Abstract
Heart disease affects millions worldwide and is a progressive condition involving loss of cardiomyocytes. The human heart has limited endogenous regenerative capacity and is thus an important target for novel regenerative medicine approaches. While cell-based regenerative therapies hold promise, cellular reprogramming of endogenous cardiac fibroblasts, which represent more than half of the cells in the mammalian heart, may be an attractive alternative strategy for regenerating cardiac muscle. Recent advances leveraging years of developmental biology point to the feasibility of generating de novo cardiomyocyte-like cells from terminally differentiated non-myocytes in the heart in situ after ischemic damage. Here, we review the progress in cardiac reprogramming methods and consider the opportunities and challenges that lie ahead in refining this technology for regenerative medicine.
Keywords: Cellular reprogramming, cardiomyocyte, fibroblasts, heart disease
Cardiomyocyte death is associated with diverse forms of heart disease, which is the leading cause of morbidity and mortality in industrialized countries. Myocardial infarctions (MI) are a major public health problem and affect more than 1 million Americans yearly. A tremendous loss of cardiomyocytes through apoptosis and/or necrosis underlies both acute and chronic MI. To compensate for the death of cardiomyocytes in the injured area, scar tissue is formed by activated fibroblasts. In addition, heart diseases related to pressure overload, such as hypertension or valvular disease, involve the death of cardiac muscle cells but the progression is slow and usually occurs over a long period of time. Human aging is associated with an irreversible loss of cardiomyocytes, which may account for the increased vulnerability of aged hearts to various risk factors1.
Unlike amphibian and fish hearts2, the human heart has limited regenerative potential. Although postnatal vertebrate cardiomyocytes undergo some degree of cell renewal, as suggested by transgenic mouse experiments3-5and a more recent human radiocarbon dating study6, the rate of cardiomyocyte renewal is very low. Agenetic fate map study in mice indicated that the endogenous regenerative capacity of the adult heart, albeit limited, largely comes from the differentiation of cardiac progenitor cells rather than replacement by existing cardiomyocytes through cell division7, 8. A major challenge moving forward is to identify such cardiac progenitor pools in the adult heart and promote their expansion and differentiation potential in vivo. In contrast, Porrello et al. reported the neonatal mouse heart has a remarkable regenerative capacity within the first 7 days of life, similar to that of the zebrafish heart9. Genetic fate mapping suggests that myocyte proliferation is the main mechanism for this regeneration9, which is reminiscent of what has been found in the adult zebrafish heart10, 11. Furthermore, in a recent report using stable isotope labeling with genetic fate-mapping in combination with multi-isotope imaging mass spectrometry, the authors concluded that pre-existing CMs are the dominant source of myocyte renewal in the adult mouse heart with or without injury5. Cardiomyocyte proliferation has been reported to be enhanced by overexpressing cyclinD212, 13 or administering factors, such as Periostin14, fibroblast growth factor-1 (FGF-1)15 or neuregulin 1 (NRG1)16 in mouse models of MI. Most recently, addition of miR-199a and miR-590 also stimulated cardiomyocyte proliferation in vitro and in vivo17. Thus, there is increasing evidence that adult mammalian cardiomyocytes have the capacity to proliferate, but whether approaches to enhance this feature are sufficient to compensate for the functional loss of damaged myocardium awaits confirmation.
Another strategy to repair an injured heart is to supply new cardiomyocytes differentiated from multipotent cardiovascular progenitor cells (CPCs) or pluripotent stem cells, including embryonic stem (ES) cells and induced pluripotent stem (iPS) cells (reviewed extensively elsewhere18-21). Cardiomyocytes derived from pluripotent stem cells are a promising source of cells due to recent advances in efficiency of human cardiomyocyte differentiation. However, the poor survival, low maturational efficiency, and limited functional integration of engrafted cells are hurdles that must still be overcome. Introduction of adult progenitor cells in animal models has positive effects after MI, but the cells do not appear to persist, pointing to a potential paracrine effect (reviewed in 22, 23). Clinical trials are underway to evaluate efficacy, with mixed results to date24.
Here, we will review a newly emerging strategy for cardiac regeneration that involves reprogramming the vast pool of non-myocytes in the heart into new cardiomyocytes25. This approach builds on recent cellular programming knowledge26 and leverages over 15 years of advances in cardiac developmental biology20 to induce a cardiomyocyte-like fate in the adult heart. Cardiac reprogramming can be induced by a discrete set of factors but appears to have critical epigenetic blocks in vitro which are significantly overcome upon reprogramming of cells in their native environment in vivo25, 27-30.We will review how advances in cellular reprogramming and developmental biology led to our more recent work in direct cardiac reprogramming and will consider the future potential of this rapidly evolving approach to cardiac regeneration.
Cellular reprogramming
For decades, the concept in the field of developmental biology had been that cells, once terminally differentiated, were relatively fixed in their cell fate. This dogma was first challenged in the 1960s by the observation that a somatic cell can obtain totipotency through nuclear transfer into an enucleated frog egg31. Cloning of Dolly the sheep in 1997 by nuclear transfer revealed that the mammalian egg also had similar ability in reprogramming an adult nucleus to the embryonic state32. In the late 80s and 90s, further evidence suggested that cell fate conversion can take place by a more direct route. Blau and colleagues demonstrated that fusion of skeletal muscle cells with fibroblasts resulted in heterokaryons that induced conversion of fibroblasts into a skeletal muscle–like phenotype33. Studies from the Weintraub lab subsequently demonstrated that fibroblasts could be converted into skeletal muscle cells in vitro with forced expression of the skeletal muscle “master regulator” gene MyoD, which encodes a basic helix-loop-helix domain-containing transcription factor34, 35. Despite the race to identify individual transcription factors that could function to guide cell fate similar to MyoD for other lineages, including cardiomyocytes, the MyoD paradigm appeared to be an exception, rather than the rule. Meanwhile, studies in model organisms showed that forced expression of individual master regulatory transcription factors containing homeobox domains could induce formation of complex body structures, which is best exemplified by the induction of ectopic eye formation on the legs of Drosophila by overexpressing eyeless, the fly orthologue of Pax636. The concept of cell fate conversion bypassing normal developmental lineage progression therefore emerged, but was still largely neglected with respect to the application of such concepts in regenerative medicine.
Takahashi and Yamanaka's milestone publication in 2006 demonstrating the creation of induced pluripotent stem cells (iPSCs)37 ushered in a new era of utilizing cellular reprogramming in regenerative medicine38-40. Co-expression of four transcription factors (Oct4, Sox2, Klf4, c-Myc) was sufficient to reprogram fibroblasts to pluripotent stem cells that had the potential to develop into viable animals41, 42. Abundant evidence has demonstrated that iPSCs can be differentiated efficiently into multiple cell types that someday could be used for regenerative therapies, disease modeling and drug discovery. A key lesson from iPSC reprogramming was the recognition that a combinatorial code involving a discrete number of regulatory factors could be sufficient to induce cell fate change43, 44.
Despite the difficulty of identifying MyoD-like factors for direct cellular reprogramming from one terminally differentiated adult somatic cell directly into another without taking a “detour” back to pluripotency, the iPSC experience raised the possibility of a combination of regulators that could together induce cell fate change, or transdifferentiation. This reprogramming strategy was first demonstrated by Zhou et al., who directly converted exocrine pancreatic cells into insulin-producing endocrine cells in the mouse pancreas with the transcription factors, Ngn3, Pdx and Mafa45. Similarly, we showed that cardiac and dermal fibroblasts could be reprogrammed into cardiomyocyte-like cells by a combination of three transcription factors, Gata4, Mef2c, and Tbx25, 27. The direct reprogramming of adult fibroblasts to neuronal-like cells was also achieved in vitro with forced expression of a combination of transcription factors46, 47 or microRNAs (miRNAs)48, and similar observations were made for conversion to the hepatocyte lineage49, 50. Unlike direct reprogramming with multiple factors, Szabo et al. showed that combining expression of only Oct4 with administration of select cytokines can reprogram human fibroblasts into hematopoietic progenitors51, while transient expression of iPSC reprogramming factors, followed by Jak/Stat inhibition, resulted in emergence of cardiomyocytes52. The advances in cardiac reprogramming are considered in more detail below.
Discovery of transcription factor-based direct cardiac reprogramming
Over the last 20 years, developmental biology studies have revealed complex and intertwined networks of signaling pathways, transcription factors and miRNAs that regulate formation and function of the heart20, 53, 54. The networks are self-reinforcing with layers of positive and negative feedback loops. Transcription factors often function in common complexes, and human mutations that disrupt their interaction can lead to similar forms of heart malformations, as seen with mutations in GATA4 and TBX555. In 2009, Takeuchi and Bruneau demonstrated that overexpression of Gata4, Tbx5 and the interacting chromatin remodeling protein, Baf60c, converts noncardiogenic mesoderm into beating cardiomyocytes in the embryo by a mechanism involving the induction of Nkx2-5 by Gata4 and Baf60c56.
The adult heart has many cell types within the organ that are normally non-cardiogenic. The vast majority of these are cardiac fibroblasts, which comprise over 50% of cells in the heart and are derived from an extracardiac structure known as the proepicardial organ57. Fibroblasts play an important structural and paracrine role supporting the neighboring myocytes58. Upon injury, cardiac fibroblasts are activated and migrate to the site of injury to create scar tissue that replaces dead myocardium. Due to the abundance of resident cardiac fibroblasts, the ability to reprogram such cells in situ would represent a powerful approach for regenerating myocardium.
We leveraged the abundant knowledge of cardiac developmental biology to attempt reprogramming of adult somatic cells into cardiomyocyte-like cells25. Despite the desire to ultimately reprogram in vivo, we established an in vitro assay to discover the minimal cocktail of factors that could convert non-cardiomycytes in the heart into a more cardiomyocyte-like phenotype. We generated transgenic mice containing the EGFP reporter downstream of the cardiomyocyte-specific αMHC (Myh6) promoter59 and isolated the EGFP-negative non-myocyte population, which consisted largely of fibroblasts. Upon retroviral introduction of 14 transcription factors, and a pool of miRNAs, neonatal αMHC-EGFP– cells activated the expression of αMHC (Myh6) and the αMHC-EGFP reporter, which allowed quantitative analysis by fluorescence activated cell sorting (FACS). The miRNAs in this setting were dispensable. Through serial deletion of one transcription factor at a time, we narrowed the required reprogramming factors to three: Gata4, Mef2c and Tbx5 (GMT). The GMT cocktail was sufficient to induce GFP expression in about 15–20% of the cells, which we termed induced cardiomyocytes (iCMs). However, the vast majority of cells were partially reprogrammed, with 5% of the total infected cell population expressing additional cardiac markers, such as cTnT, and assembling sarcomeric structures; furthermore, only ~0.5% of the αMHC−EGFP+/cTnT+ cells were capable of beating.
Despite the low percentage of fully reprogrammed cardiomyocytes, genome-wide transcriptome studies of the αMHC−EGFP+ cells (~15% of infected cells) showed that the partially reprogrammed population induced a broad cardiac transcriptional program involving thousands of genes and also silenced the fibroblast transcriptome. Moreover, the epigenetic status of iCMs was similar to neonatal endogenous cardiomyocytes at loci examined, and the reprogramming event was stable, not requiring ongoing expression of GMT. Notably, the GMT cocktail also reprogrammed tail-tip dermal fibroblasts, albeit with lower efficiency, suggesting that the presence of a cardiac progenitor pool was not necessary for the presence of iCMs.
Interestingly, the more fully reprogrammed iCMs had action potentials that were most similar to adult ventricular myocytes. This observation was in contrast to the relatively immature electrical activity noted in ES- or iPS-derived cardiomyocytes. Using a Cre-based strategy, we found that iCMs never expressed Mesp1 or Isl1, markers of early cardiac progenitors, during the process of cardiac reprogramming25. This suggested that the reprogramming event represented a direct conversion from one adult cell type to another rather than traversing through a progenitor stage. The rapidity of initial conversion and the more mature electrophysiology observed in iCMs is consistent with this interpretation.
In vivo direct cardiac reprogramming
The relatively poor quality and low efficiency of in vitro cardiac reprogramming might be due to the lack of a natural environment for cardiomyocytes on plastic dishes. Because the objective with this strategy was to harness the endogenous cardiac fibroblasts within the organ for regeneration without needing to use cell-based therapy, we attempted to deliver GMT in vivo after ischemic injury and convert non-myocytes to cardiomyocyte-like cells. Classic genetic lineage tracing studies with Periostin-Cre, Fsp1-Cre and αMHC-MerCreMer were performed to demonstrate that dividing non-myocytes infected by retroviruses could be converted into iCMs27 over a period of 4 weeks. Careful studies demonstrated that the newly born myocytes arose from in vivo conversion rather than leaky Cre expression or cell-cell fusion. Importantly, intermediate stages of reprogramming to iCMs were identified and characterized at varying time points after retroviral infection, further supporting the idea of a progressive reprogramming process.
In vivo-derived iCMs developed many characteristics of endogenous CMs. They were bi-nucleate, assembled sarcomeres and had cardiomyocyte-like gene expression by 4 weeks post-infection with GMT. Furthermore, single-cell analyses by patch clamp technology revealed that 50% of in vivo-derived iCMs closely resembled endogenous CMs with beating upon electrical stimulation and ventricular cardiomyocyte-like action potentials. The markedly improved quality of reprogramming in vivo compared to in vitro might have been due to signals from the microenvironment, exposure to the extracellular matrix, or the influence of mechanical forces while reprogramming. Importantly, we found evidence for electrical coupling of the in vivo reprogrammed iCMs with endogenous cardiomyocytes and other iCMs. In vivo delivery of GMT intramyocardially decreased scar size and attenuated cardiac dysfunction after coronary ligation, as assessed by MRI and echocardiography. As expected, the cardiomyocytes within the scar area of GMT-treated mice represented newly born iCMs as determined by lineage tracing experiments. The beneficial effects of GMT were enhanced with the addition of the pro-angiogenic and fibroblast-activating factor, Thymosin β4, which independently promotes cardiac repair60, 61.
In a similar study, Song et al.28 replicated the findings of Ieda et al.25 with GMT, and demonstrated that the addition of a bHLH domain-containing transcription factor, Hand2, could increase the efficiency of cardiac reprogramming in vitro. Hand2 was initially discovered in a search for a cardiac MyoD-like bHLH protein62, and is essential for a subset of cardiac progenitors63-65, but not sufficient to induce the cardiac phenotype. Importantly, Song et al. also showed direct conversion from non-myocyte to myocytes in vivo by retrovirally transducing fibroblasts with GMT plus Hand2 (GHMT) into hearts after coronary ligation. These in vivo induced cardiac-like myocytes were similar to the endogenous cardiomyocytes, based on their gene expression, sarcomere structure and electrophysiological features. Lineage tracing with Fsp1-Cre, an inducible Tcf21-iCre, and αMHC-MerCreMer demonstrated the origin of the induced cardiomyocyte-like cells was likely cardiac fibroblasts. This observation was further supported by the evidence that the genetically pre-labeled old myocyte pool was diluted by these newly born myocyte-like cells. Along with the emergence of new cardiomyocyte-like cells, improved heart function and reduced scar size were observed.
A third successful example of in vivo cardiac reprogramming was reported by Inagawa et al30. The authors used the same GMT cocktail and retroviral delivery method. Different from Qian et al and Song et al, immunosuppressed mice were used in an attempt to promote the survival of viral transduced cells. Reprogramming with these nude mice resulted in more iCMs with well-defined sarcomere structure. Furthermore, in order to improve the transduction of GMT in vivo, Inagawa et al generated a polycistronic retrovirus expressing GMT at near equimolar levels from the same promoter using “self-cleaving” 2A peptides that resulted in a better reprogramming efficiency. Although the global outcome of GMT introduction in this system was not clear, use of a 2A polycistronic vector and immunosuppressed mice further refined the in vivo reprogramming technology.
These three studies provide compelling evidence that the abundant non-myocyte pool in the heart, largely composed of fibroblasts, can be transdifferentiated into new cardiomyocyte-like cells in vivo after injury, resulting in regeneration of myocardium and improved cardiac function. The functional improvement can be partially explained by new myocytes that increase force generation, but a fundamental alteration in the nature of scar-producing fibroblasts could also account for part of the reduction in scar size and improvement of cardiac output.
Alternative strategies for cardiac reprogramming
The strategy involving serial deletion of one factor at a time can yield potential combinations of factors for reprogramming but, due to the complex interactions between networks, may not necessarily yield the optimal combination. To address this, Protze et al.66 screened 120 triplet combinations of 10 important developmental cardiac transcription factors expressed by lentiviruses in mouse embryonic fibroblasts (MEFs) for their ability to induce a myocyte-like phenotype. Rather than measuring the activation of a single reporter, they assayed expression of a panel of cardiac genes by qPCR. In this screen, Myocardin, Mef2c and Tbx5 were the optimal combination to convert fibroblasts into myocyte-like cells, determined by gene expression, sarcomere formation and ion channel activity, suggesting multiple combinations may exist for efficient cardiac reprogramming. Protze et al. also performed a time-course experiment and showed a progressive process involved in direct cardiac reprogramming in which a more complete cardiac phenotype arose over time.
miRNAs have important roles in cardiomyocyte decisions and are often regulated by the major cardiac transcription factors and, in turn, titrate the dosage of the key transcriptional networks54, 67. In particular, miR-1, the most abundant cardiac miRNA, promotes muscle gene expression and regulates many aspects of cardiac biology68-71. Recently, Jayawardena et al. showed that a combination of four muscle-specific miRNAs (miR-1, miR-133, miR-208, miR-499) was sufficient to transdifferentiate mouse fibroblasts into cardiomyocyte-like cells in vitro and in vivo. Remarkably, in the presence of a JAK-1 inhibitor, miR-1 alone was sufficient to reprogram the cardiac fibroblasts29.
While many groups have now successfully reprogrammed fibroblasts to cardiomyocyte-like cells in vitro, others have struggled72, highlighting the challenges to easily mastering this technique. The use of fresh, non-senescing fibroblasts, high titers of viruses expressing the reprogramming factors, and careful culture conditions are among the variables involved in achieving successful reprogramming73. Future work on standardizing the conditions and improving the conversion efficiency in vitro will be necessary to make this a routine procedure in many other laboratories.
Challenges and future directions
Despite the excitement and potential of direct cardiac reprogramming technology, several challenges remain, as would be expected for any new technology. All of the work thus far has been performed in rodents. It will be intriguing to determine if human fibroblasts can be similarly reprogrammed into iCMs. Although this might be technically challenging, identification of additional factors or a different combination of factors might allow conversion to occur in human fibroblasts given the success in human neuronal and iPSC reprogramming. Recently, successful reprogramming of human fibroblasts to an early cardiac progenitor state was reported using the transcription factors, MESP1 and ETS-152. The cardiac progenitors were multipotent and could subsequently differentiate into myocytes with calcium transients. However, direct reprogramming to a human adult-like cardiomyocyte, bypassing the embryonic stage, has not yet been reported. In addition, preclinical trials on large animal models, such as pigs, should be rigorously performed with careful monitoring of potential side effects, especially the risk of arrhythmias. Finally, new non-integrating methods for delivery of reprogramming factors and methods to increase efficiency will be important.
The low conversion rate of fibroblasts to fully reprogrammed iCMs in vitro is a major challenge for deciphering the mechanism of reprogramming. The relative inefficiency in vitro is not surprising, considering the iPSC reprogramming efficiency rate (0.01–0.1%), which is likely due to major epigenetic barriers that cells cannot easily overcome74. Unlike iPSCs, reprogrammed cardiomyocytes rapidly exit the cell cycle, making efficiency a much bigger concern. Understanding how progressive epigenetic and transcriptional changes occur temporally during direct cardiac reprogramming is an important first step towards overcoming these hurdles. As highlighted from two recent genome-wide epigenetic studies of ESC-derived CMs75, 76, temporal alterations in chromatin structure patterns lead to activation of key genes associated with heart development and function at distinctive differentiation stages. Such mechanisms might be informative for cardiac reprogramming. Second, methods aiming to improve delivery efficiency, such as the utilization of a polycistronic vector30 to control for homogenous reprogramming gene expression, nano-particle techniques for specific and efficient targeting, immunosuppression to promote survival of transduced cells30, and the administration of Thymosin β4 to mobilize and activate fibroblasts60, may increase the final number of reprogrammed iCMs. Third, techniques that enhance the maturation of cardiomyocytes should be used to promote the progression of iCMs from immature to mature stages. These include supplying the cells with cytokines at defined stages, altering the expression pattern of certain ion channel regulators, exposing cells to mechanical forces and providing extracellular matrix and/or endothelial cells. Last, small-molecule and secreted factor screens geared towards increasing iCM numbers and quality should be performed to identify externally administered factors that promote the reprogramming process.
Approaches to improve efficiency in vitro will aid in mechanistic understanding of the reprogramming process and may ultimately allow use of direct reprogramming to model human disease. Although current technology does not generate sufficient numbers of fully reprogrammed iCMs for disease modeling studies, improvements in efficiency will be valuable since iCMs appear to achieve electrical maturation that is more similar to ventricular myocytes, particularly when reprogrammed in vivo, and this has been difficult to achieve with ESC- or iPSC-derived cardiomyocytes. Generation of sufficient numbers of fully reprogrammed cells in vitro would also be valuable for drug toxicity studies and drug screening.
In contrast, current iCM technology is quite efficient for in vivo reprogramming. Thus, harnessing the vast endogenous pool of non-cardiomyocytes seems like a viable approach to regenerate heart muscle without cell-based therapy. Although this would require a gene therapy approach ideally using non-integration vectors such as adeno-associated viral (AAV) vectors, a reasonable regulatory path exists for virally mediated gene delivery with scores of FDA-approved trials underway and the recent approval of a gene therapy drug for lipoprotein lipase deficiency in Europe. Future identification of small molecules or secreted proteins that could replace each transcription factor, as has been done for iPSC reprogramming77, may allow an alternative to gene therapy. It is possible that in vivo reprogramming of cells to regenerate damaged tissue will serve as a new paradigm for many human diseases, and the lessons learned in the cardiac area will be applicable to strategies to realize this dream in other tissues. While many challenges lie ahead in advancing this nascent technology, the opportunities and the potential benefits are significant, and we are confident the field will continue to push this technology further in the years ahead.
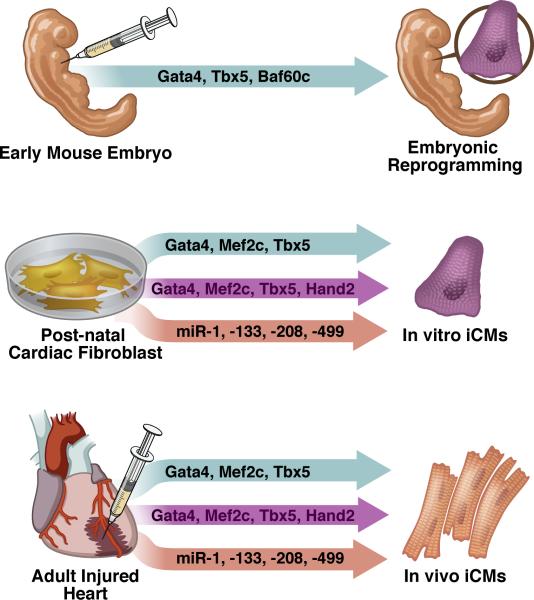
Figure 1. Approaches to direct cardiac reprogramming.
Several methods for converting non-myocytes to cardiomyocyte-like cells have been published and are summarized here. In the first example, injection of factors into the non-cardiogenic mesoderm of E7.0 embryos resulted in ectopic beating cells (E8.5 embryos shown for simplicity). In vitro delivery of various cocktails resulted in primarily partially reprogrammed cells, while in vivo delivery yielded more fully reprogrammed cardiomyocyte-like cells.
Figure 2. Direct conversion of cardiac fibroblasts into cardiomyocyte-like cells in vivo.
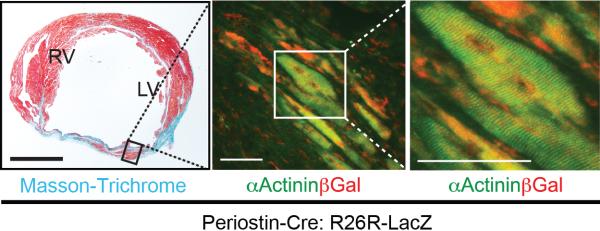
Masson-Trichrome (left panel) and immunofluorescent staining for α-Actinin and βGal (right panels) in GMT injected Periostin-Cre:R26R-lacZ mouse heart 4 weeks post-coronary ligation. Scale bars: 500 μm in the left panel, 50 μm in the right two panels.
ACKNOWLEDGMENTS
The authors thank members of the Srivastava lab for valuable discussion and contributions, B. Taylor for assistance with preparation of the manuscript, and G. Howard for editorial assistance.
SOURCES OF FUNDING
D.S. is supported by grants from NHLBI/NIH, the California Institute for Regenerative Medicine, the Younger Family Foundation, the Roddenberry Foundation, and the Whittier Foundation.
Nonstandard Abbreviations
None
Footnotes
DISCLOSURES
D.S. serves on the Scientific Advisory Board of iPierian, Inc. and RegeneRx Biopharmaceuticals, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 2.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 3.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am J Physiol. 1997;272:H220–226. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 4.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2012 doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quaini F, Urbanek K, Graiani G, Lagrasta C, Maestri R, Monica M, Boni A, Ferraro F, Delsignore R, Tasca G, Leri A, Kajstura J, Quaini E, Anversa P. The regenerative potential of the human heart. Int J Cardiol. 2004;95(Suppl 1):S26–28. doi: 10.1016/s0167-5273(04)90008-3. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassink RJ, Pasumarthi KB, Nakajima H, Rubart M, Soonpaa MH, de la Riviere AB, Doevendans PA, Field LJ. Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res. 2008;78:18–25. doi: 10.1093/cvr/cvm101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soonpaa MH, Koh GY, Pajak L, Jing S, Wang H, Franklin MT, Kim KK, Field LJ. Cyclin d1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest. 1997;99:2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 15.Engel FB, Hsieh PC, Lee RT, Keating MT. Fgf1/p38 map kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 17.Eulalio A, Mano M, Ferro MD, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies mirnas inducing cardiac regeneration. Nature. 2012 doi: 10.1038/nature11739. (advance online publication; doi:10.1038/nature11739) [DOI] [PubMed] [Google Scholar]
- 18.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441:1097–1099. doi: 10.1038/nature04961. [DOI] [PubMed] [Google Scholar]
- 21.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mummery CL, Davis RP, Krieger JE. Challenges in using stem cells for cardiac repair. Sci Transl Med. 2010;2:27ps17. doi: 10.1126/scitranslmed.3000558. [DOI] [PubMed] [Google Scholar]
- 23.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur A, Martin JF. Stem cells and repair of the heart. Lancet. 2004;364:183–192. doi: 10.1016/S0140-6736(04)16632-4. [DOI] [PubMed] [Google Scholar]
- 25.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamanaka S. Induced pluripotent stem cells: Past, present, and future. Cell Stem Cell. 2012;10:678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. Microrna-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inagawa K, Miyamoto K, Yamakawa H, Muraoka N, Sadahiro T, Umei T, Wada R, Katsumata Y, Kaneda R, Nakade K, Kurihara C, Obata Y, Miyake K, Fukuda K, Ieda M. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of gata4, mef2c, and tbx5. Circ Res. 2012;111:1147–1156. doi: 10.1161/CIRCRESAHA.112.271148. [DOI] [PubMed] [Google Scholar]
- 31.Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- 32.Gurdon JB. From nuclear transfer to nuclear reprogramming: The reversal of cell differentiation. Annu Rev Cell Dev Biol. 2006;22:1–22. doi: 10.1146/annurev.cellbio.22.090805.140144. [DOI] [PubMed] [Google Scholar]
- 33.Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- 34.Lassar AB, Paterson BM, Weintraub H. Transfection of a DNA locus that mediates the conversion of 10t1/2 fibroblasts to myoblasts. Cell. 1986;47:649–656. doi: 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- 35.Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, Miller AD. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of myod. Proc Natl Acad Sci U S A. 1989;86:5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 39.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 40.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 41.Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, Hao J, Guo CL, Ma QW, Wang L, Zeng F, Zhou Q. Ips cells produce viable mice through tetraploid complementation. Nature. 2009;461:86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- 42.Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Gifford W, Martin G, Kupriyanov S, Baldwin KK. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- 43.Sridharan R, Tchieu J, Mason MJ, Yachechko R, Kuoy E, Horvath S, Zhou Q, Plath K. Role of the murine reprogramming factors in the induction of pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buganim Y, Faddah DA, Cheng AW, Itskovich E, Markoulaki S, Ganz K, Klemm SL, van Oudenaarden A, Jaenisch R. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012;150:1209–1222. doi: 10.1016/j.cell.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. Microrna-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 50.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 51.Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 52.Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 53.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cordes KR, Srivastava D. Microrna regulation of cardiovascular development. Circ. Res. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuoka R, Cohen JC, Srivastava D. Gata4 mutations cause human congenital heart defects and reveal an interaction with tbx5. Nature. 2003;424:443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- 56.Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ. Res. 2008;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong T-T, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta-1 integrin signaling. Dev. Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gulick J, Subramaniam A, Neumann J, Robbins J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J Biol Chem. 1991;266:9180–9185. [PubMed] [Google Scholar]
- 60.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 61.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava D, Cserjesi P, Olson EN. A subclass of bhlh proteins required for cardiac morphogenesis. Science. 1995;270:1995–1999. doi: 10.1126/science.270.5244.1995. [DOI] [PubMed] [Google Scholar]
- 63.Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bhlh transcription factor, dhand. Nat Genet. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- 64.Yamagishi H, Yamagishi C, Nakagawa O, Harvey RP, Olson EN, Srivastava D. The combinatorial activities of nkx2.5 and dhand are essential for cardiac ventricle formation. Dev Biol. 2001;239:190–203. doi: 10.1006/dbio.2001.0417. [DOI] [PubMed] [Google Scholar]
- 65.Tsuchihashi T, Maeda J, Shin CH, Ivey KN, Black BL, Olson EN, Yamagishi H, Srivastava D. Hand2 function in second heart field progenitors is essential for cardiogenesis. Dev. Biol. 2011;351:62–69. doi: 10.1016/j.ydbio.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Protze S, Khattak S, Poulet C, Lindemann D, Tanaka EM, Ravens U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol. 2012 doi: 10.1016/j.yjmcc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 67.van Rooij E, Olson EN. Micrornas: Powerful new regulators of heart disease and provocative therapeutic targets. J. Clin. Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some micrornas downregulate large numbers of target mrnas. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 69.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microrna that targets hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking mirna-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 71.Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS, Srivastava D. Microrna regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2:219–229. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, Gregoire S, Engels MC, Rajarajan K, Karra R, Abel ED, Wu JC, Milan D, Wu SM. Inefficient reprogramming of fibroblasts into cardiomyocytes using gata4, mef2c, and tbx5. Circ Res. 2012;111:50–55. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Srivastava D, Ieda M. Critical factors for cardiac reprogramming. Circ Res. 2012;111:5–8. doi: 10.1161/CIRCRESAHA.112.271452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plath K, Lowry WE. Progress in understanding reprogramming to the induced pluripotent state. Nat Rev Genet. 2011;12:253–265. doi: 10.1038/nrg2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wamstad JA, Alexander JM, Truty RM, Shrikumar A, Li F, Eilertson KE, Ding H, Wylie JN, Pico AR, Capra JA, Erwin G, Kattman SJ, Keller GM, Srivastava D, Levine SS, Pollard KS, Holloway AK, Boyer LA, Bruneau BG. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell. 2012;151:206–220. doi: 10.1016/j.cell.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paige SL, Thomas S, Stoick-Cooper CL, Wang H, Maves L, Sandstrom R, Pabon L, Reinecke H, Pratt G, Keller G, Moon RT, Stamatoyannopoulos J, Murry CE. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell. 2012;151:221–232. doi: 10.1016/j.cell.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]