Abstract
Significance: Ewan Cameron reported that ascorbate, given orally and intravenously at doses of up to 10 g/day, was effective in the treatment of cancer. Double-blind placebo-controlled clinical trials showed no survival advantage when the same doses of ascorbate were given orally, leading the medical and scientific communities to dismiss the use of ascorbate as a potential cancer treatment. However, the route of administration results in major differences in ascorbate bioavailability. Tissue and plasma concentrations are tightly controlled in response to oral administration, but this can be bypassed by intravenous administration. These data provide a plausible scientific rationale for the absence of a response to orally administered ascorbate in the Mayo clinic trials and indicate the need to reassess ascorbate as a cancer therapeutic. Recent Advances: High dose ascorbate is selectively cytotoxic to cancer cell lines through the generation of extracellular hydrogen peroxide (H2O2). Murine xenograft models confirm a growth inhibitory effect of pharmacological concentrations. The safety of intravenous ascorbate has been verified in encouraging pilot clinical studies. Critical Issues: Neither the selective toxicity of pharmacologic ascorbate against cancer cells nor the mechanism of H2O2-mediated cytotoxicity is fully understood. Despite promising preclinical data, the question of clinical efficacy remains. Future Directions: A full delineation of mechanism is of interest because it may indicate susceptible cancer types. Effects of pharmacologic ascorbate used in combination with standard treatments need to be defined. Most importantly, the clinical efficacy of ascorbate needs to be reassessed using proper dosing, route of administration, and controls. Antioxid. Redox Signal. 19, 2141–2156.
Introduction
Ascorbate (vitamin C, ascorbic acid) is no stranger to controversy, as evidenced by the fact that over 40 years lapsed between James Lind's trials using citrus fruits to treat scurvy and the implementation of this practice by the Royal Navy (4). The Canadian physician William J. McCormick is largely credited with being the first to postulate that ascorbate might limit the spread of cancer (52). The idea was brought to public attention by the Scottish surgeon Ewan Cameron, who together with Douglas Rotman, expanded on McCormick's hypothesis by suggesting that ascorbate might inhibit hyaluronidases, either through direct incorporation into a hyaluronidase inhibitor complex (12) or indirectly by promoting the synthesis of one (7). Cameron and Pauling, in 1974, further hypothesized several pleiotropic effects of ascorbate in the treatment of cancer, all more likely to be carcinostatic than curative (8). Cameron and Campbell reported observational results from uncontrolled trials where cancer patients that received 10 g/day intravenous ascorbate for up to 10 days, followed by 10 g/day oral ascorbate indefinitely, showed clinical benefit ranging from decreased tumor growth to tumor regression (5, 6). Additional reports followed indicating that ascorbate treatment increased survival time substantially relative to retrospective controls (9, 10). Despite the absence of appropriate controls, these early clinical reports were considered promising and properly designed trials were requested (11).
Two controlled double-blind clinical trials were undertaken by the Mayo Clinic between 1979 and 1985. Advanced cancer patients, with prior treatment in the first trial and without it in the second, were treated with 10 g of ascorbate orally per day and compared to cancer patients treated with a placebo. No differences were observed in symptoms, side effects, or survival between groups in either trial (21, 55). A National Cancer Institute panel subsequently determined that there was insufficient evidence to demonstrate that ascorbate was beneficial after reviewing 25 case reports submitted by Cameron and Pauling [Hoffer (34)]. Ascorbate was, understandably, dismissed as a potential cancer treatment agent.
Pharmacokinetics
Questions regarding what effect the route of administration might have had on the disparity in results between Dr. Cameron's reports and the Mayo clinic studies did not arise until ascorbate pharmacokinetics were investigated. Depletion-repletion studies in healthy volunteers showed that oral doses of 30–100 mg daily produced ∼60 μM fasting plasma concentrations (Fig. 1A) (44). About 1000 mg ascorbate orally/day produced fasting plasma concentrations approaching saturation at 75–80 μM, with minimal additional increases resulting from doses as high as 2500 mg. Leukocyte ascorbate concentrations were saturated at oral doses of 100 mg/day. Bioavailability dramatically decreased at doses above 200 mg and urinary excretion was evident at 100 mg doses. Saturation of fasting plasma ascorbate concentrations in response to increasing oral doses was termed “tight control” (45).
FIG. 1.
Bioavailability of orally administered ascorbate is tightly controlled compared to intravenous administration. (A) Plasma ascorbate concentrations as a function of dose in men and women. Dotted line indicates average daily dose from intake of five servings of fruit and vegetables. [Reprinted by permission from Levine et al. (44, 45)]. (B) Plasma concentrations of ascorbate after oral and intravenous dosing in a single patient. Baseline plasma ascorbate values are represented by a dashed line. (Top) Plasma ascorbate concentrations (μM) after receiving 200 mg intravenous (•) or oral (○) ascorbate as a function of time. (Bottom) Plasma ascorbate concentrations (μM) after receiving 1250 mg intravenous (•) or oral (○) ascorbate as a function of time. [Reprinted by permission from Levine et al. (44)].
Pharmacokinetic modeling ascribed the tight control of plasma ascorbate concentrations in vivo to saturable absorption, tissue accumulation, and renal reabsorption and excretion (29). Ascorbate absorption and tissue accumulation are principally controlled by the sodium-dependent transporters SLC23A1 and SLC23A2, also denoted sodium-dependent vitamin C transporters (SVCT)1 and SVCT2, respectively. Studies in Slc23a2−/− mice, which die immediately after birth, indicate that SVCT2 is the primary transporter in the brain, pituitary, adrenals, and pancreas and responsible for a portion of the ascorbate normally found in muscle, liver, and kidney (73). In contrast, Slc23a1−/− mice showed excessive urinary excretion of ascorbate, indicative of a key role for in SVCT1 in renal reabsorption, and reduced liver accumulation (20).
Uptake of the oxidized product of ascorbate, dehydroascorbic acid, is mediated by glucose transporters 1, 3, and 4. Dehydroascorbic acid uptake may represent a mechanism for ascorbate accumulation and recycling in red blood cells and in other cells where dehydroascorbic acid formation is locally driven by oxidants, as in activated neutrophils (46, 70, 78). Dehydroascorbic acid uptake is not a major mechanism for ascorbate accumulation in most tissues, based on findings in Slc23a2−/− mice. If dehydroascorbic acid uptake were dominant, Slc23a2−/− mice should not have had severe generalized ascorbate depletion.
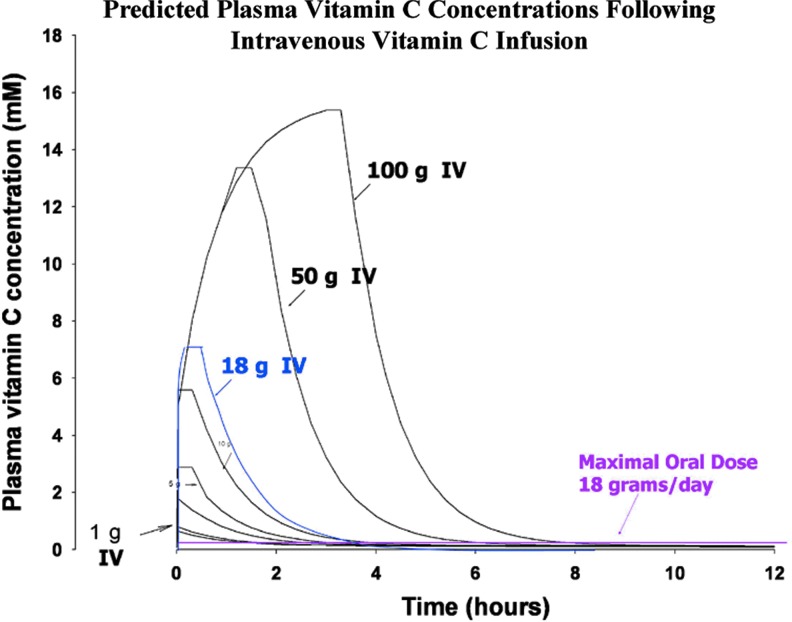
Intravenous ascorbate administration produced plasma concentrations unachievable through oral administration (Fig. 1B) (44). Riordan et al. reported that plasma concentrations of greater than 5 mM could be sustained for several hours by continuous intravenous infusion of up to 115 g over the course of 8 h in cancer patients and concentrations of ∼1.7 mM were selectively cytotoxic against four tumor cell lines in vitro (69). The realization that intravenous and oral dosing routes yielded very different plasma ascorbate concentration profiles, with potentially therapeutic levels only attainable through intravenous administration, suggested that the contradictory reports on the efficacy of high-dose ascorbate in cancer might have an underlying scientific basis (57). Additional studies were undertaken to determine the effect of route of administration on the plasma concentrations attainable from a given dose of ascorbate. Peak plasma concentrations obtained by intravenous administration of 1.25 g of ascorbate were ∼7-fold higher than those attained by the same dose given orally. Pharmacokinetic modeling predicted peak plasma levels greater than 15 mM in response to a 100 g intravenous dose, while the upper limit oral dose was predicted to yield peak values<220 μM (Fig. 2) (60). As discussed in detail below, peak pharmacologic ascorbate concentrations of 25–30 mM are achieved safely in patients and concentrations at or above 10 mM can be maintained for ∼4 h, with infusion rates of 0.5–1 g/kg per min (19, 35).
FIG. 2.
Millimolar plasma ascorbate concentrations are predicted following intravenous administration. Predicted plasma ascorbate concentrations as a function of time following infusion of variable doses of intravenous ascorbate. Pink line represents plasma concentration following oral administration of maximum dose of ascorbate (18 g/day). [Reprinted by permission from Padayatty et al. (60)]
Ascorbate parenterally administered to animals is similar to intravenous ascorbate infusions in humans (19, 35). Intraperitoneal dosing in rodents mimics intravenous infusions in humans and may be preferred due to the technical challenges associated with repeated intravenous infusions in mice and the fact that bolus ascorbate injections do not model infusions. Intraperitoneal absorption is, however, slower than intravenous infusion when equivalent doses are compared. Approximately 4 mg/g injected intraperitoneally in rodents produces ascorbate plasma concentrations over time similar to those obtained with a 0.5–1 g/kg per min infusion in humans. Rodent studies confirm that only parenteral injection (i.e., intraperitioneal administration) produces pharmacologic ascorbate concentrations similar to those obtained by intravenous infusion in humans (19, 35, 77).
To summarize, both animal and human data indicate that pharmacologic ascorbate concentrations, where ascorbate is present in sufficient quantities to act as a drug rather than a vitamin, are only achievable by parenteral administration. These observations suggest a credible reason for differential results in clinical studies of ascorbate in cancer. Animal studies and clinical ascorbate pharmacology data have fueled renewed interest in the potential therapeutic effect of high-dose ascorbate in cancer treatment.
Potential Mechanisms
Ascorbate-mediated hydrogen peroxide production
Using clinical pharmacokinetic studies as a guide for physiologically and pharmacologically relevant concentrations, the in vitro effect of ascorbate was examined using cancer cells and normal cells. Exposure to ascorbate concentrations of up to 20 mM for 1 h did not affect survival in the normal human cells tested, whereas more than half of the cancer cell lines tested showed a 50% decrease in survival after exposure to 5 mM or less (17). Cell death was dependent on hydrogen peroxide (H2O2) production mediated by extracellular ascorbate oxidation. Both apoptotic and necrotic pathways were implicated, with necrosis more prevalent at increasing doses or longer postexposure times. These studies show that pharmacological ascorbate is a precursor for H2O2 generation, which is preferentially cytotoxic to cancer cells.
This mechanism was substantiated in vivo by comparing the levels of ascorbate radical and H2O2 formation in blood and extracellular fluid in response to ascorbate delivered parenterally or by gavage in rats (18). Plasma and extracellular fluid concentrations were in pharmacological range, with peaks of ∼3 mM, and ∼8 mM, in response to intraperitoneal and intravenous administration of ascorbate, respectively. Oral dosing did not produce plasma concentrations above 100 μM. Ascorbate radical formation in the extracellular fluid was proportional to ascorbate concentration, with peaks of ∼250 nM in response to intravenous administration and ∼150 nM in response to intraperitoneal administration. Oral dosing produced less than 50 nM of ascorbate radical in extracellular fluid; blood concentrations remained below 50 nM, regardless of the route of administration. H2O2 formation required ascorbate radical concentrations attainable only with pharmacologic doses of ascorbate.
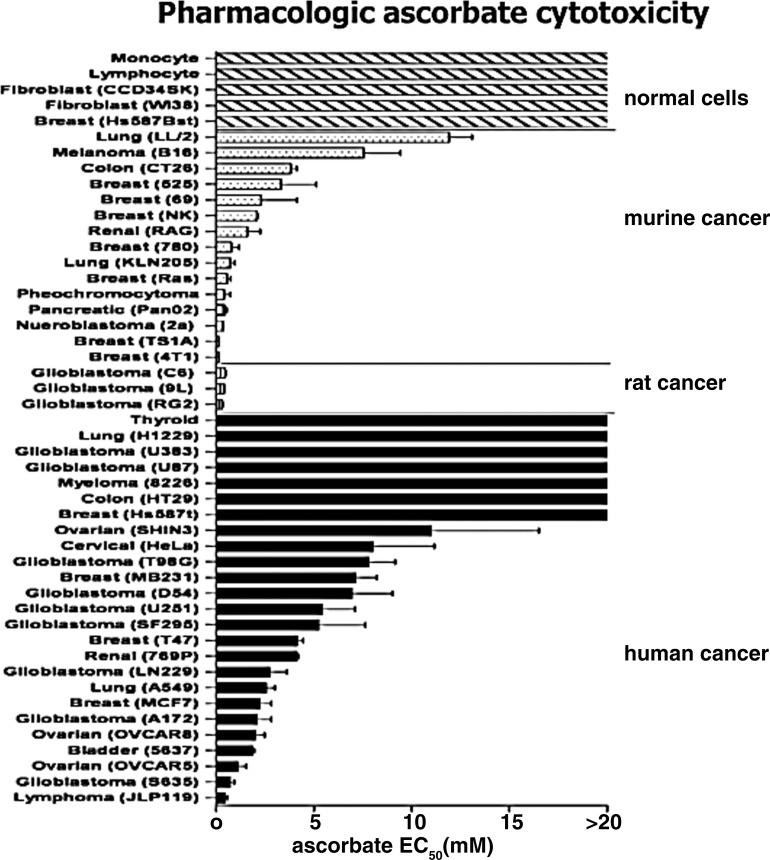
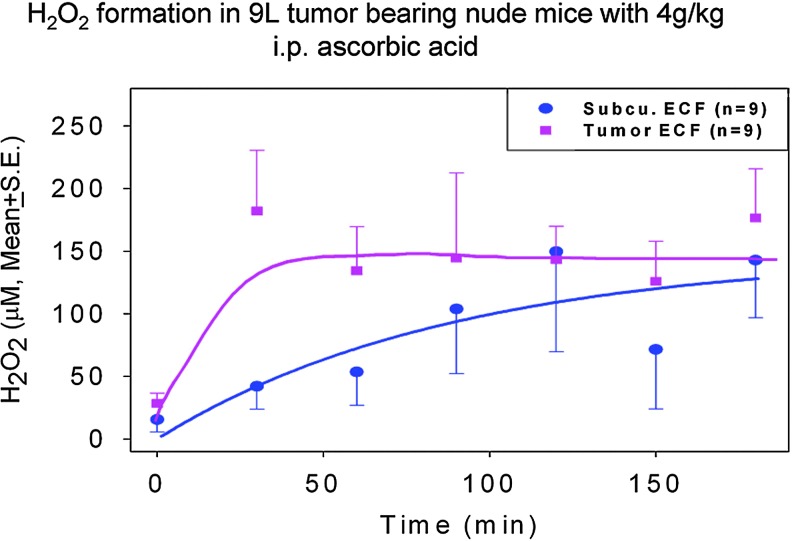
Investigation of selective cytotoxicity was expanded to 43 cancer cell lines (19). The effective concentration of ascorbate required to kill 50% of the cells (Effective dose required to kill 50% of cells [EC50]) was less than 10 mM for 34 of 43 cancer cell lines tested; normal cells remained insensitive to concentrations in excess of 20 mM (Fig. 3). The sensitivity of human ovarian carcinoma (Ovcar 5), mouse pancreatic carcinoma (Pan02), and rat glioblastoma (9L) lines to ascorbate was further examined using tumor xenografts in nude mice. Pharmacologic doses of ascorbate produced ascorbate radical and subsequent H2O2 formation in interstitial fluid but not in blood, and mice that received daily parenteral ascorbate had slower tumor growth than those that did not (Fig. 4).
FIG. 3.
Pharmacologic ascorbate concentrations are selectively cytotoxic to cancer cell lines in vitro. Effect of pharmacologic concentrations of ascorbate on oncogenic and normal cell lines. Cells were exposed to increasing concentrations of ascorbate (0–20 mM, pH 7) for 2 h, then washed and cultured for an additional 24–48 h in growth media. EC50 represents the effective concentration of ascorbate required to kill 50% of cells. EC50 was determined by either MTT or Alamar Blue viability assays. [Reprinted by permission from Chen et al. (19)].
FIG. 4.
Pharmacologic ascorbate generates H2O2 formation in a rat glioblastoma model. H2O2 formation in extracellular fluids in 9L tumor-bearing nude mice. Mice were treated with 4 g/kg of parenteral ascorbate by intraperitoneal injection and fluids were gathered from probes in the extracellular tumor tissue (square, ▪) or subcutaneous space (circle, •) at 30 min intervals. H2O2 concentrations were determined using fluorescence spectroscopy. [Reprinted by permission from Chen et al. (19)]. H2O2, hydrogen peroxide.
The contribution of intracellular metals to H2O2-mediated cytotoxicity
Verrax and Calderon also reported that parenteral administration of ascorbate in mice transiently produced pharmacologic concentrations in plasma and that these concentrations were cytotoxic to several cancer cell lines, regardless of mutations in p53 and caspase-3 (77). They confirmed that pharmacologic ascorbate produced necrotic cell death in cancer cells via the generation of H2O2. Daily administration of pharmacologic ascorbate decreased tumor growth rate in a murine hepatoma model compared to saline-treated controls. In contrast to the studies of Chen et al. (17, 77), where ascorbate-mediated killing was independent of both an intracellular and an extracellular metal chelator, cell death was inhibited by pretreatment with the intracellular chelator, desferoxamine mesylate.
Ullah et al. reported the formation of oxidative DNA damage in whole lymphocytes and isolated nuclei in response to ascorbate treatment, using physiologic concentrations in vitro (75). DNA damage could be prevented by both iron and copper chelators in whole cells, whereas only copper chelators were effective in isolated nuclei. Earlier reports indicating that cellular copper levels are increased in several cancers combined with the co-localization of copper and chromatin led these authors to hypothesize that the interaction between ascorbate and copper contributes to the selective killing of cancer cells.
Neuroblastoma cell lines were susceptible to treatment with pharmacologic ascorbate or H2O2 (22). Susceptibility was dependent on cell concentration and associated with increased ferritin release and increased lactate production. Increased ferritin production and secretion provides a continuous iron source for ascorbate-mediated H2O2 production and increased lactate production results in acidification of the surrounding extracellular fluid further enhancing the release of iron from ferritin. Increased lactate production is also associated with a decreased capacity to neutralize reactive oxygen species (ROS).
Five neuroblastoma cell lines also showed a dose-dependent decrease in transferrin receptor (TfR) expression with a corresponding decrease in intracellular iron, and increased apoptosis after treatment with 0.5–3 mM ascorbate (13). Apoptosis was indicated by depolarization of the mitochondrial transmembrane potential and global caspase activation. Pretreatment of cells with ferric ammonium citrate decreased cell death and prevented mitochondrial membrane depolarization. The relationship between TfR expression, intracellular iron levels, and ascorbate and induction of apoptosis is not elucidated in this study, but decreased intracellular iron may correspond to increased extracellular iron. Regardless, these data underscore the fact that redox active transition metals likely play an important role in ascorbate-mediated cytotoxicity.
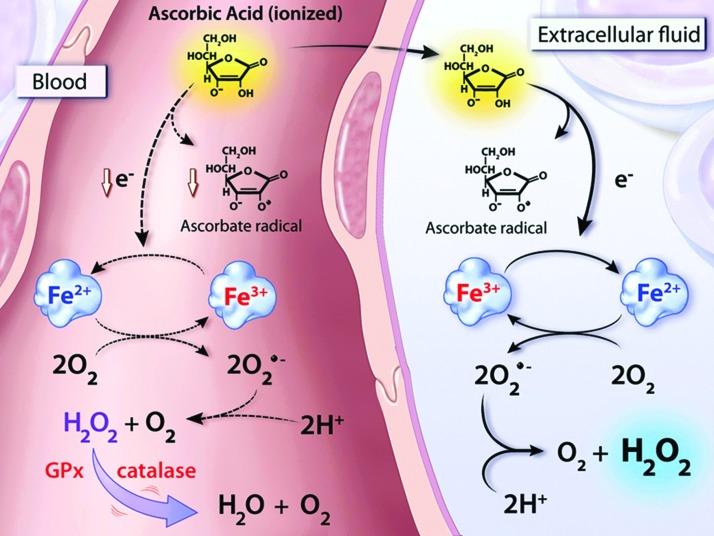
Collectively, these data support a model whereby pharmacologic ascorbate equilibrates between blood and the extracellular fluid (18). The loss of an electron results in the formation of the ascorbate radical, with the electron donated to a transition metal ion currently thought to be protein bound. The reduced metal is then available to react with molecular oxygen resulting in the generation of superoxide and subsequently H2O2. These reactions likely occur in the blood as well, but H2O2 generated in that compartment is thought to be instantly neutralized by the catalase and/or glutathione peroxidase present in erythrocytes (Fig. 5). In the presence of pharmacologic ascorbate concentrations, H2O2 is then likely to interact with another transition metal ion, such as ferrous iron in the Fenton reaction, to generate the highly reactive and damaging hydroxyl radical (39).
FIG. 5.
Pharmacologic ascorbate generates H2O2 in extracellular fluid leading to production of ROS. Proposed mechanism of ascorbate-mediated H2O2 formation in blood and extracellular fluid. After ingestion or injection, ascorbate is equally distributed between blood (left) and the extracellular fluid (right). In the extracellular fluid, the oxidation of ascorbate concomitantly forms the ascorbate radical and reduces a protein-centered metal (In this example, Fe3+ is reduced to Fe2+). Fe2+ then interacts with oxygen to form superoxide, which undergoes dismutation to H2O2. H2O2 then interacts with another transition metal to generate ROS, including the highly reactive hydroxyl radical, via Fenton chemistry. The generation of H2O2 is largely inhibited in blood by catalase and GSH peroxidase, both of which are primarily found in erythrocytes (dashed lines). [Reprinted by permission from Chen et al. (18)]. GSH, glutathione; ROS, reactive oxygen species.
Neutralization of H2O2 and superoxide
Differential expression of catalase between resistant and susceptible cells was proposed as an alternative mechanism for the selective cytotoxicity of pharmacologic ascorbate. Klingelhoeffer et al. showed that differential sensitivity to both ascorbate and H2O2 could be mitigated by treatment with exogenous catalase and was correlated with levels of endogenous catalase production in 11 cancer cell lines (41). Knock down of catalase expression in the resistant breast carcinoma line, BT-20, resulted in increased susceptibility to pharmacologic ascorbate with cell death likely resulting from apoptosis.
Three pancreatic cancer cell lines were sensitive to pharmacologic ascorbate treatment; sensitivity was reversed by pretreatment with exogenous catalase or infection of cells with an adenovirus vector containing human catalase complementary DNA (cDNA) (23). Infection of the MIA PaCa-2 cell line with an adenovirus containing human catalase cDNA preceded by a mitochondrial targeting sequence was unable to inhibit ascorbate-mediated cytotoxicity. Ascorbate treatment decreased cellular adenosine-5′-triphosphate (ATP) levels in a dose-dependent manner. This too could be reversed by pretreatment with exogenous catalase. Although overexpression of mitochondrial catalase was unable to inhibit ascorbate-mediated cell death, it was able to reverse cellular ATP depletion. Cytosolic catalase overexpression reversed both ascorbate-mediated cell death and cellular ATP depletion. Together, these data suggest that lack of mitochondria-derived ATP is unlikely to be a component of ascorbate cytotoxicity.
High-dose ascorbic acid treatment resulted in dose-dependent cytotoxicity in four human mesothelioma cell lines (74). This was associated with increased apoptosis, decreased expression of the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2), an increased number of cells with a low mitochondrial membrane potential, and increased mitochondrial superoxide production. In this study, ascorbate-mediated cell death was reversible not only by pretreatment with catalase, but also by the membrane permeable superoxide scavenger, tempol. Although somewhat speculative because tempol may interfere with Fenton chemistry through ferroxidase activity (54), these studies suggest that superoxide, in addition to H2O2, may have a pivotal role in promoting sensitivity to pharmacologic ascorbate in mesothelioma.
Pharmacologic ascorbate, but not its oxidized product dehydroascorbic acid, was selectively cytotoxic to the malignant mesothelioma cell lines, REN and MM98, compared to an immortalized human mesothelial cell line and primary mesothelial cells (66). Consistent with prior reports, cytotoxicity was associated with a dose-dependent increase in H2O2 formation; both apoptotic and necrotic cell death pathways were involved with necrosis prevailing at higher concentrations. Ascorbate-mediated cytotoxicity could be inhibited by treatment with exogenous catalase, but endogenous intracellular catalase levels were not correlated with sensitivity to ascorbate in either cancer or normal cell lines. Treatment with 1 or 5 mM ascorbate increased intracellular ROS in REN cells, but not in mesothelial cells. The malignant mesothelioma cells had increased superoxide production and upregulated expression of the superoxide-producing NADPH oxidase, NOX4. Chemical inhibition of superoxide production or small interfering RNA (siRNA) knockdown of NOX4 decreased ascorbate-mediated cytotoxicity in these cells, suggesting that increased superoxide, leading to H2O2 formation, was responsible for increased ROS production associated with selective cytotoxicity of ascorbate in these cell types.
Considered together, the evidence indicates that extracellular pharmacologic ascorbate mediates cancer cell death by H2O2 formation. Extracellular catalase prevents cancer cell death, while intracellular catalase levels have variable effects on responsiveness to pharmacologic ascorbate, depending on the cell line.
A role for transcription factors and signal transduction pathways
Whether or not differential expression of p53 plays a role in pharmacologic ascorbate action is the subject of some controversy. In one study, ascorbate cytotoxicity was independent of p53 status in five malignant cell lines (77). In contrast, Kim et al. reported that among seven malignant cell lines examined, p53-positive cells were more sensitive to both ascorbate and H2O2 treatment than those that were p53-deficient (40). To substantiate this observation, they compared the human colorectal carcinoma cell line lacking p53, HCT116−/−, with the isogenic strain expressing wild-type p53, HCT116+/+, and found that overexpression of p53 in the null strain increased sensitivity to ascorbate, whereas knockdown of p53 in HCT116+/+ enhanced cellular resistance. The human breast cancer line MCF7 showed a similar trend when p53 was knocked down. In mouse models, ascorbate treatment also more effectively slowed tumor growth arising from HCT116+/+ cells relative to its effect on HCT116−/− tumors. Not surprisingly, ascorbate treatment generated differential gene expression as a function of p53 status, with decreased transcription of antioxidant genes including catalase in the HCT116+/+ cells. Moreover, ascorbate treatment augmented ubiquitination of murine double minute (MDM2) and activated p38 mitogen-activated protein kinase (MAPK), resulting in stabilization and phosphorylation of p53 in HCT116+/+ cells. In this cell line, ascorbate-mediated cell death was independent of caspase-3.
Pharmacologic ascorbate treatment (1.5 g/kg injected intraperitoneally) upregulated hepatic expression of rapidly accelerated fibrosarcoma (Raf) kinase inhibitory protein (RKIP) and annexin A5 in a S-180 sarcoma xenograft model, coincident with decreased tumor growth and increased survival rate relative to controls (61). Messenger RNA (mRNA) levels were also upregulated, suggesting a transcriptional control mechanism. RKIP binds Raf-1, causing its dissociation from MAPK/extracellular signal-regulated kinase, ultimately resulting in disruption of MAPK signaling. The exact contribution of annexin A5 is not clear, but both proteins are thought to have a role in inhibition of metastasis.
The specificity protein (Sp) transcription factors, Sp1, Sp3, and Sp4 have been implicated in molecular mechanisms of pharmacologic ascorbate-mediated necrotic and apoptotic cell death in two human colon carcinoma cell lines (62). Ascorbate concentrations of <3 mM produced decreased expression of Sp1, Sp3, and Sp4. These decreases were recapitulated by treatment with either H2O2 or the pro-oxidant tert-butyl hydroperoxide. Both cell death and decreased protein expression mediated by ascorbate, H2O2, and tert-butyl hydroperoxide could be inhibited by glutathione and, in some instances, dithiothretol. Protein expression levels of Sp-targeted genes, including vascular endothelial growth factor (VEGF) and its receptors, the anti-apoptotic proteins Bcl-2 and survivin, cyclin D1, hepatocyte growth factor receptor, and epidermal growth factor receptor (EGFR) were also decreased by treatment with ascorbate. The authors note that cell proliferation is decreased by a lower concentration of ascorbate than is required for decreased expression of the Sp transcription factors, suggesting additional pathways are involved.
Decreased cyclooxygenase-2 (COX-2) expression may have a role in the molecular mechanism of pharmacologic ascorbate through various signaling mechanisms. The cyclooxygenases, COX-1 and COX-2, catalyze the production of prostaglandins from arachidonic acid (80). COX-1 is constitutively expressed, whereas COX-2 expression only occurs in response to certain stimuli, such as growth factors and cytokines. A role for COX-2 in ascorbate-mediated cytotoxicity was suggested in studies of the human myeloid leukemia cell line, HL60 (31). Treatment of these cells with concentrations of ascorbate up to 1.0 mM, with or without arsenic trioxide (As2O3), resulted in suppression of nuclear factor “kappa-light-chain-enhancer” of activated B cells (NF-κB). Mechanistically, ascorbate was shown to prevent degradation of the NF-κB inhibitor (IκB)-α subunit and subsequent nuclear translocation of the p65 subunit of NF-κB. Of note, glutathione has been shown to attenuate As2O3-mediated cytotoxicity, presumably by neutralization of As2O3-generated ROS (28). Consistent with pro-oxidant activity of high-dose ascorbate, these effects were dependent on glutathione levels and could be completely reversed by treatment with catalase. Moreover, ascorbate-mediated suppression of COX-2 could be recapitulated by treatment with an inhibitor of IκB degradation.
A second study connected downregulation of COX-2 to ascorbate-mediated cytotoxicity in the context of insulin-like growth factor II (IGF-II) production in the human melanoma cell line, SK-MEL-2 (43). Treatment with 1.0 mM ascorbate resulted in decreased proliferation associated with decreased production of IGF-II and proliferation could be restored by treatment with exogenous IGF-II. COX-2 expression was completely abrogated by ascorbate treatment with a corresponding decrease in prostaglandin E2 (PGE2) and the type I insulin-like growth factor receptor (IGF-1R). Chemical inhibition or siRNA-mediated knockdown of COX-2 also decreased expression of IGF-1R while treatment of cells with exogenous PGE2 rescued it, suggesting that decreased expression of IGF-1R is subsequent to downregulation of COX-2. COX-2 and IGF-II expression levels were restored when cells were pretreated with the p38 MAPK inhibitor, SB203580, and p38 MAPK was phosphorylated in response to ascorbate treatment.
On the whole, these studies suggest that multiple transcription factors and signaling pathways can potentially be involved in pharmacologic ascorbate cytotoxicity. The precise molecular mechanisms may depend on the genotypic characteristics of the cancer, the nature of the cell lines selected, the dose of ascorbate and the length of exposure, or a combination of these factors.
Cell death mechanisms: autophagy, apoptosis, and necrosis
Five of six human prostate cancer cell lines were sensitive to pharmacologic ascorbate in vitro (16). In one of the cell lines, PC-3, the mechanism involved depletion of intracellular ATP. Significant decreases in ATP were detectable within 30 min of ascorbate treatment, and ATP remained depleted throughout the time course. Proposed mechanisms for ATP depletion by ascorbate-generated H2O2 include NAD+ depletion through increased poly-ADP-ribose polymerase (PARP)-mediated DNA repair, increased mitochondrial sensitivity and the redirection of glucose through the pentose phosphate pathway to replace NADPH utilized for the regeneration of glutathione following H2O2 neutralization. Of note, in vivo support for the sensitivity of prostate cancer lines is provided by a study showing that pharmacologic ascorbate decreased the tumor weight and number of metastases generated by the hormone-refractory prostate cancer cell line PAIII in syngeneic immune-competent Lobund-Wistar rats compared with vehicle-treated controls (65). Although the in vivo study did not investigate mechanism, in the cell lines death was associated with autophagy (16). These observations are consistent with reports that autophagy, the self-digestion of cellular components through the generation of autophagosomes that fuse with lysosomes, is an oxidative stress defense that can lead to cell death (47).
Autophagy was also implicated in pharmacologic ascorbate-mediated cell death in pancreatic cancer cell lines (23). Although ascorbate was shown to deplete cellular ATP and activate PARP-1, inhibition of PARP-1 activation had no effect on either cellular ATP depletion or clonogenic survival, suggesting that PARP-1 activation is unrelated to the molecular mechanisms of ascorbate cytotoxicity. Likewise, mitochondrial targeting of catalase prevented ATP depletion (discussed above), but did not prevent cytotoxicity. These findings suggest that mitochondrial ATP depletion is also dispensable for ascorbate-mediated cell killing. Autophagy was induced in response to ascorbate, as evidenced by processing of the light chain 3 (LC3) protein and the staining pattern of a green fluorescent protein-LC3 fusion construct, and inhibited by catalase. Nude mice injected with MIA PaCa-2 cells showed decreased tumor growth and increased survival time when treated with pharmacologic ascorbate compared with treatment with saline.
Autophagy was also linked to ascorbate-mediated cell death in studies examining the sensitivity of human breast cancer cells. In a panel of nine breast cancer cell lines, sensitivity to pharmacologic ascorbate was associated with expression levels of the sodium-dependent ascorbate transporter, SVCT2 (37). Unlike other studies, ascorbate-mediated cytotoxicity was attributed to the generation of intracellular rather than extracellular ROS. Transfection of cells that had endogenously low expression of SVCT2 with a construct expressing SVCT-2 cDNA was sufficient to increase sensitivity. Likewise, siRNA-targeted knockdown of SVCT2 in the cell lines that had natively high expression levels of SVCT2 was sufficient to ameliorate sensitivity. Consistent results were obtained when these cells were xenografted into nude mice. Beclin-1, a protein that interacts with Bcl-2 and has been shown to promote autophagy, was upregulated in some cell lines in response to ascorbate treatment. LC3 was also upregulated and treatment with the autophagy inhibitor, 3-methyladenine, which functions through inhibition of phosphoinositide 3-kinase, resulted in partial rescue of cell viability.
In contrast, ascorbate-mediated cytotoxicity in breast cancer cell lines, SK-BR3 and Hs578T, involved nuclear translocation of the mitochondrial apoptosis-inducing factor (AIF) protein (36). Inhibition of mitochondrial AIF release only partially inhibited cell death. Treatment of murine melanoma B16F10 cells with 10 mM ascorbate also induced apoptosis (38). In this case, cell death was associated with the production of intracellular ROS and could be prevented by pretreatment with the antioxidant N-acetyl-l-cysteine. Apoptosis was linked to loss of mitochondrial membrane permeability and release of cytochrome c, but was insensitive to inhibition of caspase-8.
In total, these studies indicate that several cell death mechanisms may be activated simultaneously in response to ascorbate treatment in cancer cell lines. The conclusion that susceptibility to a particular type of cell death is dependent on ascorbate concentration and exposure time is reinforced. In addition, as is likely to be the case with alterations of signal transduction, activation of a particular pathway may be influenced by differences in the genotype of various cancer cell lines.
Effects on angiogenesis
High-dose ascorbate may have an effect on angiogenesis. Yeom et al. reported that ascorbate treatment slowed tumor formation in BALB/c mice inoculated with S-180 murine sarcoma cells compared with phosphate buffered saline (PBS)-treated controls (82). Pretreatment of mice with ascorbate increased survival times by 20%. The significance of this observation is uncertain, as only five mice were followed in each group. Regardless, transcription of the genes encoding the angiogenic proteins basic fibroblast growth factor, VEGF, and matrix metalloproteinase-2 (MMP-2) was decreased in tumor-bearing mice treated with ascorbate relative to those treated with PBS. Mikirova et al. reported dose-dependent inhibition of in vitro capillary-like tube formation by human endothelial cells treated with ascorbate concentrations above 2.5 mM (53). Ascorbate treatment was also associated with a dose-dependent decrease in nitric oxide production. Both studies report decreased cell migration in wound repair models (53, 82).
Transcriptional responses of several genes involved in angiogenesis and defense against oxidative stress were investigated in two hepatocellular carcinoma cell lines, HCC24/KMUH and HCC38/KMUH, in response to low (1 mM) and high (30 mM) pharmacologic ascorbate (50). Findings showed differential expression of most of the genes examined. In response to 30 mM ascorbate, both cell lines showed decreased transcription of the gene encoding the angiostatic protein C-X-C motif chemokine (CXCL)10 and increased transcription of the SOD2 gene, encoding superoxide dismutase (SOD). The genes encoding the angiogenic proteins, C-C motif chemokine (CCL2), CXCL6, and interleukin-8, and a second gene involved in the oxidative stress response, vanin 3 (VNN3), were differentially regulated by ascorbate dose, cell line, or both.
Together these studies suggest differential transcription of angiogenic factors in response to ascorbate treatment. These effects are undoubtedly complex. Additional studies are needed to precisely define the response of the cellular angiogenic program to pharmacologic ascorbate.
Effects on cell cycle
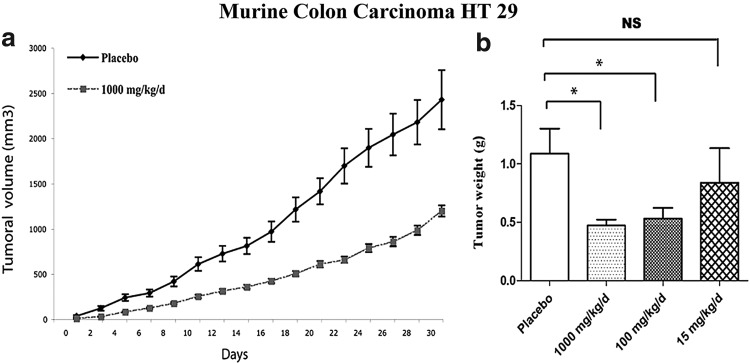
Microarray data indicate that primary normal human fibroblasts downregulated 31 genes in response to low-end pharmacologic ascorbate treatment (0.3–0.8 mM) with many of the downregulated genes encoding for either tRNA synthetases or translation initiation factors (3). As suggested by the decreased transcription of these genes, treatment of these cells and several human cancer cell lines with pharmacologic ascorbate concentrations resulted in decreased proliferation. Ascorbate at a concentration of 3 mM arrested the cell cycle at S phase in both healthy fibroblasts and the Burkitt's lymphoma-derived Raji cell line. Mice xenografted with HT29 colon adenocarcinoma cells showed decreased tumor volume and weight corresponding to prolonged survival when treated by intraperitoneal injection with 1 g/kg ascorbic acid daily relative to placebo-treated controls (Fig. 6). Consistent with the microarray data, quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) experiments examining RNA extracted from tumors following ascorbate treatment indicate decreased tRNA synthetase and translation initiation factor mRNAs compared with the levels in control mice. A different study described effects of pharmacological ascorbate on the cell cycle; 1 mM ascorbate treatment produced an increase in the number of DU-145 prostate carcinoma cells in the G0/G1 phase (27). Others also reported cell cycle arrest at the G1 phase in the murine melanoma line, B16F10, when cells were treated with ascorbate doses of 0.2 mM or less (30).
FIG. 6.
Pharmacologic ascorbate decreaseses tumor growth in mice. Growth and weight of murine colon carcinoma HT 29 tumors after exposure to pharmacologic ascorbate. (a) Tumor growth of murine colon carcinoma in mice treated by daily intraperitoneal injection of 1000 mg/kg (dashed lines) or placebo (solid lines). (b) After 1 month of daily treatment with either placebo, 1000 mg/kg, 100 mg/kg or 15 mg/kg of intraperitoneal ascorbate, mice were sacrificed and the tumors were weighed. *Indicates statistical significance of p<0.05. [Reprinted by permission from Belin et al. (3)].
Cell cycle arrest was associated with the molecular mechanism of ascorbate-mediated cytotoxicity in the human melanoma cell line, A375.S2 (49). Pharmacologic ascorbate doses ranging from 2 to 16 mM resulted in a dose-dependent decrease in cell viability resulting from a combination of apoptotic and necrotic mechanisms. Apoptosis occurred via a mitochondria-dependent pathway, as evidenced by dose-dependent increases in cytosolic Ca2+, combined with decreases in mitochondrial membrane potential, non-cleaved caspase-3, and Bcl-2 protein expression. RT-PCR analyses of ascorbate-treated cells indicated decreased transcription of cyclin-dependent kinases CDK2, CDK4, cyclin A and E along with increased transcription of p53 suggesting an arrest at the G1/S phases of the cell cycle. Cell cycle arrest was confirmed by flow cytometry. As a group, these studies suggest that pharmacologic ascorbate is associated with cell cycle arrest in certain neoplastic cell lines and that this may occur in vivo as well. The phase at which the cell cycle is arrested appears to depend on the properties of the cancer cell type and the ascorbate dose.
Interactions with Standard Treatments
Synergy and antagonism of chemotherapeutics
Pharmacologic ascorbate has been shown to interact with several cancer chemotherapeutics in vitro and in vivo, often enhancing cancer cell death as functions of cell and drug type. In vitro analyses of the effect of pretreatment of DU-145 prostate carcinoma cells with 1 or 2 mM ascorbate, in addition to several chemotherapeutics, on cell viability showed variable results depending on the drug investigated (27). Ascorbate treatment enhanced the effectiveness of docetaxel, epirubicin, and 5-fluorouracil (5-FU), as evidenced by shifted dose–response curves. The ascorbate-mediated decrease in half maximal inhibitory dose (IC50) of docetaxel and 5-FU showed a linear dependency on ascorbate concentration. In the case of irinotecan, ascorbate treatment decreased the final number of viable cells. Ascorbate had no effect on oxaliplatin or vinorelbin cytotoxicity.
Pretreatment of esophageal cancer cell lines with 20 mM ascorbate enhanced the cytotoxicity of both cisplatin and 5-FU (1). Both chemotherapeutics increased nuclear translocation of NF-κB and activator protein 1 (AP-1) DNA binding activity. Ascorbate dose-dependently decreased NF-κB nuclear translocation; doses >10 mM decreased AP-1 DNA binding activity.
Additive and synergistic effects of ascorbate were found using the human breast cancer cell lines, MCF-7 and MDA-MB-231, although ascorbate doses were in the physiological range (42). Addition of 1 and 100 μM of ascorbate to cell cultures improved activity of doxorubicin, cisplatin, and paclitaxel. Ascorbate was synergistic with doxorubicin at both concentrations in both cell lines, and the effect was dose-dependent in MDA-MB-231 cells. Ascorbate was synergistic with cisplatin in MCF-7 cells at both concentrations while only 100 μM was effective in MDA-MB-231 cells. When combined with paclitaxel, neither concentration improved the activity of drug alone in MCF-7 cells while the lower concentration had a synergistic effect and the higher concentration had an additive effect in MDA-MB-231 cells. Further investigation of combination therapy using higher doses of ascorbate is warranted.
Studies examining the interaction of pharmacologic ascorbate with several chemotherapeutics in the p53-deficient malignant mesothelioma cell line, REN, had mixed results (51). Ascorbate strongly antagonized the efficacy of imatinib, etoposide, and the potential agent taurolidine. In this cell line, ascorbate was also antagonistic, albeit to a lesser extent, in combination with paclitaxel and the vitamin E analog, (+)-α-tocopherol. Additive effects were obtained when ascorbate was used in combination with either cisplatin or tyrphostin. Ascorbate was synergistic in combination with either gemcitabine or the natural green tea extract epigallocatechin-3-gallate. These two combinations also resulted in increased lactate dehydrogenase release, indicative of necrosis, and increased caspase-3 activity, suggesting the synergistic effects were mediated by more than one cell death pathway.
Parental administration of ascorbate in combination with the synthetic vitamin K3 inhibited tumor growth and metastases in a Lewis lung carcinoma xenograft model (14). Low (100 mg/kg ascorbate plus 1 mg/kg vitamin K3) and high (1000 mg/kg ascorbate plus 10 mg/kg vitamin K3) doses decreased tumor volume, lung metastases, and protein expression levels of MMP-2 and −9 in the lungs of tumor-bearing mice relative to untreated controls in a dose-dependent manner. This combination also decreased plasma activity levels of MMP-2, MMP-9, and urokinase plasminogen activator, which is required for activation of MMPs. Expression levels of the MMP inhibitors, tissue inhibitor of metalloproteinases, TIMP-1 and −2, plasminogen activator inhibitor-1, and nonmetastatic protein 23 homolog 1 were increased in treated mice relative to controls. Ascorbate plus vitamin K3 decreased cell proliferation, as evidenced by a dose-dependent decrease in proliferating cell nuclear antigen-positive cells in the lung tissue of treated mice compared to tumor-bearing control mice. The data from this model suggest that this combination may have a specific anti-metastatic effect by inhibiting degradation of the extracellular matrix. Unfortunately, toxicity found in a phase I study of vitamin K3 alone may limit further clinical exploration in combination with ascorbate (48).
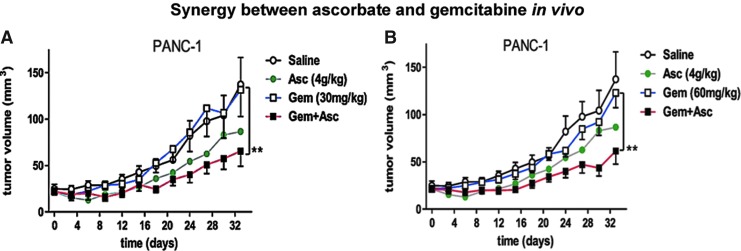
Pharmacologic ascorbate enhanced gemcitabine activity in a study of eight pancreatic cancer cell lines (24). In all cell lines, gemcitabine combined with ascorbate was more effective than gemcitabine alone. Ascorbate augmented cell killing per unit gemcitabine, suggesting that the gemcitabine dose could be lowered when the two are used in combination to achieve the same antitumor effect. Ascorbate's effect was mediated by H2O2 formation, as evidenced by the capacity of catalase to reverse the cytotoxicity of the combination. Although previously linked to the chemosensitivity of pancreatic cancer cells, no connection was found between the epithelial-to-mesenchymal transition phenotype and the response to the combination of gemcitabine and ascorbate. Sensitivity to gemcitabine alone or in combination with ascorbate was positively associated with growth rate. These data were confirmed in vivo in athymic mice xenografted with either the gemcitabine-sensitive murine cell line, PAN-O2, or the relatively gemcitabine-resistant human cell line, PANC-1. In both instances, gemcitabine plus ascorbate produced a significantly larger decrease in tumor volume than gemcitabine alone. This was particularly pronounced in the gemcitabine-resistant PANC-1 tumor (Fig. 7).
FIG. 7.
Pharmacologic ascorbate enhances the effect of gemcitabine in pancreatic cancer xenograft models. Comparison of gemcitabine and ascorbate treatments on PANC-1 tumor volumes in mice. Mice were intraperitoneally injected with either saline, 4 g/kg of pharmacologic ascorbate, 30 g/kg (A) or 60 g/kg (B) of gemcitabine, or both ascorbate and gemcitabine at the indicated concentrations every 4 days. **indicates a p value<0.002 using a paired two-tailed t-test. Reprinted by permission from Espey et al. (24). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Physiologic levels of ascorbate were shown to antagonize the activity of the proteasome inhibitor, bortezomib, in the human myeloma cell line, RPM18226, in vitro and in a severe combined immunodeficiency (SCID) mouse xenograft model (64). Ascorbate treatment showed a dose-dependent reduction in bortezomib-mediated growth inhibition of RPM18226 cells across a dose range of 30–250 μM. Ascorbate at a concentration of 125 μM decreased bortezomib-mediated poly-ubiquitination of proteins and partially reversed bortezomib-mediated inhibition of 20S proteasome activity. Inhibition of cytotoxicity by ascorbate-enriched plasma was inversely correlated with the concentration of ascorbate up to 125 μM. Bortezomib treatment decreased tumor growth relative to controls in xenografted SCID mice. The tumor volume from mice treated with 40 mg/kg of ascorbate per day in addition to bortezomib did not significantly differ from vehicle-treated controls. It was noted by the authors, and should be re-emphasized here, that even normal physiologic levels of ascorbate, obtainable from foods, inhibited bortezomib. These data indicate that chemotherapeutic response should be investigated carefully with respect to ingestion of nutrients and supplements, and these highlight the importance of route of delivery and dosage in determining the mechanisms of ascorbate activity and subsequent downstream effects.
Effects of the oxidized product of ascorbate, dehydroascorbic acid, were examined in combination with chemotherapeutic drugs using K562 leukemia and RL lymphoma cells, and a lymphoma xenograft model (32). These studies show that pretreatment with 500 μM dehydroascorbic acid can prevent mitochondrial membrane permeabilization and decrease the efficacy all drugs tested in vitro and in vivo. As noted elsewhere, the concentration of dehydroascorbic acid used is biologically irrelevant and potentially harmful. The results cannot be extrapolated to the effects of ascorbate (25, 63).
Thus, ascorbate, in both physiological and pharmacological concentrations interacts with a variety of drugs. In some instances, these interactions are potentially beneficial. Synergistic, additive, and antagonistic effects all have potentially important clinical implications; continued investigation of the interaction of ascorbate with chemotherapeutics is necessary.
Synergy with radiotherapy
Sensitivity of both primary and immortalized glioblastoma multiforme cells to radiation was enhanced by exposure to pharmacologic ascorbate (33). One Gray (Gy) radiation in combination with 0.5 mM ascorbate decreased viability of primary cells by 15%–20% and viability of the mouse astrocytoma cell line, GL261, by 25% compared with treatment with 1 Gy radiation alone. A primary human cell line showed a 4-fold to 10-fold increase in cell death when treated with radiation doses ranging from 3 to 6 Gy plus 5 mM ascorbate relative to the level obtained with radiation alone. Combination treatment also increased the number of double strand breaks in DNA relative to the number produced by radiation alone and blocked the repair-associated G2/M cell cycle arrest in glioblastoma cells, but not normal astrocytes, that occurred in response to radiation alone. Cell killing was facilitated, in part, by ascorbate-mediated H2O2 production and occurred by necrosis rather than apoptosis or autophagy.
Likewise, 5 mM ascorbate combined with 2 Gy radiation decreased cell viability and produced higher levels of DNA fragmentation at earlier time points than radiation alone in the human leukemia cell line, HL60 (72). Cell death in response to radiation or 5 mM ascorbate was associated with apoptotic mechanisms, whereas necrotic cell death resulted from 20 mM ascorbate treatment. Caspase-3 and −9 were activated by all treatment regimens; caspase-8 activity increased in response to ascorbate treatment or ascorbate combined with radiation, but not in response to radiation alone. Correspondingly, inhibition of caspase-8 decreased the level of DNA fragmentation produced by combination treatment or ascorbate alone, but not radiation alone, while inhibition of caspase-3 and −9 decreased the level of DNA fragmentation in all treatment groups. Although cytosolic translocation of mitochondrial cytochrome c was evident in response to all treatments, expression levels of the pro-apoptotic BCL2-associated X protein protein were only increased in response to treatment with ascorbate alone or ascorbate combined with radiation. These studies suggest that pharmacologic ascorbate enhances radiation-induced tumor cell death.
A key limitation of experiments testing ascorbate plus radiation is that they have only been conducted in vitro. Pharmacologic ascorbate could be a radiosensitizing agent for tumor treatment in vivo. However, animal experiments are lacking. Appropriate dosing and timing of radiation in relation to pharmacologic ascorbate concentrations are unknown and must be determined precisely. It is essential to test whether ascorbate plus radiation accelerates or reduces collateral damage to host tissue, which has been inadvertently irradiated because of proximity to (i.e., adjacency to) tumor tissue. To avoid harm, such experiments must be performed in animals before testing pharmacologic ascorbate as a potential radiosensitizer in humans.
Safety
Relatively widespread human use has generated substantial information on the safety of intravenous pharmacologic ascorbate (59). Recent surveys of intravenous ascorbate use by complementary and alternative medicine (CAM) practitioners indicate that administration is fairly commonplace; fatigue, infection, and cancer were the most commonly cited indications. Adverse effects reported by CAM practitioners were relatively rare and included, but were not limited to, fatigue, vein irritation, kidney stones, hemolysis, nausea, and vomiting. Adverse events from the Food and Drug Administration reporting system were included; adverse effects were reported in 77 patients treated with 1 g of ascorbate or less but confounding variables hindered any assignment of causality. Several factors identified through literature searches have been reported to predispose patients to life-threatening intravenous ascorbate-mediated side effects: renal impairment or failure; glucose-6-dehydrogenase deficiency; and paroxysmal nocturnal hemoglobinuria. Due to the body of evidence supporting generation of H2O2 by pharmacologic ascorbate, administration to patients with iron overload is likely contraindicated (26, 58).
Case studies also provide valuable information on the safety and tolerability of pharmacologic ascorbate, both alone and in combination with standard cancer treatments. For example, Riordan et al. reported on cases of renal cell carcinoma, breast cancer, colorectal cancer, pancreatic cancer, and non-Hodgkin's lymphoma where intravenous ascorbate was administered in doses ranging from 15 to 100 g up to three times per week without evidence of toxicity (68). Several combination therapies were used in this report, including 5-FU and leucovorin plus 100 g intravenous ascorbate twice weekly, gemcitabine plus 75 g intravenous ascorbate twice weekly, and radiation therapy in conjunction with 15 g intravenous ascorbate twice weekly. No toxicities were reported with these combinations and remission occurred in two of the three cases, indicating that ascorbate treatment was not toxic at these doses or in these combinations for this small group of patients.
Another case series study, which included a subset of the above cases, included independent confirmation of diagnosis by a pathologist at the National Cancer Institute and examined supplement medication usage in detail (58). These patients received ascorbate infusions for 10 months or longer. In addition to confirming safety and tolerability of up to 65 g intravenous ascorbate in patients, this study also indicated that ascorbate administration appears to be safe when given in conjunction with a wide variety of supplements. It should be noted that two of the three patients presented in this series were also taking N-acetylcysteine supplements. Experimental evidence indicates that N-acetylecysteine, like catalase, can inhibit the activity of pharmacologic ascorbate in vitro (38). Therefore, the potential interaction between them might warrant additional study. Parenteral glutathione is another alternative therapy frequently used in conjunction with pharmacologic ascorbate that interferes with ascorbate-mediated cytotoxicity (15).
The most important line of evidence confirming the safety of intravenous ascorbate in cancer patients is derived from clinical studies performed to date. A pilot study conducted by Riordan et al. indicates that doses of ∼10–50 g of ascorbate daily produced relatively minor side effects in the majority of the 24 cancer patients who participated in the study (67). However, in this study ascorbate was continuously infused for 8 weeks, which is not how pharmacologic ascorbate is administered by others and is not practical for widespread use. Although ascorbate concentrations were not clear, the doses used at the indicated infusion rate would be expected to produce very low pharmacologic concentrations.
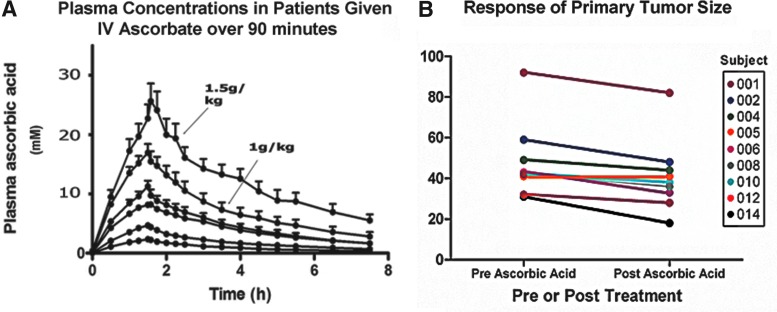
A phase I trial to define a tolerated dose and treatment interval of intravenous ascorbate therapy was conducted at McGill University in 24 patients with varied advanced and treatment-refractory cancers (35). One-third of the patients had previously received three or more courses of treatment with standard therapies. Patients were prescreened for glucose-6-phosphate dehydrogenase deficiency, renal impairment, and the potential formation of clinically silent kidney stones. The study utilized a design of dose escalation. For each 4 week treatment cycle, 5–7 patients received a fixed dose three times weekly, with escalation to the next dose if no dose-limiting toxicity occurred. Doses ranged from 0.4 to 1.5 g/kg. Most adverse effects were mild and clinical laboratory measures were unaffected by treatment, other than a slight increase above normal limits for urinary oxalic acid excretion in the cohort receiving 1.5 g/kg. Peak ascorbate concentrations in plasma as a function of dose were predictable with less than 10% error. The 1.5 g/kg dose produced plasma concentrations greater than 10 mM for ∼4 h and concentrations greater than 5 mM for almost twice as long. The data confirmed that peak plasma concentrations of ascorbate previously predicted to be attainable do, in fact, occur in vivo (Fig. 8A). Although no patients had objective tumor responses and all eventually progressed, two patients had unexpectedly stable disease and received greater than six cycles of ascorbate.
FIG. 8.
Pharmacologic ascorbate concentrations are attainable and safe in patients. (A) Plasma concentrations in patients given intravenous ascorbate over a period of 90 min. Infusion doses are (from top to bottom) 1.5, 0.9, 0.6, 0.4, 0.2, and 0.1 g/kg. The data points represent the mean value of plasma concentration from either five or six patients as a function of time. Peak plasma values>20 mM are transiently attainable in humans. [Reprinted by permission from Hoffer et al. (35)]. (B) Pre- and post-treatment tumor size in millimeters for metastatic pancreatic cancer patients treated with pharmacologic ascorbate (50, 75, or 100 g per infusion three times weekly) in combination with intravenous gemcitabine (1 g/m2 once per week) and erlotinib (100 mg per day orally). Gemcitabine was administered for seven consecutive weeks; ascorbate and erlotinib were administered for 8 weeks [Reprinted by permission from Monti et al. (56)].
Despite lack of cures, reasons for optimism from the McGill phase I trial remain. The patients had advanced cancers that had already proven resistant to as many as three courses of conventional therapy. The course of therapy was short compared to reported cases where responses were documented (58) (Levine, M. unpublished observations). Consistent with this pattern, two patients in the McGill trial had unexpectedly stable disease with at least 6 months of ascorbate. Emerging data, as discussed below, support the concept for longer-term treatment. In addition, it may be difficult to detect clinical benefit from pharmacologic ascorbate by itself in small patient cohorts with progressive disease. To enhance ability to detect benefit, it may be necessary to use ascorbate in combination with standard therapy, and early in disease treatment rather than when other therapies have failed. This approach is supported by in vitro evidence, and in vivo data from xenograft models as discussed in detail above. Selection of tumors can be based on preclinical evidence combined with case reports.
Accordingly, a recent phase I, dose escalation, open label trial at Thomas Jefferson University examined safety and efficacy of intravenous ascorbate given to newly diagnosed patients with stage IV metastatic pancreatic ductal adenocarcinoma, in combination with the pyrimidine analog, gemcitabine, and the EGFR inhibitor, erlotinib (56). Fourteen patients were enrolled, three patients died from progressive disease during the course of the study, and two patients withdrew. The remaining nine patients completed the study. Three patients in each cohort received 50, 75, or 100 g of ascorbic acid per infusion over 90–120 min three times per week for 8 weeks. Patients were also treated with 100 mg erlotinib per day, by mouth, for 8 weeks and 1 g/m2 gemcitabine intravenously once per week for 7 weeks. At the beginning and end of the treatment cycle, patients had X-ray computerized tomography imaging, evaluated by an expert radiologist who was blinded to patient treatment. Twenty-four adverse events were reported in total; eight of these were serious but all were most likely attributable to disease progression or chemotherapeutic treatment rather than ascorbate. Consistent with the data obtained in the previous trial, plasma ascorbate levels exceeded 25 mM in patients treated with 100 g per infusion. Eight of nine patients who completed 8 weeks of treatment had decreased primary tumor size after treatment (Fig. 8B), and the remaining patient was stable without primary tumor increase. Secondary lesions showed improvement in two, were stable in five, and progressed in the remaining two patients. With addition of the three patients who died from progressive disease during treatment, 7 of 12 patients had stable disease by Response evaluation criteria in solid tumors (RECIST) 1.0 standards. Although the average overall and progression-free survival were similar to previously reported survival data from patients treated with gemcitabine/erlotinib alone, this may be explained by the short treatment with ascorbate. It is encouraging and unexpected that eight of nine patients had decreased tumor size, and that no patients had primary tumor progression during treatment.
Encouraging data emerged from a recent phase I trial at the University of Iowa examining toxicity and effects of intravenous ascorbate plus gemcitabine in patients with stage IV pancreatic adenocarcinoma (79). Nine patients completed at least one 4-week treatment cycle and were evaluable. For each cycle, patients received 1000 mg/m2 intravenous gemcitabine once weekly for three consecutive weeks followed by 1 week of rest. Intravenous ascorbate doses, administered twice weekly for all 4 weeks, were tailored to achieve peak plasma concentrations >20 mM. Ascorbate treatment continued until disease progression, defined by RECIST. Severe adverse events were not observed, and toxicities attributed to ascorbate were relatively mild, including nausea, diarrhea, and dry mouth. Mean progression-free survival was 26 weeks and mean overall survival was 13 months. Of note, in prior large trials using gemcitabine for advanced pancreatic cancer, average progression-free and overall survivals were ∼9 weeks and 6 months, respectively. Consistent with cell and animal studies, ascorbate radical was detectable in whole blood containing ascorbate concentrations exceeding 19 mM but not in samples containing physiologic (μM) concentrations of ascorbate. F2-isoprostanes, a marker of systemic oxidative stress, were decreased after intravenous ascorbate treatment in all patients, and intravenous ascorbate did not significantly alter glutathione or glutathione disulfide concentrations in erythrocytes. These results suggest a tumor-specific, rather than global, pro-oxidant effect of ascorbate. Although hampered by a small sample size, these data suggest potential efficacy and are consistent with the hypothesis that prolonged duration of ascorbate treatment may be required to unmask clinical efficacy. Combined, the Iowa and Jefferson studies provide a strong foundation for a phase II trial to determine efficacy of pharmacologic ascorbate in combination with gemcitabine in advanced pancreatic adenocarcinoma.
For patients, quality of life is a key issue. In a self-assessment administered to 39 terminal cancer patients treated with 10 g of intravenous ascorbate at 3 day intervals combined with daily administration of 4 g orally for 1 week, quality of life was reportedly improved on several scales (81). Specifically, patients rated physical, emotional, and cognitive function higher and several symptoms, including nausea, vomiting, loss of appetite, and fatigue, lower after ascorbate treatment. Similarly, advanced cancer patients in the phase I trial who received intravenous ascorbate doses >0.4 g/kg sustained quality of life for the duration of the trial (35). However, neither of these trials had a placebo control. How intravenous ascorbate might mediate improved well being is unknown; further examination with appropriate placebo controls may be worthwhile.
In summary, intravenous ascorbate therapy is safe, well tolerated, and has minimal side effects compared to most standard agents, as verified by both case studies and early clinical trials. Additionally, intravenous ascorbate may contribute to maintaining quality of life. Current trials are investigating the potential efficacy of pharmacologic ascorbate given over longer durations in combination with standard chemotherapies.
Future Directions
Available data provide a solid scientific rationale for the continued investigation of parenteral ascorbate as a chemotherapeutic agent. The data show that pharmacologic ascorbate functions through the generation of extracellular H2O2 with selective cytotoxicity against several cancer types in vitro and in animal models. It should be noted, however, that xenograft mouse models have several limitations for preclinical drug testing and many drugs that initially looked promising in these systems were not efficacious in the clinic (71).
Several important scientific questions remain. It is not fully understood why there is selective toxicity against cancer but not normal cells, nor is there clarity about molecular mechanisms downstream of H2O2 that produce cancer cell death. Identification of clinical biomarkers indicating sensitivity would be extremely useful in selecting patients who might benefit from ascorbate treatment.
Additional studies on the effect of pharmacologic ascorbate when used in combination with standard chemotherapy or radiotherapy are warranted. Many patients seek pharmacologic ascorbate treatment in conjunction with their standard care, making it imperative to determine which combinations have synergistic effects, which have antagonistic effects, and which are neutral. To optimally ensure appropriate combinations, these studies should be done with ascorbate doses ranging from physiologic to pharmacologic.
Several clinical pharmacology issues remain. The toxic dose and tolerated frequency intervals have not been described to date. Given widespread use by CAM practitioners, definition of these limits and potential adverse effects has clinical value (59). The concentration of H2O2 generated as a function of ascorbate dose has implications for correct dosing and should be investigated in people (18), as should changes in intracellular concentrations of ascorbate as a function of parenteral dose in various cell types.
In direct contrast to existing cancer treatments, pharmacologic ascorbate is relatively well tolerated by patients and largely appears to lack toxicity. Thus, if efficacious, ascorbate may indicate a new class of therapeutic agent. However, pharmacologic ascorbate itself is unlikely to cure cancer. Clinical effects of ascorbate are likely to be cytostatic and/or synergistic, suggesting the need for long-term intravenous use. Ascorbate pharmacotherapy may be a chronic therapy, more intensive than insulin treatment in diabetes but less risky and intensive than hemodialysis in patients with end-stage renal disease. Potentially rare adverse events may not have been uncovered yet, but as with most drugs, they are likely to exist.
Despite years of research and thousands of patients who have been treated with intravenous ascorbate, the question of efficacy remains. Although full mechanism of action is the scientific ideal and generally required for the translation of other therapeutics, the overall safety and tolerability of parenteral ascorbate combined with the urgent need for new therapeutic strategies in many cancers suggests that clinical studies may be performed in parallel with basic scientific investigations. Indeed, acetaminophen and aspirin were each in use for nearly 100 years before compelling mechanisms of action were described, for the simple reason that benefit outweighed risk (2, 76). For the same reason, we believe that larger phase I and phase II trials are justified now to expand testing of pharmacologic ascorbate therapy. Ascorbate can be added to conventional first line treatments and continued until disease progression occurs. Optimal trials should include two arms: ascorbate plus conventional therapy and conventional therapy alone. Ideally, placebo infusions can be incorporated to the conventional treatment arm, although such a strategy adds complexity, expense, and risk associated with any repeated infusion. Tumors that can be considered for such trials are those similar to metastatic stage IV pancreatic carcinoma, meaning tumors with poor treatment options and rapid progression. In addition to pancreatic cancer, candidate tumors include metastatic melanoma; metastatic lung (small and non-small cell); inoperable glioblastoma; anaplastic thyroid carcinoma; inoperable (multifocal) hepatoma; aggressive non-Hodgins B cell lymphomas in patients who are marginal candidates for bone marrow transplant; and patients over 60 with acute leukemias who are not candidates for bone marrow transplant. For these tumors, if pharmacologic ascorbate is beneficial, data might emerge after several months of treatment. Because there is no patent potential, pharmaceutical support cannot be counted on to sponsor trials. Public and non-profit funds will be required to drive research. Despite gaps in knowledge and funding uncertainties, patients with cancer deserve no less than to have pharmacologic ascorbate tested rigorously as a treatment agent.
Abbreviations Used
- 5-FU
5-fluorouracil
- AIF
apoptosis-inducing factor
- AP-1
activator protein 1
- As2O3
arsenic trioxide
- ATP
adenosine-5′-triphosphate
- Bcl-2
B-cell lymphoma 2
- CAM
complementary and alternative medicine
- CCL
C-C motif chemokine
- CDK
cyclin-dependent kinase
- cDNA
complementary DNA
- COX
cyclooxygenase
- CXCL
C-X-C motif chemokine
- EC50
effective dose required to kill 50% of cells
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- GSH
glutathione
- Gy
Gray
- H2O2
hydrogen peroxide
- IC50
half maximal inhibitory dose
- IGF-1R
type I insulin-like growth factor receptor
- IGF-II
insulin-like growth factor II
- IκB
NF-κB inhibitor
- LC3
light chain 3
- MAPK
mitogen-activated protein kinase
- MDM2
murine double minute
- MMP
matrix metalloproteinase
- mRNA
messenger RNA
- NF-κB
nuclear factor “kappa-light-chain-enhancer” of activated B cells
- NOX4
NADPH oxidase
- PARP
poly-ADP-ribose polymerase
- PBS
phosphate buffered saline
- PGE2
prostaglandin E2
- Raf
rapidly accelerated fibrosarcoma
- RECIST
response evaluation criteria in solid tumors
- RKIP
Raf kinase inhibitory protein
- ROS
reactive oxygen species
- RT-PCR
reverse transcriptase–polymerase chain reaction
- SCID
severe combined immunodeficiency
- siRNA
small interfering RNA
- SOD
superoxide dismutase
- Sp
specificity protein
- SVCT
sodium-dependent vitamin C transporters
- TfR
transferrin receptor
- TIMP
tissue inhibitor of metalloproteinases
- tRNA
transfer RNA
- VEGF
vascular endothelial growth factor
- VNN3
vanin 3
Acknowledgments
We are indebted to the patients and healthy volunteers who agreed to participate in the clinical studies reviewed in this article. This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH) (ZIA DK053212). The opinions are those of the authors and do not represent official NIH policy.
References
- 1.Abdel-Latif MM, Raouf AA, Sabra K, Kelleher D, and Reynolds JV. Vitamin C enhances chemosensitization of esophageal cancer cells in vitro. J Chemother 17: 539–549, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, Bucher B, Galzi JL, Sterner O, Bevan S, Hogestatt ED, and Zygmunt PM. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Delta(9)-tetrahydrocannabiorcol. Nat Commun 2: 551, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Belin S, Kaya F, Duisit G, Giacometti S, Ciccolini J, and Fontes M. Antiproliferative effect of ascorbic acid is associated with the inhibition of genes necessary to cell cycle progression. PLoS One 4: e4409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bown SR. Scurvy: How a Surgeon, a Mariner, and a Gentleman Solved the Greatest Medical Mystery of the Age of Sail. New York: Thomas Dunne Books, 2004, p 254 [Google Scholar]
- 5.Cameron E. and Campbell A. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact 9: 285–315, 1974 [DOI] [PubMed] [Google Scholar]
- 6.Cameron E, Campbell A, and Jack T. The orthomolecular treatment of cancer. III. Reticulum cell sarcoma: double complete regression induced by high-dose ascorbic acid therapy. Chem Biol Interact 11: 387–393, 1975 [DOI] [PubMed] [Google Scholar]
- 7.Cameron E. and Pauling L. Ascorbic acid and the glycosaminoglycans. An orthomolecular approach to cancer and other diseases. Oncology 27: 181–192, 1973 [DOI] [PubMed] [Google Scholar]
- 8.Cameron E. and Pauling L. The orthomolecular treatment of cancer. I. The role of ascorbic acid in host resistance. Chem Biol Interact 9: 273–283, 1974 [DOI] [PubMed] [Google Scholar]
- 9.Cameron E. and Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 73: 3685–3689, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron E. and Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 75: 4538–4542, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron E, Pauling L, and Leibovitz B. Ascorbic acid and cancer: a review. Cancer Res 39: 663–681, 1979 [PubMed] [Google Scholar]
- 12.Cameron E. and Rotman D. Ascorbic acid, cell proliferation, and cancer. Lancet 1: 542, 1972 [DOI] [PubMed] [Google Scholar]
- 13.Carosio R, Zuccari G, Orienti I, Mangraviti S, and Montaldo PG. Sodium ascorbate induces apoptosis in neuroblastoma cell lines by interfering with iron uptake. Mol Cancer 6: 55, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen MF, Yang CM, Su CM, Liao JW, and Hu ML. Inhibitory effect of vitamin C in combination with vitamin K3 on tumor growth and metastasis of Lewis lung carcinoma xenografted in C57BL/6 mice. Nutr Cancer 63: 1036–1043, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Stone J, Sullivan G, Drisko JA, and Chen Q. Anti-cancer effect of pharmacologic ascorbate and its interaction with supplementary parenteral glutathione in preclinical cancer models. Free Radic Biol Med 51: 681–687, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Yu J, Chalmers B, Drisko J, Yang J, Li B, and Chen Q. Pharmacological ascorbate induces cytotoxicity in prostate cancer cells through ATP depletion and induction of autophagy. Anticancer Drugs 23: 437–444, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, and Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A 102: 13604–13609, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, and Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A 104: 8749–8754, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, and Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A 105: 11105–11109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, Margolis S, Padayatty S, Sun H, Wang Y, Nussbaum RL, Espey MG, and Levine M. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest 120: 1069–1083, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creagan ET, Moertel CG, O'Fallon JR, Schutt AJ, O'Connell MJ, Rubin J, and Frytak S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med 301: 687–690, 1979 [DOI] [PubMed] [Google Scholar]
- 22.Deubzer B, Mayer F, Kuci Z, Niewisch M, Merkel G, Handgretinger R, and Bruchelt G. H(2)O(2)-mediated cytotoxicity of pharmacologic ascorbate concentrations to neuroblastoma cells: potential role of lactate and ferritin. Cell Physiol Biochem 25: 767–774, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Du J, Martin SM, Levine M, Wagner BA, Buettner GR, Wang SH, Taghiyev AF, Du C, Knudson CM, and Cullen JJ. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin Cancer Res 16: 509–520, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, and Chen Q. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med 50: 1610–1619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espey MG, Chen Q, and Levine M. Comment re: vitamin C antagonizes the cytotoxic effects of chemotherapy. Cancer Res 69: 8830; author reply 8830–8831, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Fleming RE. and Ponka P. Iron overload in human disease. N Engl J Med 366: 348–359, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Fromberg A, Gutsch D, Schulze D, Vollbracht C, Weiss G, Czubayko F, and Aigner A. Ascorbate exerts anti-proliferative effects through cell cycle inhibition and sensitizes tumor cells towards cytostatic drugs. Cancer Chemother Pharmacol 67: 1157–1166, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grad JM, Bahlis NJ, Reis I, Oshiro MM, Dalton WS, and Boise LH. Ascorbic acid enhances arsenic trioxide-induced cytotoxicity in multiple myeloma cells. Blood 98: 805–813, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LR, Jr., Wang Y, and Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res 14: 1133–1139, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Hahm E, Jin DH, Kang JS, Kim YI, Hong SW, Lee SK, Kim HN, Jung da J, Kim JE, Shin DH, Hwang YI, Kim YS, Hur DY, Yang Y, Cho D, Lee MS, and Lee WJ. The molecular mechanisms of vitamin C on cell cycle regulation in B16F10 murine melanoma. J Cell Biochem 102: 1002–1010, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Han SS, Kim K, Hahm ER, Lee SJ, Surh YJ, Park HK, Kim WS, Jung CW, Lee MH, Park K, Yang JH, Yoon SS, Riordan NH, Riordan HD, Kimler BF, Park CH, Lee JH, and Park S. L-ascorbic acid represses constitutive activation of NF-kappaB and COX-2 expression in human acute myeloid leukemia, HL-60. J Cell Biochem 93: 257–270, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Heaney ML, Gardner JR, Karasavvas N, Golde DW, Scheinberg DA, Smith EA, and O'Connor OA. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res 68: 8031–8038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herst PM, Broadley KW, Harper JL, and McConnell MJ. Pharmacological concentrations of ascorbate radiosensitize glioblastoma multiforme primary cells by increasing oxidative DNA damage and inhibiting G2/M arrest. Free Radic Biol Med 52: 1486–1493, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Hoffer LJ. Proof versus plausibility: rules of engagement for the struggle to evaluate alternative cancer therapies. CMAJ 164: 351–353, 2001 [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L, and Miller WH, Jr., Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol 19: 1969–1974, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Hong SW, Jin DH, Hahm ES, Yim SH, Lim JS, Kim KI, Yang Y, Lee SS, Kang JS, Lee WJ, Lee WK, and Lee MS. Ascorbate (vitamin C) induces cell death through the apoptosis-inducing factor in human breast cancer cells. Oncol Rep 18: 811–815, 2007 [PubMed] [Google Scholar]
- 37.Hong SW, Lee SH, Moon JH, Hwang JJ, Kim DE, Ko E, Kim HS, Cho IJ, Kang JS, Kim DJ, Kim JE, Shin JS, Jung DJ, Jeong YJ, Cho BJ, Kim TW, Lee JS, Kang JS, Hwang YI, Noh DY, Jin DH, and Lee WJ. SVCT-2 in breast cancer acts as an indicator for L-ascorbate treatment. Oncogene [Epub ahead of print]; DOI: 10.1038/onc.2012.1762012 [DOI] [PubMed] [Google Scholar]
- 38.Kang JS, Cho D, Kim YI, Hahm E, Yang Y, Kim D, Hur D, Park H, Bang S, Hwang YI, and Lee WJ. L-ascorbic acid (vitamin C) induces the apoptosis of B16 murine melanoma cells via a caspase-8-independent pathway. Cancer Immunol Immunother 52: 693–698, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 149: 43–50, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Lee SD, Chang B, Jin DH, Jung SI, Park MY, Han Y, Yang Y, Il Kim K, Lim JS, Kang YS, and Lee MS. Enhanced antitumor activity of vitamin C via p53 in cancer cells. Free Radic Biol Med 53: 1607–1615, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Klingelhoeffer C, Kammerer U, Koospal M, Muhling B, Schneider M, Kapp M, Kubler A, Germer CT, and Otto C. Natural resistance to ascorbic acid induced oxidative stress is mainly mediated by catalase activity in human cancer cells and catalase-silencing sensitizes to oxidative stress. BMC Complement Altern Med 12: 61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurbacher CM, Wagner U, Kolster B, Andreotti PE, Krebs D, and Bruckner HW. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett 103: 183–189, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Lee SK, Kang JS, Jung da J, Hur DY, Kim JE, Hahm E, Bae S, Kim HW, Kim D, Cho BJ, Cho D, Shin DH, Hwang YI, and Lee WJ. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol 216: 180–188, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, and Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A 93: 3704–3709, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine M, Wang Y, Padayatty SJ, and Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A 98: 9842–9846, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Tu H, Wang Y, and Levine M. Vitamin C in mouse and human red blood cells: an HPLC assay. Anal Biochem 426: 109–117, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Ishdorj G, and Gibson SB. Reactive oxygen species regulation of autophagy in cancer: Implications for cancer treatment. Free Radic Biol Med 53: 1399–1410, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Lim D, Morgan RJ, Jr., Akman S, Margolin K, Carr BI, Leong L, Odujinrin O, and Doroshow JH. Phase I trial of menadiol diphosphate (vitamin K3) in advanced malignancy. Invest New Drugs 23: 235–239, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Lin SY, Lai WW, Chou CC, Kuo HM, Li TM, Chung JG, and Yang JH. Sodium ascorbate inhibits growth via the induction of cell cycle arrest and apoptosis in human malignant melanoma A375.S2 cells. Melanoma Res 16: 509–519, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Lin ZY. and Chuang WL. Pharmacologic concentrations of ascorbic acid cause diverse influence on differential expressions of angiogenic chemokine genes in different hepatocellular carcinoma cell lines. Biomed Pharmacother 64: 348–351, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Martinotti S, Ranzato E, and Burlando B. In vitro screening of synergistic ascorbate-drug combinations for the treatment of malignant mesothelioma. Toxicol In Vitro 25: 1568–1574, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Mc CW. Cancer: a collagen disease, secondary to a nutritional deficiency. Arch Pediatr 76: 166–171, 1959 [PubMed] [Google Scholar]
- 53.Mikirova NA, Ichim TE, and Riordan NH. Anti-angiogenic effect of high doses of ascorbic acid. J Transl Med 6: 50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell JB, Samuni A, Krishna MC, DeGraff WG, Ahn MS, Samuni U, and Russo A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry 29: 2802–2807, 1990 [DOI] [PubMed] [Google Scholar]
- 55.Moertel CG, Fleming TR, Creagan ET, Rubin J, O'Connell MJ, and Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med 312: 137–141, 1985 [DOI] [PubMed] [Google Scholar]
- 56.Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, Pillai MV, Newberg AB, Deshmukh S, and Levine M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One 7: e29794, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Padayatty SJ. and Levine M. New insights into the physiology and pharmacology of vitamin C. Cmaj 164: 353–355, 2001 [PMC free article] [PubMed] [Google Scholar]
- 58.Padayatty SJ, Riordan HD, Hewitt SM, Katz A, Hoffer LJ, and Levine M. Intravenously administered vitamin C as cancer therapy: three cases. Cmaj 174: 937–942, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, and Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One 5: e11414, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, and Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 140: 533–537, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Park S, Ahn ES, Lee S, Jung M, Park JH, Yi SY, and Yeom CH. Proteomic analysis reveals upregulation of RKIP in S-180 implanted BALB/C mouse after treatment with ascorbic acid. J Cell Biochem 106: 1136–1145, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Pathi SS, Lei P, Sreevalsan S, Chadalapaka G, Jutooru I, and Safe S. Pharmacologic doses of ascorbic acid repress specificity protein (Sp) transcription factors and Sp-regulated genes in colon cancer cells. Nutr Cancer 63: 1133–1142, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patterson JW. Course of diabetes and development of cataracts after injecting dehydroascorbic acid and related substances. Am J Physiol 165: 61–65, 1951 [DOI] [PubMed] [Google Scholar]
- 64.Perrone G, Hideshima T, Ikeda H, Okawa Y, Calabrese E, Gorgun G, Santo L, Cirstea D, Raje N, Chauhan D, Baccarani M, Cavo M, and Anderson KC. Ascorbic acid inhibits antitumor activity of bortezomib in vivo. Leukemia 23: 1679–1686, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Pollard HB, Levine MA, Eidelman O, and Pollard M. Pharmacological ascorbic acid suppresses syngeneic tumor growth and metastases in hormone-refractory prostate cancer. In Vivo 24: 249–255, 2010 [PMC free article] [PubMed] [Google Scholar]
- 66.Ranzato E, Biffo S, and Burlando B. Selective ascorbate toxicity in malignant mesothelioma: a redox Trojan mechanism. Am J Respir Cell Mol Biol 44: 108–117, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Riordan HD, Casciari JJ, Gonzalez MJ, Riordan NH, Miranda-Massari JR, Taylor P, and Jackson JA. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J 24: 269–276, 2005 [PubMed] [Google Scholar]
- 68.Riordan HD, Riordan NH, Jackson JA, Casciari JJ, Hunninghake R, Gonzalez MJ, Mora EM, Miranda-Massari JR, Rosario N, and Rivera A. Intravenous vitamin C as a chemotherapy agent: a report on clinical cases. P R Health Sci J 23: 115–118, 2004 [PubMed] [Google Scholar]
- 69.Riordan NH, Riordan HD, Meng X, Li Y, and Jackson JA. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses 44: 207–213, 1995 [DOI] [PubMed] [Google Scholar]
- 70.Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, and Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem 272: 18982–18989, 1997 [DOI] [PubMed] [Google Scholar]
- 71.Sharpless NE. and Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov 5: 741–754, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Shinozaki K, Hosokawa Y, Hazawa M, Kashiwakura I, Okumura K, Kaku T, and Nakayama E. Ascorbic acid enhances radiation-induced apoptosis in an HL60 human leukemia cell line. J Radiat Res 52: 229–237, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, and Nussbaum RL. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med 8: 514–517, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Takemura Y, Satoh M, Satoh K, Hamada H, Sekido Y, and Kubota S. High dose of ascorbic acid induces cell death in mesothelioma cells. Biochem Biophys Res Commun 394: 249–253, 2010 [DOI] [PubMed] [Google Scholar]
- 75.Ullah MF, Khan HY, Zubair H, Shamim U, and Hadi SM. The antioxidant ascorbic acid mobilizes nuclear copper leading to a prooxidant breakage of cellular DNA: implications for chemotherapeutic action against cancer. Cancer Chemother Pharmacol 67: 103–110, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231: 232–235, 1971 [DOI] [PubMed] [Google Scholar]
- 77.Verrax J. and Calderon PB. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med 47: 32–40, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Russo TA, Kwon O, Chanock S, Rumsey SC, and Levine M. Ascorbate recycling in human neutrophils: induction by bacteria. Proc Natl Acad Sci U S A 94: 13816–13819, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Welsh JL, Wagner BA, Van't Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, Yee NS, Bodeker KL, Du J, Roberts LJ, 2nd, Drisko J, Levine M, Buettner GR, and Cullen JJ. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol 71: 765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu KK. Cyclooxygenase 2 induction: molecular mechanism and pathophysiologic roles. J Lab Clin Med 128: 242–245, 1996 [DOI] [PubMed] [Google Scholar]
- 81.Yeom CH, Jung GC, and Song KJ. Changes of terminal cancer patients' health-related quality of life after high dose vitamin C administration. J Korean Med Sci 22: 7–11, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yeom CH, Lee G, Park JH, Yu J, Park S, Yi SY, Lee HR, Hong YS, Yang J, and Lee S. High dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J Transl Med 7: 70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]