Abstract
The concepts of absolute electronegativity, χ, and absolute hardness, η, are incorporated into molecular orbital theory. A graphic and concise definition of hardness is given as twice the energy gap between the highest occupied molecular orbital and the lowest unoccupied molecular orbital. Useful correlations can now be made between chemical behavior, visible-UV absorption spectra, optical polarizability, ionization potentials, and electron affinities.
Keywords: polarizability, visible-ultraviolet spectra, ionization potential, electron affinity
Full text
PDF
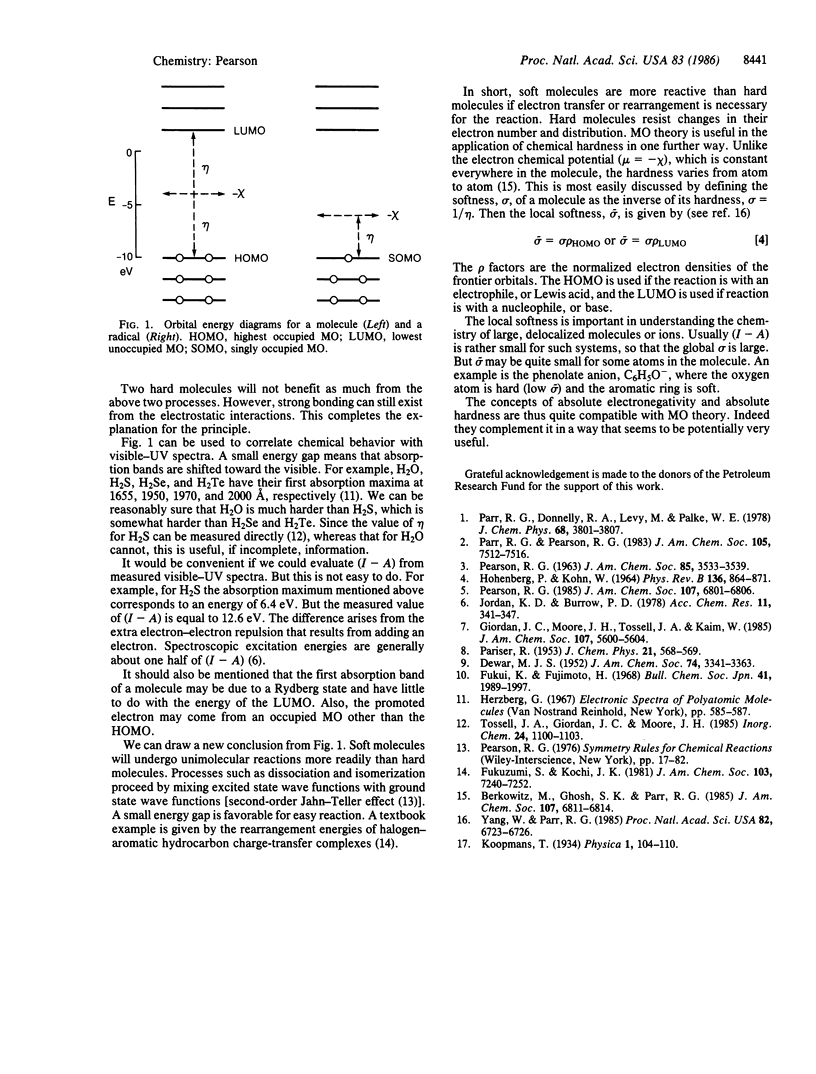
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Yang W., Parr R. G. Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6723–6726. doi: 10.1073/pnas.82.20.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]



