36).
The in vivo vasodilatory activity of the sGC activator BAY 60-2770 is more pronounced in the presence of L-NAME. Similar results have already been shown in isolated rat aortas and small mesenteric arteries with the sGC activator BAY 58-2667 in the presence of L-NAME, confirming the protection of native sGC from oxidation by endogenous NO (14). Therefore, this enhanced vasodilatory effect might be a consequence of sGC oxidation, which results in a potentiation of sGC activity as already shown at the isolated enzyme by BAY 60-2770 and BAY 58-2667 (16, 35).
There may be several advantages of the use of an sGC activator such as BAY 60-2770 compared with NO donors or PDE inhibitors to counteract the vasoconstrictive effects of HBOCs. Since BAY 60-2770 does not generate NO similar to SNP, but still activates sGC, it might be feasible in the future to add the drug along with a HBOC, resulting in an effect that does not cause vasoconstriction after infusion. Furthermore, there is no risk of NO-induced formation of methemoglobin or peroxynitrite formation as observed with NO donors. sGC activators (such as BAY 60-2770 and Cinaciguat) can even activate oxidized sGC or heme-deficient sGC (23, 37). Therefore, patients with cardiovascular disease who have reduced endothelial function due to oxidative stress and are expected to have more oxidized sGC might additionally benefit from the use of sGC activators along with the administration of HBOCs.
SCD is a chronic hemolytic disease that is characterized by acute vaso-occlusive complications, pain, acute chest syndrome, and the development of pulmonary hypertension (41). Cell-free plasma hemoglobin levels in SCD patients are elevated due to saturation of hemoglobin scavenging systems (haptoglobin), and plasma NO consumption values are much higher than controls (25). Subpopulations of SCD patients demonstrate blunted responses to nitroglycerin and SNP (25, 41). Patients with SCD are, therefore, another potential patient group that may benefit from treatment with sGC stimulators and sGC activators. Future experiments with sGC stimulators or sGC activators in transgenic sickle cell mice will have to confirm whether the beneficial effects of these agents are maintained in chronic models of increased vascular cell-free hemoglobin.
In conclusion, not only sGC stimulators but especially sGC activators, which activate oxidized sGC and are independent of NO, may represent promising new approaches to bypass the NO-scavenging effects that result in hypertension and platelet aggregation after infusion of HBOC solutions, or after hemolysis in hemolytic diseases or transfusion of stored RBCs.
Materials and Methods
Animals
This study was reviewed and approved by the ethical committee for animal subjects of the Erasmus MC—University Medical Center Rotterdam, Rotterdam, The Netherlands and the University of Pittsburgh, Pittsburgh, PA, the United States. Care and handling of the animals were in accordance with the guidelines for Institutional and Animal Care and Use Committees. A total of 102 experiments with Wistar male rats (Charles River) with a body weight of 338±26 g (mean±SD) were included in this study. For the open chest hemodynamic measurements, 10 Wistar male rats (Charles River) with a body weight of 385±49 g (mean±SD) were used.
Materials
SNP was prepared at a concentration of 25 mg/ml by the pharmacy of the Erasmus University. Sildenafil citrate was obtained from Santa Cruz Biotechnology. BAY 41-8543 (2-[1-[2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5(4-morpholinyl)-4,6-pyrimidine-diamine) and BAY 60-2770 (4-({(4-carboxybutyl)[2-(5-fluoro-2-{[4′-(trifluoro-methyl) biphenyl-4-yl]methoxy}phenyl)ethyl]amino}methyl) benzoic acid were a gift from Bayer Healthcare Wuppertal. BAY compounds were dissolved in three steps. First, Transcutol was added; subsequently, Cremophor EL was added; and finally, Ringer's was added. In each step, the mixture was heated to 45°C and sonicated for 10 min. The final solution contained 10% Transcutol, 10% Cremophor EL, and 80% Ringer's. Hemoglobin was purified as previously described (11). Briefly, RBCs were washed, lysed with deionized water, separated from the membrane fraction by sedimentation, and pelleted in liquid nitrogen for storage at −80°C. For preparation of hemoglobin and cyano-methemoglobin, hemoglobin solutions were incubated with a fivefold molar excess (vs. heme) of either potassium ferricyanide (for methemoglobin) or potassium ferricyanide/potassium cyanide (for cyano-methemoglobin) and incubated for 30 min at 4°C. After incubation, the hemoglobin samples were run through a G-25 column (PD-10, GE Healthcare Life Sciences) equilibrated with Ringer's solution to remove the excess of ferricyanide and cyanide. The collected samples were concentrated with Amicon Ultra centrifugal devices (50 kDa MW cutoff, Millipore) a concentration around 8.1 mM. Ringer's was obtained from Baxter, and Voluven was obtained from Fresenius-Kabi. Oxyglobin was purchased from Dechra.
Surgical preparation
Rats were mechanically ventilated, and four vessels were cannulated, as described in detail in a previous study (5, 24) with some modifications; while body temperature was maintained between 36.5°C and 37.5°C. Rats were anesthetized with an intraperitoneal injection of a mixture of 90 mg/kg ketamine (Alfasan), 0.5 mg/kg medetomidine (Sedator®; Eurovet Animal Health), and 0.05 mg/kg atropine sulfate (Centrafarm). A fluid-filled catheter was inserted into the right carotid artery catheter and connected to a pressure transducer (AD instruments) that was connected to a data acquisition system (Powerlab 8/30; AD Instruments) for continuous monitoring of mean arterial pressure (MAP) and heart rate. MAP was calculated with the use of the formula: (systolic blood pressure − diastolic blood pressure)/3 + diastolic blood pressure. In addition, the jugular vein was cannulated to infuse maintenance anesthesia (ketamine, 58 mg/kg/h in Ringer's solution). The femoral artery was cannulated for blood withdrawal and arterial blood-gas sampling. The femoral vein was cannulated for the infusion of the hemoglobin solutions and drugs. Inspiratory pressure (range 15–18 mbar) was adjusted to keep arterial PCO2 values between 35 and 45 mm Hg (4.7–6.0 kPa) during surgery and baseline and were left unmodified during the rest of the experiment. An inspiratory/expiratory ratio of 2, a breathing rate of ∼60 breaths per minute, and a positive end-expiratory pressure of 4 mbar were used.
Experimental protocol
After surgery, the rats were allowed to stabilize for 30 min, after which 0.2 ml of blood was drawn for blood gas analysis (ABL 800; Radiometer). As replacement for the drawn blood, 0.2 ml of Voluven was infused and the rats were allowed to stabilize for 10 min, after which the experiments were started. In case of pre-infusion with vasodilators, infusion of SNP (0.4 μg/kg/min) or sildenafil (10 μg/kg/min) was started at an infusion speed of 7.5 ml/kg/hr and continued for the whole duration of the experiment. BAY 41-8543 and BAY 60-2770 were infused as a bolus dose of 10 μg/kg (200 μl of 0.5 mg/ml stock solution) or 1 μg/kg (200 μl of 0.1 mg/ml stock solution), respectively, for ∼5 min at an infusion speed of 7.5 ml/kg/h. Ringer's solution was used as a control. Fifteen minutes after administration of the vasodilatory drugs when the MAP had reached a plateau, 175 mg/kg purified human hemoglobin (13 g/dl; 1.35 ml/kg) was infused at 7.5 ml/kg/h for ∼10 min. Responses in blood pressure and heart rate were recorded continuously for approximately an hour after the end of the hemoglobin infusion. In another set of experiments, a volume of 1.35 ml/kg of purified human hemoglobin was infused at 7.5 ml/kg/hr for ∼10 min. When a plateau in MAP was reached, the various vasodilators were infused in four stepwise escalating doses, starting at the dose used at pre-infusion. Doses were increased by a factor of 3, 10, and 30, respectively. 175 mg/kg purified human hemoglobin, methemoglobin, cyano-methemoglobin, and Oxyglobin, all of which were at 13 g/dl and 1.35 ml/kg, were infused at a speed of 7.5 ml/kg/h for ∼10 min. Plasma heme concentrations were estimated based on the dilution of the hemoglobin stock (heme concentration 8.1 mM) in the blood volume of the rat (60 ml/kg).
Open chest hemodynamic assessments in vivo
In separate experiments, rats were prepared and anesthetized as described in the surgical preparation section. Once the baseline blood gases were established, the thorax was entered via an incision at the lower costal margin of the eighth rib. The pericardium was opened at the apex, and an apical incision was made to place a 1.9 Fr or smaller high-fidelity micromanometry pressure-volume conductance catheter (Scisense, Inc.) along the long axis of the right ventricle. Initial pressure-volume measurements were obtained; then, the vena cava was isolated and briefly occluded to reduce venous return for determination of end-systolic pressure relationships. Right ventricular pressure, volume, and pulmonary artery pressure data were recorded. After data had been recorded from the right ventricle, the catheter was removed with direct visualization of hemostasis, and the micromanometer catheter was placed in the left ventricle for pressure-volume acquisition. These data and heart rate were continuously displayed, recorded, and saved with commercially available software (Iox2; Emka). After right and left ventricle pressure, volume, and pulmonary and aorta pressures had been established, infusion of the hemoglobin solutions was initiated. Hemodynamic parameters were recorded during the whole infusion and when the infusion was finished, the vena cava was occluded with the catheter in the left and right ventricle. After recording of hemodynamic parameters for an additional 60 min, occlusion of vena cava was performed in order to obtain the end-systolic pressure relations at the end point in the left and right ventricle.
Hemodynamic data analysis
At least 10 consecutives cardiac cycles were recorded and used in the analysis from each baseline and oxy-hemoglobin condition. Heart rate, systolic and diastolic pressures were measured. Parameters of systolic and diastolic ventricular function such as cardiac output, ejection fraction, relaxation factor, chamber compliance, and parameters of arterial afterload compliance (i.e., effective arterial elastance) were calculated from the hemodynamic data.
Statistical analysis
Values are presented as mean±SEM. Differences in MAP between hemoglobin infusion preinfused with Ringer's (control) and prefusion with the drugs were analyzed by t-test. Differences in MAP between infusion of drugs before and after hemoglobin infusion were analyzed by t-test without correction for multiple comparisons. Differences in MAP between infusion with hemoglobin followed by infusion with L-NAME and infusion of L-NAME followed by hemoglobin were analyzed by a paired t-test. Differences of various doses of drugs in MAP after Hb infusion or L-NAME infusion were analyzed by a two-way ANOVA with Bonferroni correction for multiple comparisons. Differences in hemodynamic variables after Ringer's and hemoglobin infusion in the open chest experiments were determined by paired t-test. We considered p<0.05 to be significant.
Acknowledgments
This study was partly funded by Bayer Pharma AG (www.grants4targets.com), Wuppertal, Germany, and the Vascular Medicine Institute, University of Pittsburgh, Pittsburgh, PA, the United States.
Dr. Gladwin received research support from NIH grants R01HL098032, RO1HL096973, and PO1HL103455, the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Abbreviations Used
- cGMP
cyclic guanosine monophosphate
- eNOS
endothelial NO synthase
- HBOC
hemoglobin-based oxygen carriers
- L-NAME
L-NG-nitroarginine methyl ester
- MAP
mean arterial pressure
- NO
nitric oxide
- PDE-5
phosphodiesterase-5
- RBC
red blood cell
- SCD
sickle cell disease
- sGC
soluble guanylate cyclase
- SNP
sodium nitroprusside
Author Disclosure Statement
H.C.C. has served as a consultant for Bayer, Gilead, Pfizer, and Merck. J.P.S., E.M.B., and H.T. are full-time employees at Bayer Pharma.
References
- 1.Badejo AM, Jr., Nossaman VE, Pankey EA, Bhartiya M, Kannadka CB, Murthy SN, Nossaman BD, and Kadowitz PJ. Pulmonary and systemic vasodilator responses to the soluble guanylyl cyclase stimulator, BAY 41-8543, are modulated by nitric oxide. Am J Physiol Heart Circ Physiol 299: H1153–H1159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, and Zapol WM. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology 116: 637–647, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourque SL, Whittingham HA, Brien SE, Davidge ST, and Adams MA. Role of endothelin-1 in the hyper-responsiveness to nitrovasodilators following acute NOS inhibition. Br J Pharmacol 165: 1992–1999, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derbyshire ER. and Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem 81: 533–559, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, and Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 124: 465–476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle MP. and Hoekstra JW. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem 14: 351–358, 1981 [DOI] [PubMed] [Google Scholar]
- 7.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, and Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 350: 886–895, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Heim JM, Gottmann K, Weil J, Haufe MC, and Gerzer R. Is cyclic GMP a clinically useful marker for ANF action? Z Kardiol 77Suppl 2: 41–46, 1988 [PubMed] [Google Scholar]
- 9.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, and Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood 109: 3088–3098, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu W, Jin R, Zhang J, You T, Peng Z, Ge X, Bronson RT, Halperin JA, Loscalzo J, and Qin X. The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood 116: 1613–1622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z, Louderback JG, Goyal M, Azizi F, King SB, and Kim-Shapiro DB. Nitric oxide binding to oxygenated hemoglobin under physiological conditions. Biochim Biophys Acta 1568: 252–260, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Ignarro LJ, Byrns RE, Buga GM, and Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res 61: 866–879, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Jeffers A, Gladwin MT, and Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic Biol Med 41: 1557–1565, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemp-Harper B, Luk J, Favaloro JL, Stasch JP, and Schmidt HHHW. Oxidised sGC: a novel therapeutic target in the vasculature. BMC Pharmacol 7: S5, 2007 [Google Scholar]
- 15.Kim-Shapiro DB, Schechter AN, and Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol 26: 697–705, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Knorr A, Hirth-Dietrich C, Alonso-Alija C, Harter M, Hahn M, Keim Y, Wunder F, and Stasch JP. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60–2770 in experimental liver fibrosis. Arzneimittelforschung 58: 71–80, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Mendes-Silverio CB, Leiria LO, Morganti RP, Anhe GF, Marcondes S, Monica FZ, De Nucci G, and Antunes E. Activation of haem-oxidized soluble guanylyl cyclase with BAY 60–2770 in human platelets lead to overstimulation of the cyclic GMP signaling pathway. PLoS One 7: e47223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moncada S. and Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Nagasu H, Satoh M, Kidokoro K, Nishi Y, Channon KM, Sasaki T, and Kashihara N. Endothelial dysfunction promotes the transition from compensatory renal hypertrophy to kidney injury after unilateral nephrectomy in mice. Am J Physiol Renal Physiol 302: F1402–F1408, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Natanson C, Kern SJ, Lurie P, Banks SM, and Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA 299: 2304–2312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ott IM, Alter ML, von Websky K, Kretschmer A, Tsuprykov O, Sharkovska Y, Krause-Relle K, Raila J, Henze A, Stasch JP, and Hocher B. Effects of stimulation of soluble guanylate cyclase on diabetic nephropathy in diabetic eNOS knockout mice on top of angiotensin II receptor blockade. PLoS One 7: e42623, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer RM, Ferrige AG, and Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Pankey EA, Bhartiya M, Badejo AM, Jr., Haider U, Stasch JP, Murthy SN, Nossaman BD, and Kadowitz PJ. Pulmonary and systemic vasodilator responses to the soluble guanylyl cyclase activator, BAY 60–2770, are not dependent on endogenous nitric oxide or reduced heme. Am J Physiol Heart Circ Physiol 300: H792–H802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raat NJ, Verhoeven AJ, Mik EG, Gouwerok CW, Verhaar R, Goedhart PT, de Korte D, and Ince C. The effect of storage time of human red cells on intestinal microcirculatory oxygenation in a rat isovolemic exchange model. Crit Care Med 33: 39–45, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, and Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 8: 1383–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Roger S, Paysant J, Badier-Commander C, Cordi A, Verbeuren TJ, and Feletou M. Anti-aggregating effect of BAY 58-2667, an activator of soluble guanylyl cyclase. Vascul Pharmacol 53: 281–287, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Rother RP, Bell L, Hillmen P, and Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 293: 1653–1662, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Sachdev V, Kato GJ, Gibbs JS, Barst RJ, Machado RF, Nouraie M, Hassell KL, Little JA, Schraufnagel DE, Krishnamurti L, Novelli EM, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Castro OL, Taylor JGt, Hannoush H, Goldsmith JC, Gladwin MT, and Gordeuk VR. Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and United Kingdom. Circulation 124: 1452–1460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt HHHW, Schmidt PM, and Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. In: Handbook of Experimental Pharmacology. cGMP Generators, Effectors and Therapeutic Implications, edited by Hofmann F, Schmidt HHHW, and Stasch JP. Berlin-Heidelberg: Springer-Verlag, 2009, pp. 309–339 [DOI] [PubMed] [Google Scholar]
- 30.Schmidt P, Schramm M, Schroder H, and Stasch JP. Mechanisms of nitric oxide independent activation of soluble guanylyl cyclase. Eur J Pharmacol 468: 167–174, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Silverman TA. and Weiskopf RB. Hemoglobin-based oxygen carriers: current status and future directions. Anesthesiology 111: 946–963, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Stasch JP, Dembowsky K, Perzborn E, Stahl E, and Schramm M. Cardiovascular actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41-8543: in vivo studies. Br J Pharmacol 135: 344–355, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stasch JP, Kazda S, and Neuser D. Different effects of ANP and nitroprusside on cyclic GMP extrusion of isolated aorta. Eur J Pharmacol 174: 279–282, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Stasch JP, Pacher P, and Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, Schramm M, Schroeder W, Schroder H, Stahl E, Steinke W, and Wunder F. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol 136: 773–783, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY.HS AK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W, and Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surmeli NB. and Marletta MA. Insight into the rescue of oxidized soluble guanylate cyclase by the activator cinaciguat. Chembiochem 13: 977–981, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai EJ, Liu Y, Koitabashi N, Bedja D, Danner T, Jasmin JF, Lisanti MP, Friebe A, Takimoto E, and Kass DA. Pressure-overload-induced subcellular relocalization/oxidation of soluble guanylyl cyclase in the heart modulates enzyme stimulation. Circ Res 110: 295–303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vermeulen Windsant IC, Hanssen SJ, Buurman WA, and Jacobs MJ. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg 142: 1–11, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Kramer S, Loof T, Martini S, Kron S, Kawachi H, Shimizu F, Neumayer HH, and Peters H. Enhancing cGMP in experimental progressive renal fibrosis: soluble guanylate cyclase stimulation vs. phosphodiesterase inhibition. Am J Physiol Renal Physiol 290: F167–F176, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Wood KC, Hsu LL, and Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med 44: 1506–1528, 2008 [DOI] [PubMed] [Google Scholar]