Abstract
Background and Aims: Advanced glycation end products (AGEs) contribute to cardiovascular disease in patients with hemodialysis (HD). We have recently found that carnitine levels are inversely associated with skin AGE levels in HD patients. We examined whether l-carnitine supplementation reduced skin AGE levels in HD patients with carnitine deficiency.
Methods: This was a single-center study. One hundred and two HD patients (total carnitine levels <50 μmol/L) were enrolled and randomized to either oral administration of l-carnitine (900 mg/day) (n=51) or control (n=51). After 6 months, metabolic and inflammatory variables, including serum levels of carnitine, were measured. Skin AGE levels were determined by evaluating skin auto-fluorescence with an AGE-reader.
Results: There were no significant differences of clinical variables at baseline between the control and l-carnitine therapy group. Thirty-two patients did not complete the assessment or treatment of the study. Oral l-carnitine supplementation for 6 months significantly increased low-density lipoprotein cholesterol (LDL-C), triglycerides, total, free, and acyl carnitine levels, while it decreased alanine transaminase, acyl/free carnitine ratio, β2-microglobulin, and skin AGE values. Change in total carnitine values from baseline (Δtotal carnitine) and Δfree carnitine were inversely associated with Δskin AGE levels in l-carnitine-treated patients (p=0.036 and p=0.016, respectively). In multiple regression analysis, Δfree carnitine was a sole independent determinant of Δskin AGEs (R2=0.178).
Conclusions: The present study demonstrated that oral l-carnitine supplementation significantly decreased skin AGE levels in HD patients with carnitine deficiency. These observations suggest that supplementation of l-carnitine might be a novel therapeutic strategy for preventing the accumulation of tissue AGEs in carnitine-deficient patients with HD.
Introduction
Reducing sugars can react non-enzymatically with the amino groups of proteins to initiate a complex series of rearrangements and dehydrations, and then to produce a class of irreversibly cross-linked, fluorescent moieties, termed advanced glycation end products (AGEs).1–3 Oxidative stress and reactive carbonyl compounds could contribute to the formation and accumulation of AGEs, which have been known to progress in a normal aging process, and at an accelerated rate under diabetes or end-stage renal failure, thereby playing a role in the development and progression of various age- and diabetes-related disorders such as cardiovascular disease (CVD), osteoporosis, Alzheimer disease, and cancer growth and metastasis in these subjects.4–14 Recently, tissue accumulation levels of AGEs can be evaluated non-invasively by measuring skin autofluorescence (SAF) with an AGE reader.15,16 Indeed, SAF has been shown to correlate with AGE accumulation levels from skin biopsies in diabetes, renal failure, and control subjects.15,17 Furthermore, SAF is also associated with AGE-related functional and structural derangements of the vessels and myocardium.15,18,19 Therefore, SAF is now becoming an accepted clinical method for assessing the skin accumulation levels of AGEs in humans.15,16,19
Carnitine, a natural substance that could contribute to transport long-chain fatty acids from the cytoplasm to mitochondria, has been known to play a central role in fatty acid β-oxidation and subsequent adenosine triphosphate (ATP) production in a variety of cells.20 Furthermore, because carnitine regulates the function of mitochondrial respiratory chain and oxidative stress generation as well,20 carnitine deficiency might be involved in muscle weakness, cardiac hypertrophy, and accelerated atherosclerosis in hemodialysis (HD) patients.21–23
We have found that serum carnitine levels are inversely associated with skin accumulation levels of AGEs evaluated by SAF in patients with HD.23 Given the inhibitory potential of l-carnitine on formation of AGEs,24 our previous observations suggest a causative role of carnitine deficiency in AGE accumulation in HD patients, which could lead to the increased risk for AGE-related various disorders in these subjects. However, the effect of l-carnitine supplementation on skin AGE levels remains unknown. In this study, we examined whether oral l-carnitine supplementation for 6 months could actually reduce skin accumulation levels of AGEs in patients with HD by measuring the SAF with an AGE reader. We also studied which anthropometric, metabolic, and inflammatory variables, including serum carnitine levels, were the independent correlates of SAF in l-carnitine–treated HD patients.
Methods
Patients and study protocol
This study was a prospective, randomized, comparator-controlled, single-center trial involving 6 months of study drug administration and follow-up. In all, 102 HD patients (mean age, 67.5±12.7 years old; mean duration of HD, 99.5±85.3 months), whose serum total carnitine levels were less than 50 μmol/L, were enrolled in this study. Age- and sex-matched healthy subjects (n=75, mean age 65.4±10.3 years old) were used as a control. HD patients were randomly assigned to oral l-carnitine therapy group (900 mg/day) (n=51) and control group (n=51), and were followed-up for 6 months. At baseline and after 6 months of treatment, HD patients underwent a complete history, physical examination, and determination of blood chemistries just before the HD session. Patients were dialyzed for 4–5 hr with high-flux dialyzers three times a week.
Informed consent was obtained from all subjects, and the study protocol was approved by the Institutional Ethics Committees of Kurume University School of Medicine and Sugi Cardiovascular Medicine Hospital, Japan. This work was conducted in accordance with the Declaration of Helsinki. This trial was registered with the University Hospital Medical Information Network clinical trials database (UMIN000010953).
Data collection
Medical history was ascertained by a questionnaire. Blood pressure was measured in the sitting position using an upright standard sphygmomanometer just before starting HD. Vigorous physical activity and smoking were avoided for at least 30 min before blood pressure measurement.
Blood was drawn from an arteriovenous shunt just before starting the HD session for determinations of hemoglobin, total protein, albumin, lipids (low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides), blood urea nitrogen, creatinine, uric acid, calcium, phosphate, and C-reactive protein (CRP). Whole parathyroid hormone (PTH) was evaluated by an immunoradiometric assay (IRMA; Allegro I-PTH, Nichols Institute, San Juan Capistrano, CA). β2-microglobulin (β2-MG) was measured by a latex immunoagglutination assay (Eiken Chemical Co., Ltd. Tokyo, Japan). Serum carnitine levels were determined by enzyme cycling methods, as described previously.25 Other blood chemistries were measured at standard enzymatic methods as described previously (Wako Pure Chemical Industries, Ltd, Osaka, Japan). HD adequacy was evaluated by a single-pool fractional clearance of body water for urea (Kt/V).26 Tissue accumulation levels of AGEs were evaluated quantitatively by measuring SAF with an AGE reader according to the supplier's recommendations (DiagOptics BV, Groningen, Netherlands).15 We measured SAF just before HD sessions on non-shunt arm. Use of skin creams was prohibited when measuring the SAF.
Statistical analysis
Data are presented as mean±standard deviation (SD). Use of renin–angiotensin system (RAS) inhibitors and statins and the presence or absence of diabetes mellitus were coded as dummy variables. Because triglycerides and whole PTH levels were not normally distributed, log-transformed values were used for analysis. An unpaired t-test was performed to compare clinical valuables among healthy subjects, controls, and the l-carnitine group. The difference of CRP between two groups at baseline was performed by non-parametric analysis with a Mann–Whitney test. To examine the difference of valuables between baseline and 6 months after the treatment, a paired t-test was performed. In the case of CRP, non-parametric analysis with a Wilcoxon signed rank test was performed. To determine the independent correlates of change of SAF from baseline (Δskin AGEs), multiple stepwise regression analysis was performed. Statistical significance was defined as p<0.05. All statistical analyses were performed with SPSS ver.20 (Chicago, IL).
Results
Demographic data at baseline
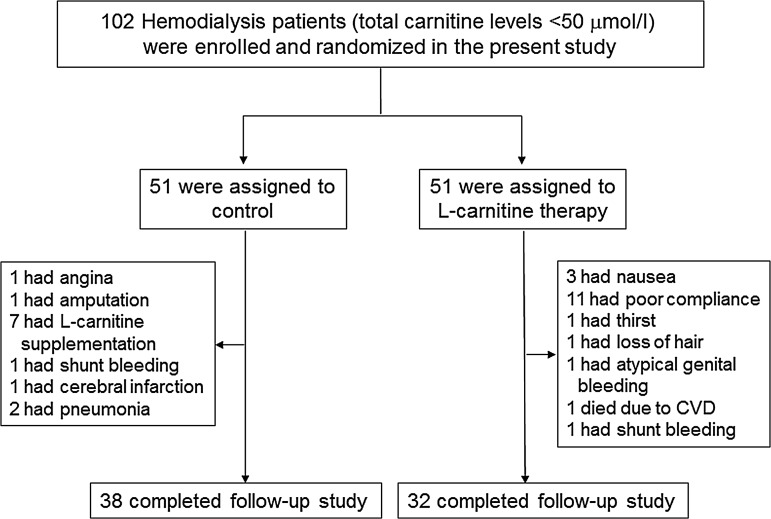
Thirty-two patients did not complete the assessment or treatment of the study. Finally, 70 patients completed the study (n=38 in the control group and n=32 in the l-carnitine group) (Fig. 1). In the group of l-carnitine therapy, 19 (37.3%) subjects dropped out from the study due to poor adherence (n=11), nausea (n=3), thirst (n=1), hair loss (n=1), atypical genital bleeding (n=1), shunt bleeding (n=1), and death from cardiovascular disease (CVD) (n=1). In the control group, 13 (25.5%) dropped out due to l-carnitine supplementation (n=7), pneumonia (n=2), CVD (N=3), and shunt bleeding (n=1).
FIG. 1.
Enrollment, randomization, and follow-up of the study patients. CVD, cardiovascular disease.
Demographic data at baseline are shown in Table 1. There were no significant differences of baseline data between the two groups, including metabolic, hemodynamic, anthropometric, and inflammatory variables. At baseline, total and free carnitine levels were significantly lower, whereas acyl carnitine, acyl/free carnitine ratio, and SAF were higher in HD patients compared with healthy subjects (total, free, acyl carnitine, acyl/free carnitine ratio, and SAF in HD and control subjects; 36.3±7.6 vs. 59.3±10.6 μmol/L (p<0.001), 21.7±4.5 vs. 47.3±9.2 μmol/L (p<0.001), 14.6±3.9 vs. 12.1±3.7 μmol/L (p<0.01), 0.69±0.18 vs. 0.26±0.09 (p<0.001), and 3.17±0.80 vs. 2.25±0.44 (p<0.001), respectively.
Table 1.
Clinical Characteristics of the Patients
| Control group | L-carnitine group | p | |
|---|---|---|---|
| Number of patients |
38 |
32 |
|
| Age (years old) |
67.0±13.2 |
68.0±12.4 |
0.732 |
| Sex (no.) (male/female) |
22/16 |
22/10 |
|
| HD durationa (months) (range) |
91.3 (2–442) |
109.2 (2–371) |
0.386 |
| Body mass index (kg/m2) |
21.5±4.6 |
22.3±3.17 |
0.422 |
| Systolic blood pressure (mmHg) |
152.0±22.7 |
154.0±26.9 |
0.737 |
| Hemoglobin (g/dL) |
10.7±1.2 |
10.8±0.8 |
0.621 |
| Total protein (g/dL) |
6.55±0.49 |
6.35±0.49 |
0.091 |
| Albumin (g/dL) |
3.65±0.32 |
3.71±0.29 |
0.423 |
| BUN (mg/dL) |
60.2±12.9 |
58.9±13.3 |
0.655 |
| Serum Cr (mg/dL) |
9.42±2.32 |
10.30±2.18 |
0.109 |
| Uric acid (mg/dL) |
7.37±1.10 |
7.10±1.08 |
0.307 |
| Corrected Ca (mg/dL) |
9.02±0.63 |
9.07±0.52 |
0.764 |
| Phosphate (mg/dL) |
4.43±1.06 |
4.43±0.73 |
0.978 |
| LDL-C (mg/dL) |
73.8±22.6 |
64.4±19.8 |
0.072 |
| HDL-C (mg/dL) |
52.9±17.8 |
52.2±15.7 |
0.927 |
| Triglyceridesa (mg/dL) (range) |
111(45–537) |
90(46–261) |
0.207 |
| Whole PTHa (pg/mL) (range) |
66(12–187) |
56(15–220) |
0.358 |
| CRP (mg/dL) |
0.35±0.37 |
0.37±0.72 |
0.146 |
| β2-MG (mg/dL) |
31.3±7.6 |
30.1±6.8 |
0.483 |
| Total carnitine (μmol/L) |
36.0±7.8 |
36.7±7.6 |
0.713 |
| Free carnitine (μmol/L) |
21.7±5.4 |
21.6±4.5 |
0.901 |
| Acyl carnitine (μmol/L) |
14.3±3.4 |
15.1±4.5 |
0.382 |
| Acy/free ratio |
0.67±0.14 |
0.71±0.21 |
0.344 |
| Kt/V |
1.61±0.24 |
1.54±0.21 |
0.253 |
| SAF (arbitrary units) |
3.11±0.78 |
3.24±0.81 |
0.510 |
| Diabetes (no.) (−/+) |
25/13 |
24/8 |
0.290 |
| Medication | |||
| RAS inhibitors (no.) (−/+) |
12/26 |
14/18 |
0.301 |
| Statins (no.) (−/+) | 30/8 | 22/10 | 0.338 |
Values are shown as mean±standard deviation (SD) or range.
These variables are shown in the original scale after using log-transformed values.
No., number; HD, hemodialysis; BUN, blood urea nitrogen; Cr, creatinine; Ca, calcium; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; PTH, parathyroid hormone; CRP, C-reactive protein; β2-MG, β2-microglobulin; SAF, skin autofluorescence; RAS, renin–angiotensin system.
Effects of l-carnitine supplementation on clinical variables
Total, free, acyl carnitine, LDL-C, and triglycerides levels just before the HD session were significantly increased by l-carnitine supplementation for 6 months, whereas alanine transaminase (ALT), acyl/free carnitine ratio, and β2-MG levels were decreased (Table 2). After 6-month observation periods, total protein and albumin levels were significantly decreased in control group (Table 2).
Table 2.
Effects of l-Carnitine Supplementation on Clinical Variables in Hemodialysis Patients
| |
Control group |
L-carnitine group |
||||
|---|---|---|---|---|---|---|
| Variables | Pre-treatment | Post-treatment | p | Pre-treatment | Post-treatment | p |
| Total protein |
6.54±0.49 |
6.34±0.49 |
0.005 |
6.35±0.50 |
6.31±0.38 0.592 |
|
| Albumin |
3.65±0.32 |
3.51±0.34 |
0.002 |
3.71±0.29 |
3.70±0.26 |
0.716 |
| AST |
15.4±5.8 |
14.9±6.7 |
0.532 |
14.8±6.7 |
12.7±7.6 |
0.116 |
| ALT |
12.8±5.5 |
12.5±8.1 |
0.756 |
12.8±9.4 |
9.8±8.7 |
0.024 |
| BUN |
60.4±13.0 |
61.5±14.4 |
0.600 |
58.8±13.3 |
54.6±12.9 |
0.107 |
| Serum Cr |
9.42±2.35 |
9.62±2.55 |
0.168 |
10.30±2.18 |
10.00±2.26 |
0.138 |
| Uric acid |
7.36±1.10 |
7.55±1.44 |
0.294 |
7.10±1.08 |
7.14±1.22 |
0.775 |
| Corrected Ca |
9.03±0.64 |
8.94±0.53 |
0.339 |
9.07±0.51 |
9.00±0.52 |
0.511 |
| Phosphate |
4.49±1.02 |
4.34±1.32 |
0.609 |
4.43±0.73 |
4.63±1.17 |
0.390 |
| LDL-C |
73.8±22.5 |
79.1±30.1 |
0.103 |
64.5±19.8 |
75.0±19.5 |
0.002 |
| HDL-C |
52.9±17.9 |
50.9±17.2 |
0.272 |
52.2±15.7 |
51.4±19.1 |
0.589 |
| Triglyceridesa |
111(45–537) |
105(39–508) |
0.166 |
90(46–261) |
111(44–230) |
0.015 |
| Whole PTHa |
66(12–187) |
52(10–122) |
0.057 |
56(14–220) |
48(11–136) |
0.270 |
| CRP |
0.35±0.37 |
0.62±1.65 |
0.106 |
0.37±0.72 |
0.44±1.17 |
0.054 |
| Total carnitine |
36.0±7.8 |
34.5±8.1 |
0.121 |
36.7±7.6 |
219.9±77.5 |
<0.001 |
| Free carnitine |
21.7±5.4 |
21.2±5.5 |
0.403 |
21.6±4.5 |
138.0±48.4 |
<0.001 |
| Acyl carnitine |
14.3±3.4 |
13.4±3.6 |
0.063 |
15.1±4.5 |
81.9±33.5 |
<0.001 |
| Acyl/free ratio |
0.67±0.14 |
0.65±0.14 |
0.231 |
0.71±0.21 |
0.60±0.12 |
0.001 |
| Skin AGEs |
3.11±0.78 |
2.99±0.75 |
0.072 |
3.24±0.82 |
2.99±0.82 |
0.027 |
| β2-MG | 31.3±7.6 | 30.0±8.7 | 0.453 | 30.1±6.8 | 27.2±5.1 | 0.003 |
Values are shown as mean±standard deviation (SD) or range.
These variables are shown in the original scale after using log-transformed values.
AST, aspartate transaminase; ALT, alanine transaminase; BUN, blood urea nitrogen; Cr, creatinine; Ca, calcium; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; PTH, parathyroid hormone; CRP, C-reactive protein; AGEs, advanced glycation end products; β2-MG, β2-microglobulin.
Effects of l-carnitine supplementation on skin AGE levels
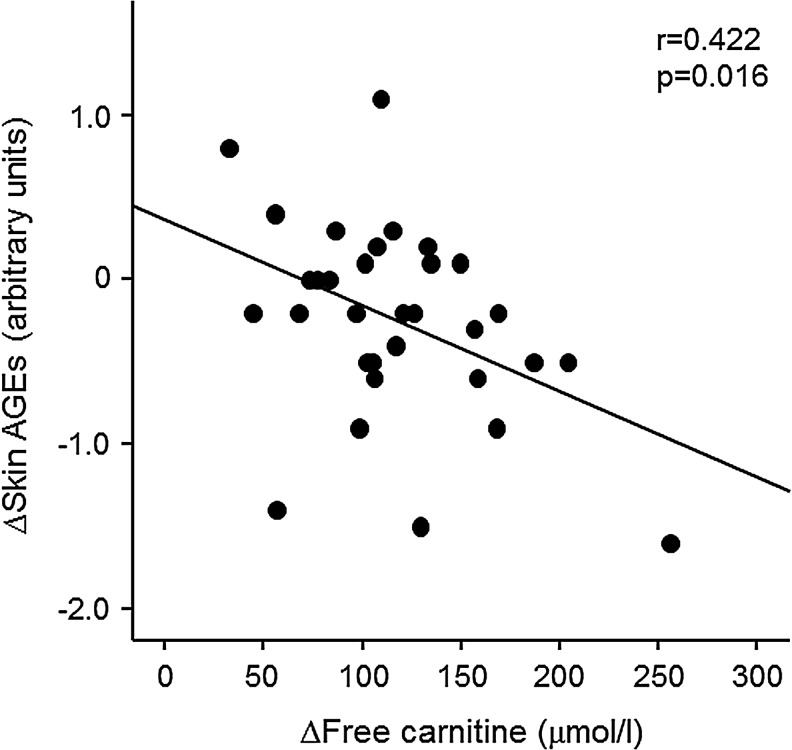
After 6-month treatments, skin AGE levels measured by SAF were significantly decreased in the l-carnitine therapy group, but not in the control group (Table 2). When comparing the ΔSAF between control and l-carnitine group, there was no significant difference (only trend) of ΔSAF between the two groups (−0.12±0.39 vs. −0.24±0.60, p=0.283). Univariate analysis revealed that Δtotal carnitine and Δfree carnitine were inversely correlated with ΔSAF in l-carnitine–treated HD patients (r=0.372, p=0.036 and r=0.422, p=0.016, respectively) (Table 3 and Fig. 2). As shown in Table 3, in multiple stepwise regression analysis, Δfree carnitine was a sole independent determinant of ΔSAF (R2=0.178).
Table 3.
Univariate and Multiple Regression Analysis for the Correlates of ΔSkin Advanced Glycation End Products
| |
Univariate |
Multiple stepwise regression |
||||
|---|---|---|---|---|---|---|
| SE | β | p | SE | β | p | |
| ΔTotal protein |
0.278 |
−0.006 |
0.975 |
|
|
|
| ΔAlbumin |
0.448 |
−0.122 |
0.507 |
|
|
|
| ΔAST |
0.014 |
−0.035 |
0.851 |
|
|
|
| ΔALT |
0.015 |
−0.039 |
0.834 |
|
|
|
| ΔBUN |
0.007 |
−0.294 |
0.102 |
|
|
|
| ΔSerum Cr |
0.097 |
0.012 |
0.102 |
|
|
|
| ΔUric acid |
0.148 |
−0.026 |
0.890 |
|
|
|
| ΔCorrected Ca |
0.181 |
0.229 |
0.208 |
|
|
|
| ΔPhosphate |
0.081 |
0.067 |
0.715 |
|
|
|
| ΔLDL-C |
0.006 |
−0.096 |
0.600 |
|
|
|
| ΔHDL-C |
0.013 |
−0.007 |
0.969 |
|
|
|
| ΔTriglycerides |
0.274 |
−0.041 |
0.824 |
|
|
|
| ΔWhole PTH |
0.003 |
0.052 |
0.777 |
|
|
|
| ΔCRP |
0.093 |
−0.017 |
0.926 |
|
|
|
| Δβ2-MG |
0.021 |
0.072 |
0.694 |
|
|
|
|
ΔTotal carnitine |
0.001 |
−0.372 |
0.036 |
|
|
|
|
ΔFree carnitine |
0.002 |
−0.422 |
0.016 |
0.002 |
−0.422 |
0.016 |
| ΔAcyl carnitine |
0.003 |
−0.259 |
0.152 |
|
|
|
| ΔAcy/free ratio | 0.604 | 0.024 | 0.898 | |||
R2=0.178
AST, aspartate transaminase; ALT, alanine transaminase; BUN, blood urea nitrogen; Cr, creatinine; Ca, calcium; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; PTH, parathyroid hormone; CRP, C-reactive protein; β2-MG, β2-microglobulin.
FIG. 2.
Correlation between Δskin advanced glycation end products (AGEs) and Δfree carnitine in l-carnitine–treated hemodialysis (HD) patients (n=32). AGEs, advanced glycation end products.
Discussion
We found here that: (1) Total, free, and acyl carnitine levels were significantly lower in HD patients, whereas tissue accumulation levels of AGEs evaluated by SAF were higher, (2) 900 mg of l-carnitine supplementation daily for 6 months dramatically increased all the carnitine fraction levels (total, free, and acyl carnitine values) and reduced skin AGE levels, (3) Δtotal and Δfree carnitine levels were inversely associated with Δskin AGEs, and (4) Δfree carnitine values were a sole independent determinant of Δskin AGEs in l-carnitine–treated HD patients.
In vitro study has shown that l-carnitine significantly inhibits the AGE-modification of bovine serum albumin,24 and its anti-glycating capacity is more potent than that of aminoguanidine, a prototype inhibitor of AGEs.24,27 Furthermore, administration of l-carnitine has been reported to reduce skin glycated collagen levels and improve insulin resistance in fructose-fed rats.24 In the present study, l-carnitine supplementation not only increased serum carnitine levels, but also reduced skin AGE values, and there was a significant and independent correlation between Δfree carnitine and Δskin AGE values in HD subjects. Because we have previously shown that SAF is inversely associated with serum carnitine levels in patients with HD,23 our present findings further suggest the causative role of carnitine deficiency in AGE accumulation in uremic subjects on HD.
Tissue accumulation levels of AGEs evaluated by SAF are elevated in HD patients.15,16 Furthermore, we, along with others, have shown that SAF is correlated with high-sensitivity CRP and the carotid pulsatility index and could predict future cardiovascular events and death in end-stage renal disease patients undergoing HD.15,16 These observations suggest that reduction of AGE accumulation in the skin might be a novel therapeutic target for preventing CVD in HD subjects. Given the facts that: (1) Carnitine levels were associated with the increased risk of CVD in uremic patients,28 (2) administration of l-carnitine attenuated the development and progression of atherosclerosis in animal model,29 and (3) l-carnitine supplementation improved endothelial dysfunction and ameliorated cardiac function in patients with HD,28 carnitine deficiency might be involved in the progression of CVD partly by stimulating tissue AGE accumulation. Therefore, suppression of the AGE accumulation by l-carnitine supplementation might play a protective role against CVD in HD subjects.
β2-MG has been shown to contribute to HD-related amyloidosis.30,31 Furthermore, its serum levels are positively correlated with inflammatory variables30 and inversely associated with circulating endothelial progenitor cell number.31 These findings suggest that increased β2-MG could contribute to impaired endothelial cell repair, thus being involved in CVD in HD subjects. In this study, l-carnitine supplementation significantly reduced serum β2-MG levels in patients with HD. Since Simone et al. reported that high-dose l-carnitine therapy decreased serum β2-MG levels, which was associated with amelioration of immunologic and metabolic parameters in patients with acquired immunodeficiency disease syndrome,32 so l-carnitine supplementation might exert beneficial effects in HD patients partly via suppression of β2-MG levels.
Malnutrition is one of the strongest predictors for disabilities and high mortality rate in patients with HD.33 We have recently shown that serum albumin and LDL-C levels are significantly decreased in HD patients, and these levels were positively associated with serum carnitine levels.23 In the present study, after 6-month observation periods, total protein and albumin levels were significantly decreased in control group, whereas LDL-C and triglycerides levels were increased in the l-carnitine–treated group. These observations suggest that some type of malnutrition may be involved in decreased carnitine levels in HD subjects and that l-carnitine supplementation might improve the conditions of malnutrition in uremic patients.
Limitations
In this study, we used only SAF to assess tissue AGEs. This might be a weak methodology because it is nonspecific. Thus, it would be helpful to examine whether l-carnitine supplementation could actually decrease tissue levels of AGEs with more specific methods.
In the present study, SAF not only decreased in the intervention group but also in the control group, although the change did not reach significance in the latter group. Furthermore, there was no significant difference (only trend) of ΔSAF between the two groups. Therefore, the effects of l-carnitine supplementation on SAF could be modest.
Some of the data points on ΔSAF over 6 months were >1 arbitrary unit change. However, because we measured SAF just before HD sessions and that the use of skin creams was prohibited when measuring the SAF, it was unlikely that these factors could affect the present results.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Welfare, and Scientific Research (C) (no. 25461239) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (K.F) and by Grants of MEXT-Supported Program for the Strategic Research Foundation at Private Universities, the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (S.Y.).
Author Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Brownlee M, Cerami A, Vlassara H.Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 1988;318:1315–1321 [DOI] [PubMed] [Google Scholar]
- 2.Grandhee SK, Monnier VM.Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J Biol Chem 1991;266:11649–11653 [PubMed] [Google Scholar]
- 3.Dyer DG, Blackledge JA, Thorpe SR, Baynes JW.Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem 1991;266:11654–11660 [PubMed] [Google Scholar]
- 4.Miyata T, Ueda Y, Yamada Y, Izuhara Y, Wada T, Jadoul M, Saito A, Kurokawa K, van Ypersele de Strihou C.Accumulation of carbonyls accelerates the formation of pentosidine, an advanced glycation end product: Carbonyl stress in uremia. J Am Soc Nephrol 1998;9:2349–2356 [DOI] [PubMed] [Google Scholar]
- 5.Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, Friedman EA, Cerami A, Vlassara H.Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med 1991;325:836–842 [DOI] [PubMed] [Google Scholar]
- 6.Vlassara H.Recent progress in advanced glycation end products and diabetic complications. Diabetes 1997;46(Suppl 2):S19–S25 [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Ueda Y, Suzuki T, Yamada S, Takeuchi M, Fukami K, Ueda S, Adachi H, Matsui T, Okuda S, Yamagishi S.Positive association of serum levels of advanced glycation end products and high mobility group box-1 with asymmetric dimethylarginine in nondiabetic chronic kidney disease patients. Metabolism 2009;58:1624–1628 [DOI] [PubMed] [Google Scholar]
- 8.Yamagishi S.Advanced glycation end products and receptor-oxidative stress system in diabetic vascular complications. Ther Apher Dial 2009;13:534–539 [DOI] [PubMed] [Google Scholar]
- 9.Kilhovd BK, Juutilainen A, Lehto S, Ronnemaa T, Torjesen PA, Birkeland KI, Berg TJ, Hanssen KF, Laakso M.High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: A population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol 2005;25:815–820 [DOI] [PubMed] [Google Scholar]
- 10.Kilhovd BK, Juutilainen A, Lehto S, Ronnemaa T, Torjesen PA, Hanssen KF, Laakso M.Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: A population-based 18 year follow-up study. Diabetologia 2007;50:1409–1417 [DOI] [PubMed] [Google Scholar]
- 11.Furuya R, Kumagai H, Miyata T, Fukasawa H, Isobe S, Kinoshita N, Hishida A.High plasma pentosidine level is accompanied with cardiovascular events in hemodialysis patients. Clin Exp Nephrol 2012;16:421–426 [DOI] [PubMed] [Google Scholar]
- 12.Mitome J, Yamamoto H, Saito M, Yokoyama K, Marumo K, Hosoya T.Nonenzymatic cross-linking pentosidine increase in bone collagen and are associated with disorders of bone mineralization in dialysis patients. Calcif Tissue Int 2011;88:521–529 [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi M, Kikuchi S, Sasaki N, Suzuki T, Watai T, Iwaki M, Bucala R, Yamagishi S.Involvement of advanced glycation end-products (AGEs) in Alzheimer's disease. Curr Alzheimer Res 2004;1:39–46 [DOI] [PubMed] [Google Scholar]
- 14.Schupp N, Dette EM, Schmid U, Bahner U, Winkler M, Heidland A, Stopper H.Benfotiamine reduces genomic damage in peripheral lymphocytes of hemodialysis patients. Naunyn Schmiedebergs Arch Pharmacol 2008;378:283–291 [DOI] [PubMed] [Google Scholar]
- 15.Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, Thorpe SR, Baynes JW, Navis G, Gans RO, Smit AJ.Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol 2005;16:3687–3693 [DOI] [PubMed] [Google Scholar]
- 16.Nagano M, Fukami K, Yamagishi S, Sakai K, Kaida Y, Matsumoto T, Hazama T, Tanaka M, Ueda S, Okuda S.Tissue level of advanced glycation end products is an independent determinant of high-sensitivity C-reactive protein levels in haemodialysis patients. Nephrology (Carlton) 2011;16:299–303 [DOI] [PubMed] [Google Scholar]
- 17.Mulder DJ, Water TV, Lutgers HL, Graaff R, Gans RO, Zijlstra F, Smit AJ.Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: An overview of current clinical studies, evidence, and limitations. Diabetes Technol Ther 2006;8:523–535 [DOI] [PubMed] [Google Scholar]
- 18.Januszewski AS, Sachithanandan N, Karschimkus C, O'Neal DN, Yeung CK, Alkatib N, Jenkins AJ.Non-invasive measures of tissue autofluorescence are increased in type 1 diabetes complications and correlate with a non-invasive measure of vascular dysfunction. Diabet Med 2012;29:726–733 [DOI] [PubMed] [Google Scholar]
- 19.Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, Smit AJ.Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care 2006;29:2654–2659 [DOI] [PubMed] [Google Scholar]
- 20.Evans AM, Fornasini G.Pharmacokinetics of L-carnitine. Clin Pharmacokinet 2003;42:941–967 [DOI] [PubMed] [Google Scholar]
- 21.Carvajal K, Moreno-Sánchez R.Heart metabolic disturbances in cardiovascular diseases. Arch Med Res 2003;34:89–99 [DOI] [PubMed] [Google Scholar]
- 22.Ascunce RR, Nayar AC, Phoon CK, Srichai MB.Cardiac magnetic resonance findings in a case of carnitine deficiency. Tex Heart Inst J 2013;40:104–105 [PMC free article] [PubMed] [Google Scholar]
- 23.Adachi T, Fukami K, Yamagishi S, Kaida Y, Ando R, Sakai K, Adachi H, Otsuka A, Ueda S, Sugi K, Okuda S.Decreased serum carnitine is independently correlated with increased tissue accumulation levels of advanced glycation end products in haemodialysis patients. Nephrology (Carlton) 2012;17:689–694 [DOI] [PubMed] [Google Scholar]
- 24.Rajasekar P, Anuradha CV.L-Carnitine inhibits protein glycation in vitro and in vivo: Evidence for a role in diabetic management. Acta Diabetol 2007;44:83–90 [DOI] [PubMed] [Google Scholar]
- 25.Takahashi M, Ueda S, Misaki H, Sugiyama N, Matsumoto K, Matsuo N, Murao S.Carnitine determination by an enzymatic cycling method with carnitine dehydrogenase. Clin Chem 1994;40:817–821 [PubMed] [Google Scholar]
- 26.Daugirdas JT.Linear estimates of variable-volume, single-pool Kt/V: An analysis of error. Am J Kidney Dis 1993;22:267–270 [DOI] [PubMed] [Google Scholar]
- 27.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S.Beneficial effects of metformin and irbesartan on advanced glycation end products (AGEs)-RAGE-induced proximal tubular cell injury. Pharmacol Res 2012;65:297–302 [DOI] [PubMed] [Google Scholar]
- 28.van Es A, Henny FC, Kooistra MP, Lobatto S, Scholte HR.Amelioration of cardiac function by L-carnitine administration in patients on haemodialysis. Contrib Nephrol 1992;98:28–35 [DOI] [PubMed] [Google Scholar]
- 29.Sayed-Ahmed MM, Khattab MM, Gad MZ, Mostafa N.L-carnitine prevents the progression of atherosclerotic lesions in hypercholesterolaemic rabbits. Pharmacol Res 2001;44:235–242 [DOI] [PubMed] [Google Scholar]
- 30.Kuragano T, Kida A, Furuta M, Nanami M, Otaki Y, Hasuike Y, Nonoguchi H, Nakanishi T.The impact of beta2-microglobulin clearance on the risk factors of cardiovascular disease in hemodialysis patients. ASAIO J 2010;56:326–332 [DOI] [PubMed] [Google Scholar]
- 31.Jourde-Chiche N, Dou L, Sabatier F, Calaf R, Cerini C, Robert S, Camoin-Jau L, Charpiot P, Argiles A, Dignat-George F, Brunet P.Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J Thromb Haemost 2009;7:1576–1584 [DOI] [PubMed] [Google Scholar]
- 32.De Simone C, Tzantzoglou S, Famularo G, Moretti S, Paoletti F, Vullo V, Delia S.High dose L-carnitine improves immunologic and metabolic parameters in AIDS patients. Immunopharmacol Immunotoxicol 1993;15:1–12 [DOI] [PubMed] [Google Scholar]
- 33.Akdag I, Yilmaz Y, Kahvecioglu S, Bolca N, Ercan I, Ersoy A, Gullulu M.Clinical value of the malnutrition-inflammation-atherosclerosis syndrome for long-term prediction of cardiovascular mortality in patients with end-stage renal disease: A 5-year prospective study. Nephron Clin Pract 2008;108:c99–c105 [DOI] [PubMed] [Google Scholar]