Abstract
Over the last few decades, the achievements and progress in the field of medical imaging have dramatically enhanced the early detection and treatment of many pathological conditions. The development of new imaging modalities, especially non-ionising ones, which will improve prognosis, is of crucial importance. A number of novel imaging modalities have been developed but they are still in the initial stages of development and serious drawbacks obstruct them from offering their benefits to the medical field. In the 21 st century, it is believed that nanotechnology will highly influence our everyday life and dramatically change the world of medicine, including medical imaging. Here we discuss how nanotechnology, which is still in its infancy, can improve Terahertz (THz) imaging, an emerging imaging modality, and how it may find its way into real clinical applications. THz imaging is characterised by the use of non-ionising radiation and although it has the potential to be used in many biomedical fields, it remains in the field of basic research. An extensive review of the recent available literature shows how the current state of this emerging imaging modality can be transformed by nanotechnology. Innovative scientific concepts that use nanotechnology-based techniques to overcome some of the limitations of the use of THz imaging are discussed. We review a number of drawbacks, such as a low contrast mechanism, poor source performance and bulky THz systems, which characterise present THz medical imaging and suggest how they can be overcome through nanotechnology. Better resolution and higher detection sensitivity can also be achieved using nanotechnology techniques.
Introduction
Nanotechnology is one of the newest fields of technology and science that has attracted the attention of the scientific community, since it is believed to possess the potential to entirely change our everyday life as we know it up to now. It is quite complicated to identify the origins of nanotechnology; however, no-one can deny the fact that the inspiring lecture of R. Feynman (29 December 1959) “There is plenty of room at the bottom” is the keystone to the field of nanotechnology 1. This talk was so amazing for its time that many believe that it represented the birth of the new scientific field of nanotechnology.
Not only are the origins of nanotechnology complicated, but also the definition of the term ‘nanotechnology’ is not as straightforward as it sounds since the field is very recent and there are many conflicting opinions. The first part of the words nanotechnology and nanoscience, the word nano, comes from the Greek word ‘nannos’, which means a very short man 2 and indicates that we are referring to the technology and science that deal with the physical phenomena/technology in the nanoscale. Generally, we call nanotechnology the manipulation and study of the properties of objects that are, in at least one of their dimensions, smaller than 100 nm.
The importance of nanotechnology is the fact that on the nanometre scale, dimensions of materials are essential to characterise their properties 3. At such dimensions, materials possess new physical properties or exhibit new physical phenomena. At such small dimensions, the properties of matter are completely different from what we have been taught and new uncommon properties are observed 4. The new properties arise from the fact that at these dimensions the surface area per volume is increased and the material properties obey the rules of quantum mechanics and not the classical physics of the macroscopic scale 5. Therefore, nanotechnology has not only to do with small dimensions, but also with new novel physical properties 2.
The emerging applications of nanotechnology are so powerful that many scientists believe that it has the potential to radically change the world as we know it and some of them are even wondering whether nanotechnology can push forward the next 'nano-industrial revolution’ 6, 7. One area that has been very promising is the application of nanotechnology to medicine 5, the so-called nanomedicine. Through the developing field of nanomedicine, nanotechnology and medicine come together so that existing therapies and medical techniques can be improved 8. Due to its significance for humans, nanomedicine has become one of the most crucial branches of nanoscience. It is considered to be the great challenge of medicine of the 21 st century, mainly in three key areas: diagnosis, treatment and regenerative medicine 9.
A scientific and technological area with so many expectations will inevitably also positively affect the field of medical imaging and radiology. The field of medical imaging is very broad and since the discovery of X-rays, many non-invasive imaging modalities have been invented. Each modality presents its unique characteristics and its intrinsic limitations, and there are differences in their ionising or non-ionising nature, sensitivity, resolution, complexity, time of data acquisition, physical principles, performance conditions, provided information and of course the financial costs. Although the field of medical imaging has a quite long history, new innovative imaging modalities emerge in order to reduce the limitations and expand the capabilities of the existing modalities 10. Unfortunately a ‘perfect and ideal’ imaging modality has not yet been developed and the existing modalities are characterised by different limitations. According to Boulaiz et al. 8, an ideal imaging modality should have a non-invasive nature, high sensitivity and the ability to provide information on cell survival, function and localisation.
An area that attracts the interest of researchers is the use of non-invasive and non-ionising radiation for medical-imaging purposes. It has been stated that there is a revolution in non-invasive imaging modalities 11 and imaging modalities that do not use ionising radiation minimise patient’s risks, enable imaging repeatability and in many cases are non-invasive and reduce patient’s suffering. According to Wallace et al. 12 there is a gap between microscopy and medical imaging and consequently current efforts are focusing on developing non-ionising modalities that can fill this gap. One of the most recent and attractive modalities that satisfy these requirements is Terahertz (THz) imaging 13– 16.
THz radiation, also called ‘sub-millimetre radiation’ or ‘T-rays’, is generally defined as the frequency range from 100 GHz to 10 THz 17 and is actually the gap between the infrared (IR) and microwaves 13 ( Figure 1). This region of the electromagnetic spectrum remained unexplored for many years since there were not appropriate sources (electronic or optical) 18 available, although the characteristics of this radiation are unique ( Table 1) and there are a number of potential applications. The development of ultra-short optical pulse lasers and the growth of semiconductor microfabrication techniques pushed for the expansion of THz radiation technology 19.
Figure 1. The THz spectrum.
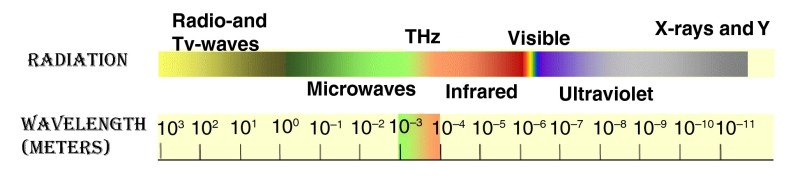
It can be seen that THz radiation is the gap between the infrared and microwaves.
Table 1. Characteristics of radiation at 1 THz.
| Characteristics | |
|---|---|
| Period | 1 ps |
| Wavelength | 300 μm |
| Wave number | 33 cm -1 |
| Photon energy | 4.1 meV |
| Equivalent temperature | 47,6 K |
This paper aims to investigate whether nanotechnology can reform a specific imaging modality, THz imaging, and support it in order to overcome its limitations. Generally, it is believed that nanotechnology has the potential to change medicine, and modify it so as to enhance therapy and medical imaging techniques. Many of the proposed applications still remain far beyond what is now possible to achieve using nanotechnology techniques. The purpose of this work is to review the possible applications of not futuristic applications of nanotechnology-supported THz imaging modalities. THz imaging uses non-invasive radiation and although it is among the most attractive emerging imaging modalities, it has not yet become fully established. In order to achieve this, the limitations and drawbacks of the state-of-the-art THz imaging set-ups are first identified. Then nanotechnology-based techniques that were used to improve THz imaging are presented. How THz imaging can help nanotechnology and ethical/risk issues are briefly discussed. The focus is on both the improvement of the current imaging set-ups (detectors, emitting sources, etc.) and the use of nanotechnology-based contrast agents (nano-particles, nano-rods, etc.) to enhance their signals from specific targets.
Method
In the next section (Background), the current status of THz imaging is briefly presented and the limitations of these unique imaging modalities are presented. In the following section, the results of a systematic review on how nanotechnology can support THz imaging are presented. The searching of relevant material for the review was performed based on electronic resources including the online databases Scopus, Web of Science and PubMed. Google and Google Scholar were used for finding extra material. Furthermore, references found in the initial articles were also used. To capture the relevant studies the keywords and indexing terms that were used included: THz/THz imaging and nanotechnology/nanoscience, nano contrast agents, THz nano-imaging, terahertz nanoscopy. The searching procedure included the following limits: for the nanotechnology-supported THz imaging modalities, only articles published in English between January 2000 and January 2012 were included.
Background
General
THz radiation’s applications are expanding so quickly that they have an outstanding potential and social impact 20. These applications can be expanded from medical, science and pharmaceutical applications to material non-destructive testing and security purposes. There is special interest in biomedical applications, such as the use of THz radiation as an imaging modality and for spectroscopy studies 21, which have the potential for a serious clinical impact 11. The first biomedical THz imaging was demonstrated in 1995 22 and since then a new non-invasive imaging modality has emerged.
Since THz radiation has a long wavelength it can penetrate many materials 23. Furthermore, polar molecules are sensitive to THz waves and consequently the detection of different hydration levels from tissues can be achieved 13. The biomedical applications of THz waves are a consequence of the fact that THz radiation is sensitive to water and, what is more, biological molecules' characteristic energies lie in the THz region 24. This is very important considering that water is one of the most important components of the tissue 25. Actually, water molecules and all polar liquids absorb all the frequencies in the THz band. As a consequence, THz waves cannot penetrate moist tissue, a fact that enables both the development of imaging set-ups in transition (for in vitro studies) and reflection geometry (for in vitro and in vivo imaging) 11. THz radiation is characterised by its ability to penetrate organic materials without ionisation, to distinguish different materials according to their water content and the fact that it can help the clarification of the unknown dynamics in the area of condensed matter physics (e.g. molecular recognition and protein folding) 26.
In the case of THz medical imaging, the contrast mechanism arises from the fact that different absorption spectra and refractive indices characterise the different biological tissues when they interact with waves in the THz region 17. Consequently, images and information from normal or pathological tissues (with abnormalities) can be obtained. Even from the very first demonstration of imaging from Hu and Nuss 22, it was shown that the different water content of two different tissues (porcine muscle and fat) was the contrast mechanism 12. Initial studies have demonstrated that biochemical and morphological features of the tissue provide contrast in images formed with THz pulses 25.
When THz radiation is used for spectroscopy, the key factor is the fact that the energy of the vibrational and rotation molecules (like proteins and DNA) corresponds to that of the THz photons 25. THz spectroscopy can be used to investigate a variety of phenomena of great importance to scientists and engineers 27. An interesting biomedical application is the demonstration of using THz spectroscopy to detect mutations and biomolecule conformational changes 11. Also, the diagnosis and imaging of cancer is one very promising application of THz imaging technology 28– 32. This is a consequence of the fact that there is difference in the water content between healthy tissues and cancerous tumours and, what is more, there are differences due to cell alterations and abnormal protein density alterations that result in larger THz absorption and refractive index 24, 33. Although there are many expectations on this technique, the studies seem not to support any clinical application which can achieve high cancer detection rate 24.
THz imaging modalities
Although the field of medical imaging in the last decades has seen great improvements and has significantly contributed towards a better medical practice both for diagnosis and therapy, THz is still developing in all of its components from technological concepts/set-ups to possible applications. THz-material/tissue interactions and the physical/biological mechanisms involved are still not well established and more research is still needed.
The lack of appropriate, low expense and compact-size emitters and detectors of T-rays has meant that the THz band has remained unexplored and unused for many years. Initial inappropriate set-ups were developed with expensive and bulky sources (e.g. free electron lasers, thermal sources), while the detectors demonstrated poor performance, like the liquid helium-cooled bolometers 11. In the last decades, a number of novel techniques (like the ultrafast optical switch, the nonlinear method and quantum cascade lasers) innovated the field of THz optoelectronics 19. The THz imaging systems can be separated into two main categories: Passive (also named Incoherent) and Active (also named Coherent) Pulsed or Continuous 34, 35. Here, we focus on the set-ups of the active categories, since these are the most extensively used for medical imaging purposes, while passive set-ups are most widely used for security purposes in airports and for weapon detection 36, 37. Pulsed and continuous THz imaging set-ups are both still in development, but pulsed systems are more widely used 24.
At present there are many competitive techniques for generating THz waves (continuous or pulsed). The different THz sources can be separated into three basic categories: electronic sources, photonic sources and quantum cascade lasers. There are also some other smaller categories that are either emerging or not very popular, like p-germanium THz lasers 38– 40 and uni-travelling-carrier photodiode 13, 41, but they are not the focus here.
There are several ways of detecting THz radiation. There are a large number of published articles explaining the spectrum of the existing detectors and the new principles that are used for detecting T-rays are very broad 42. They can be separated into direct detection (with Schottky-barried diodes and bolometers), heterodyne detection (like with super conducting hot electron bolometers) and heterodyne detection that uses photonical-generated THz local oscillators (electronic or photonic mixers) 43. Photoconductors (PCs), electro-optic (EO) materials and photodiodes (PDs) are frequently used as photonic mixers. Direct and heterodyne detection are also referred to as incoherent and coherent detection, respectively 44. Which method of detection will eventually be applied is mainly determined by the type of THz source that is used in the same set-up 26 or which characteristics of the THz waves are intended to be detected.
The THz imaging systems, and THz technology in general, can be separated on whether they uses continuous waves (CWs) or pulses. CW THz is traditionally used for astronomy (like the study of Big Bang radiation), environmental monitoring and plasma diagnostics. Optically Pumped Terahertz Laser is a characteristic CW THz source 26. For medical imaging, pulse THz sources are more attractive and have made THz imaging a challenging field for medical imaging.
The term “THz time-domain spectroscopy” (THz-TDS) refers to the technique where THz pulse methods are used for spectroscopy studies 19. The same method system can be used for the formation of 2- and 3-D images. THz pulse-imaging is a quite simple methodology and a pump and probe beam are used. The pump beam interacts with the sample, and the detection of the coherent signal is obtained by combining the probe laser with the THz radiation 25. The image of the subject can be built up due to the selective absorption of the THz radiation 26. Consequently, the detector receives the signals with delay 26 and by scanning the sample an image can be formed with each pixel representing the different time-series, which are the different adsorption characteristics 25. The obtained data can then be processed with fast Fourier transform so as to move from the time to the frequency domain 26.
The characteristics of THz radiation and already developed imaging set-ups also enable the use of this technique as an endoscope-based procedure 45. The researchers involved in this study believed that if some limitations could be overcome (like the fact that water and the side-walls of the organs have a similar refractive index and power absorption), THz endoscopes would be a valuable tool for detecting tissue changes within the human body 45.
Limitations of THz imaging
Unfortunately, THz imaging modalities are still characterised by a number of quite significant limitations. Particularly, THz radiation remained unexplored for many years due to the fact that detectors of THz waves were characterised by poor signal-to-noise ratio and slow processing. A second limitation was that the emitters were able to produce only incoherent and low-brightness THz radiation 25 and some sources require cryogenic operating temperatures 20. The development of both electronic and optical sources that emit at the THz spectrum is difficult to implement but very beneficial 17. Some of the initial problems that THz technology has faced have found some solutions but there are also a number of significant drawbacks that still remain ( Table 2) 23, 46.
Table 2. Advantages and limitations/drawbacks of THz imaging.
| Advantages | Drawbacks/limitations |
|---|---|
| Non-ionising radiation; is considered safe for biological imaging. | Limited penetration depth and THz waves cannot penetrate into the human body due to high water component. |
| Sensitive to water component. Biological molecules' characteristic energies lie in the THz region (the energy of vibrational and rotational molecules correspond to that of the THz photons). | Difficulties in the development of appropriate THz sources
• Measuring speeds and scanning times require improvement. • Bulky systems due to their components, like the use of fs lasers. • System costs are relatively high (mainly due to the use of fs lasers). • Problems in transferring THz waves and difficulties in achieving distance sensing in air over several metres. • Need to improving systems with a large signal-to-noise ratio. • Some THz sources cannot be used at room temperature. |
| It can perform non-destructive testing and contact-free imaging or characterisation of the sample. | |
| Compared with microwaves, THz waves possess shorter wavelength and consequently greater spatial resolution can be achieved. | |
| The long wavelength of the THz photons enables the THz radiation to penetrate many materials. | |
| THz radiation is not heavily affected by Rayleiyh scattering. | Limited imaging resolution due to long wavelength. |
| Fills the ‘gap’ in medical imaging modalities. | Applications still in research. |
| Can perform spectroscopy. Medical imaging can be combined with spectroscopy information. Biochemical and morphological features can be provided simultaneously. | Low contrast between healthy and pathological tissues. |
The first drawback of THz imaging is a consequence of the nature of THz waves. Their long wavelength results in limitations in the imaging resolution compared with imaging modalities that use shorter wavelengths 47. Due to the long wavelength, THz imaging can illustrate features in the range 1–3 mm, which is not enough for biomedical applications 11. The limited penetration depth due to the high body-water component has until now limited possible applications and THz studies to surface tissues such as skin 48, 49, teeth 50 and the cornea 51. The use of the 0.5 THz frequency gives the highest contrast between normal and cancerous tissues, but this minimized the later resolution of THz imaging 33. Furthermore, the contrast between healthy and pathological tissues is too low and there is a need for contrast agents 33. Humphreys et al. 11 also stressed the need for well informed and well organised databases that include the different responses of different tissues to THz radiation.
As was shown in the previous section, the use of femtosecond (fs) lasers is a common method for emitting THz radiation in the optical-to-THz conversion. The use of these sources make the commercialisation of THz imaging set-ups difficult due to both the high cost and the bulky dimensions of fs lasers 17. This is why quantum cascade lasers are very attractive in the field, since they are expected to be cheaper and of appropriate size. Finally, it must be noted that although the potentials are great, the penetration depth of THz radiation is limited. Consequently, until now research has been concentrated on wound healing, burn diagnosis dermatology and dentistry, where a high depth is not required and the probed tissue is accessible without the need for waveguides 11, 25. Furthermore, the majority of the THz applications are still in the research phase, except for a few examples from the TeraView Company (Cambridge, UK), which has developed set-ups and techniques for detecting cancerous cells 26. Currently, there are a number of companies that produce THz-related technology including Picometrix Inc. (Michigan, USA), Zomega Terahertz Corporation (New York, USA), Nikon Corporation (Tokyo, Japan), Toptica Photonics (Munich, Germany) and Hamamatsu Photonics (Tokyo, Japan), T-Ray Science Inc. (Vancouver, Canada).
Nanotechnology for THz imaging
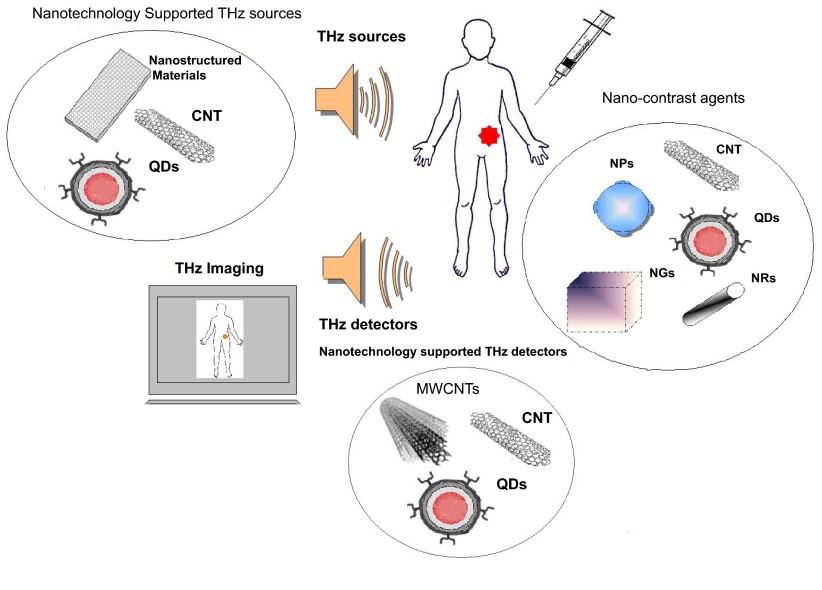
As in all the emerging imaging modalities, THz presents some drawbacks that do not allow it to find its place in every day medical use. As was shown, these limitations cover a wide range from the low-performance of emitting sources to the low sensitivity or selectivity to pathological tissues. In order to overcome these drawbacks researchers are expanding their efforts in many different directions. Nanotechnology-based techniques seems to be a crucial key tool in their efforts to improve these imaging modalities. We believe that nanotechnology has the potential to improve the performance of imaging modalities. The next sections aim to discuss how current nanotechnology techniques can directly enhance THz medical imaging modalities. It will be demonstrated that nanotechnology can support THz imaging through several concepts, from using nanoparticles as contrast agents to the development of new THz sources and/or detectors ( Figure 2).
Figure 2. Nanotechnology-supported THz imaging.
Nanotechnology methods are used in all the components of THz imaging: contrast agents, sources and detectors (CNT: Carbon Nanotubes, QDs: Quantum Dots, NPs: Nanoparticles, NRs: nanorods, NGs: nanocages, WCNTs: multi-walled CNTs).
Nanocontrast agents for THz imaging
One area of THz imaging that nanotechnology could innovate is the use of contrast agents, also called contrast media or probes. Generally, contrast agents are used in order to increase image contrast from healthy and pathological tissue areas or molecules. Many contrast agents have been proposed and used for the existing imaging modalities (e.g. MRI contrast agents) 52– 54, but in the case of THz imaging, very few studies have been published. Although this area is in the very early stages, the results are very positive and open new horizons for the clinical application of THz medical imaging. By using contrast agents, it will be possible to enhance the sensitivity of cancer diagnosis by targeting tumours and by enabling the use of higher THz frequencies, which will allow better resolution 33.
Nanotechnology can innovate THz imaging in this direction by the use of nanoparticles as contrast agents. With the term 'nanoparticles', a broad range of particles with dimensions in the nanoscale is implied, such as spherical particles, carbon nanotubes (CNT), fullerenes, quantum dots (QDs), cantilevers, nanorods (NRs), nanoshells, nanocages (NGs), nanowires and various metal and metal oxide nanoparticles. Nanoparticles that are characterised by their ability to produce surface plasmons, the so-called plasmon nanoparticles, are particularly interesting as they can be used for imaging and therapy purposes 55. Gold (Au) is the most commonly used metal for fabricating nanoparticles for biomedical applications due to its biocompatibility, strong scattering around local surface plasmon (LSP) resonance wavelengths and the ability to accept the bio-conjunction process 56.
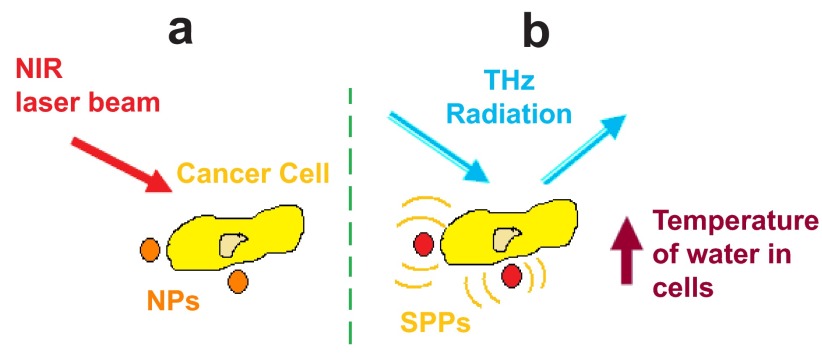
Nanoparticle contrast agent techniques take advantage of the physical phenomenon known as the hyperthermia effect that occurs due to surface plasma polaritons (SPPs) when near-infrared laser beams irradiate nanoparticles. As a consequence of this phenomenon, the temperature of water in cancer cells (which are probed with nanoparticles) rises and since the THz signal is sensitive to water temperature alterations 57, cancer cells can be probed and imaged ( Figure 3).
Figure 3. The hyperthermia effect.
a) First, the cancer cells are probed with nanoparticles (NPs) and are then irradiated with near-infrared (NIR) laser beams. b) After irradiation, surface plasma polaritons (SPPs) occur and as a result the temperature of water in the cancer cells is increased. Consequently, the cancer cells can be probed and imaged with THz radiation since the THz signal is sensitive to water temperature alterations.
A significant work on this direction was published by Oh, Son and their colleagues in a series of four recent papers 33, 58– 60. The new methodology was called nanoparticle-contrast-agent-enabled terahertz imaging 58. Initially, hydroxyapatite gold nanocomposites and gold nanorods (GNRs) were studied and it was shown that contrast agents can enhance sensitivity in THz signals and can be bound in cancer cells and can consequently target cancerous tumours 33. Their next in vitro experiments were performed in cancer cells with and without GNRs 58. Their results showed that although there were not any significant differences in THz reflection images, the enhancement was high under IR irradiation and the differential mode enabled cancer diagnosis. They also demonstrated that tumours could be identified by monitoring the signal at a point without the need of imaging.
Oh et al. expanded their experiments in vivo by acquiring THz images in tumours of mice 24 hours after the injection of GNRs and the high sensitivity of the differential technique was shown 59. Finally, in a recent publication THz molecular imaging (TMI) was demonstrated to be sufficiently sensitive to detect 15 mM of nanoprobes in vivo 60. What is more, it was characterised in linear proportion to the nanoparticle concentration, which is a very useful quantification property. For their experiments, Oh et al. used a reflection-mode THz imaging set-up accompanied by a laser in the IR region for the surface plasma polariton induction 59.
Other research groups are also working in the field and Lee et al. 24 studied gadolinium oxide (Gd 2O 3) nanoparticles as possible terahertz imaging contrast agents. Their results demonstrated that these kinds of particles are appropriate for terahertz medical imaging since their interaction with THz waves is very strong (the power absorption is ~3 orders higher than water) 24. Moreover, as Gd 2O 3 nanoparticles are already used as MRI multi-functional contrast agents, their use for THz studies might enable the combination of the two imaging modalities (MRI-THz) and the combination and enhancement of the offered information.
Apart from the previously mentioned advantages of the use of nanoparticles as nanoprobes (nanocontrast agents), it is believed that they can offer further possibilities. The simultaneous use of the nanoparticles as hyperthermia therapeutic agents and THz imaging can achieve both diagnosis in early stages of cancer 59 and therapy. Furthermore, THz imaging techniques can be applied for monitoring drug-delivery processes 59, 60 and finally, the use of infrared laser beams for imaging with THz set-ups opens the horizons for real practical THz endoscopy 58.
Nanotechnology-supported new THz sources
One of the most suitable candidates for the development of compact THz sources is the quantum dot (QD) system, although the emission in the THz range has not yet been accomplished. QDs have dimensions between nanometres to a few microns and are characterised by the fact that they contain a tiny droplet of free electrons. Their size, shape and number of electrons can be precisely controlled depending on their possible applications. QDs are very attractive due to their intrinsic discreet energy level. After confirmation of the long carrier relaxation times and the ability to control these times, the way forward for QD-based THz optoelectronic devices was opened 61. Takatori et al. 62 demonstrated that InAc/GaAs (indium monoarsenide/Gallium arsenide) QDs have the potential to be intense terahertz emitters and recently the generation of THz radiation from InAc/GaAs quantum-based photoconductive antennae was achieved 63. Moreover, a novel methodology for varying the QD growth parameters for manipulating the band gap in the THz emission range has been demonstrated 64. In order to achieve 40 meV differences between intra-bands (E 1 and E 2), which is a necessary condition for intra-band THz emission, the effect of growth and monolayer coverage on the energy difference between the ground and excited states of two types of QD structures was investigated 64. In a recent publication, a method for generating dual (or multiple) high-power THz difference frequency from a single QD laser diode was demonstrated 65.
One other way that nanotechnology can innovate the THz sources, is the novel production of pulsed THz radiation by using nanostructured materials. It has been demonstrated by a group in Glasgow, UK, that appropriate nano-engineered surfaces can enhance THz radiation through surface plasmons (SPs) under a femtosecond laser simulation 66. Initially, the emission of terahertz signal due to SPs has been confirmed from metal grating 66, nanoparticle (ZnSe) surface 67 and nanograin surface (ZnSe) 68, while the studies have been expanded to different types of metal surfaces (mainly gold), such as nanoparticles, nanoparticle rings and pyramid-shaped particles 69. When light interacts with metallic nanosurfaces, non-linear optical phenomena are enhanced due to the strong interactions and the high field strengths that are produced 66. This concept enables the production of a terahertz pulse due to a new process of rectification. Gao and colleagues stated that this phenomenon is a consequence of the electrons' acceleration, which is a result of the surface plasmon excitation and believed that the mechanism is related to the multiphoton photoelectric effect 69. In the case of surface nanoparticles, the mechanism of THz emission can be described in terms of dipole orientation 67.
Apart from QDs and nanostructure materials, nanotubes, and especially CNTs, is another innovative nanotechnology area that is expected to highly influence THz emission and detection (see next section) of terahertz radiation. CNTs are molecular-scale tubes of graphitic carbon and were invented in 1991 by Iijima at Nec Fundamental Research Laboratories (Tsukuba Science City, Japan) 70 and can be used in a variety of ways. Simulation studies have shown that CNT antenna properties can be improved by controlling the length, inter-tube distance and the number of nanotube elements so as to achieve better design of CNT-based sources/detectors for THz studies 71. Recently, the traverse vibration of a novel composite NT was studied 72. This NT was synthesized by coating CNT with piezoelectric zinc oxide (ZnO), which is a bio-safe and biocompatible material. The results showed than ZnO-CNT can be used for gigahertz/terahertz electromechanical nanoresonators. What is more, the tubular shape of CNT offers sharp tips that are appropriate for field emission, and consequently with the discovery of CNT a new class of field emitters was generated 73. CNT bundle arrays have been developed as components of cathodes and have shown very promising results 73. The CNTs were arranged as arrays and found to be appropriate for cold cathodes and able to operate at low voltages. It is believed that a high field enhancement, which produces efficient field emission, is caused by rearrangement of the free ends and outliers under an applied field, and is the reason of the bundle arrays' high emission 73. Furthermore, in their work Manohara et al. 73 showed that a highly compact field emission electron gun can be formed with monolithic integration of multiple electrodes. This technique enables electron-beam shaping and a novel miniature electron gun can be fabricated. In an older study, Manohara and colleagues presented the Nanoclystron, which is a novel micro-tube THz source 74. In their circuit, the THz emission is achieved by CNTs, which are performing as electron emitters, and silicon-based reflex klystron-type cavities 74. The use of highly ordered CNT arrays for THz emission has also recently demonstrated 73.
Gamziha et al. (2011) are working towards miniaturised vacuum electronic devices that will be able to be used as high power THz sources 75. One of the methods they have proposed is nanomaching, which offers many advantages such as rapid prototyping of any circuit. Their recent work on the development of a 0.22 THz circuit using nanocomputer numerical control (NCNC) presented very promising results. NCNC combined with UV lithography was also used by Shin et al. 76 in order to develop a Travelling wave tube circuit for high power and broadband terahertz applications. The circuit was fabricated with ~50 nm surface roughness and a cascaded nanocomposite cathode was synthesized, a fact which opens the way for the future development of watt-level terahertz radiation sources.
Nanotechnology-supported new THz detectors
In the previous section, it was shown that QDs are very attractive for the generation of new THz sources. QDs could also innovate the existing sensors for detecting and sensing terahertz radiation. QDs have been shown to be able to detect single THz photons with or without the assistance of a magnetic field 77. Furthermore, carbon nanotube quantum dots can be used for developing highly sensitive detectors, which can also be frequency adjustable in the THz region 78, 79. Also, in a very recent publication, nanoscale carbon material was used for fabricating a tuneable quantum-dot sensing device 80. Additionally, QD-type detectors can expand the performance of THz detectors at temperatures where state-of-the art THz detectors have limited sensitivity 81. A novel sensor consisting of a QD (GaAS/AlGaAs QD), an electron reservoir and a superconducting single-electron transistor was presented with a cutting-edge performance 82. The detector operating at temperatures below 1 K was able to perform single-THz photon counting by relying on photon-to-plasmon and plasmon-to-charge conversion, followed by charge measurement in a single-shot mode.
As in the case of QDs, nanotubes/CNTs can also be used for detection purposes with several ways as nanoantenna 71, 83, bolometers 84 and even by using their mechanical properties 85. Furthermore, CNTs can be coated with bio-safe and biocompatible materials, like piezoelectric zinc oxide (ZnO), and be safely used as terahertz electromechanical nanoresonators 72. Their unique characteristics, like small junction areas, high electron mobilities and low estimated capacitances make them more attractive than solid-state components 42. CNTs, especially multi-walled CNTs, can be used as antennas in THz detectors since it has been shown that they interact with light in the same manner as simple dipole radio antennas 83. The polarisation and the length antenna effect are the key phenomena that can be used in optoelectronic devices. Furthermore, simulations have shown that CNT antenna arrays have better performance than single CNTs, and optimal design for receivers can be achieved. Very recently a novel resonant detector of THz radiation based on mechanically floating CNTs was presented 85. The detector consists of two electrically coupled single-wall-CNTs, which lie parallel over an insulator and are characterised by a proportional response of the plasma-mechanical resonance to the mechanical oscillation quality factor. The detector output signal depends on an AC displacement current that occurs between the CNTs due to plasma-mechanical oscillations of CNTs. Nanotubes can also be used as bolometers. Of course the need for new, better performing, detectors has not been driven only from the field of THz medical imaging. Astrophysicists studying the universe radiation at THz radiation require improvement of the sensitivity of current bolometers 86. To achieve this goal, the bolometers should be thermally isolated from the environment and have a very small capacity 87. The development of nanobolometers would deliver the required characteristics and enables high sensitivity of even single THz photons 88. The development of this kind of nanotechnology-based detectors pushes research to its current limits and possible future applications might be found in areas other than that of astronomy.
For some researchers, the development of novel electronic and semiconductor devices might improve and overcome many of the drawbacks that characterise current THz technology. In this direction, Balocco and his colleagues demonstrated novel planar nanodiodes, which are able both to emit and detect THz radiation at room temperature 20. The diode concept relies on asymmetric device nanochannels and showed high efficiency and speed sensitivity. Additionally, nanostructure physics principles and an antennae approach were used for fabricating a compact THz detector performing at room temperature 89. The inventors believed that these structures could contribute to the development of cost effective, compact and room-temperature operating THz emitters and detectors. One other significant limitation of current THz systems is that the detector and probe are not located close enough. As a consequence there is an affect on the sensitivity. New opportunities for high-resolution imaging can be achieved by integrating all the detection components on a semiconductor chip 90. In the future nanotechnology could facilitate this by providing the tools for minimising the dimensions of all the components of THz imaging set-ups. Generally, there are many perspectives on nanotechnology concerning terahertz electronics and many novel electronic components are expected to be developed 91.
Nanotechnology for general THz purposes
In this section, it will be briefly discussed how nanotechnology can support THz imaging set-ups in areas that do not correspond directly to one of the previous discussed concepts (THz sources, detectors and contrast agents).
As already mentioned, terahertz radiation remained for many years unexplored and consequently a complete characterisation of its properties, physical characteristics and occurring phenomena have not yet been fully understood. An area that was affected by the absence of high-power THz sources is the study of the THz non-linear phenomena. In this direction, new sources that use nanotechnology will provide a useful tool for researchers in this field. Furthermore, other nanotechnology-based techniques could help the study of THz non-linear principles. For instance, nanostructures (nanoslits and nanogap split-ring resonators) were used in order to enhance the electric field and extend THz experiments into the non-linear regime 92. The understanding of the THz-related non-linear phenomena might allow their application for medical imaging or other biomedical purposes as it happens with the non-linear optical phenomena.
One important limitation of THz imaging techniques is the limited imaging resolution 47. One way that nanotechnology could help in minimising this drawback is the guide and focus of THz radiation by the use of SPPs on corrugated wires 93. In addition, THz propagation on wires is the key area for the development of probes for biological investigations. It has been demonstrated that propagation on wires with a size around a nanometre can be achieved 94. The small size of the wire opens the way for the development of MicroElectroMechanical Systems with sub-micrometre spatial resolution 94. These systems can be applied for the THz spectroscopy of biomolecules in biological entities. Other nanotechnology techniques can also be applied in the same direction for the collection of THz spectroscopic signatures from individual biological molecules. For instance, a single-electron source and a THz radiation detection cell can compose a coupled three-quantum-dot structure that can be applied for single-molecule spectroscopy studies 95. These novel THz spectroscopy techniques can be simultaneously used in the future with THz imaging methods.
THz imaging for nanotechnology
The relationship between nanotechnology and THz is bidirectional, in the sense that the concurrent developments can contribute to both technologies. THz modalities have helped the expansion of nanotechnology. For instance, THz has made significant contributions in the study of semiconductor nanocrystals and quantum dots in recent years 23. It is widely believed that terahertz nanoscopy will advance the development of new novel nanostructures since it overcomes the limited spatial resolution due to the diffraction limits of the traditional optical microscopy techniques 96. Terahertz nanoscopy could be achieved by the development of novel probes for THz-scattering near-field optical microscopy (THZ-SNOM). The same concept has also been applied in the IR region with very promising results 97. In the scattering-type of SNOM, optical amplitude and phase images with nanoscale resolution are formed. In this technique, Atomic Force Microscope tips are illuminated with laser and the elastically scattering light is recorded interferometrically 98. Moreover, a resolution better than 40 nm was recently achieved by using IR and THz illumination 96, since the resolution depends on the sharpness of the tip and not to the wavelength. Compared with other imaging modalities, it has the advantage of rich spectral contrast and consequently it can provide information concerning chemical composition, structural status and conductivity 99. The possible applications of the technique are many and very promising. Recently it has been demonstrated that the method can be used for quantitative mapping of the local carrier concentration and mobility at the nanometre scale of different materials 100. Finally, one very interesting field concerning nanotechnology is the development of techniques and methods that can be used for detecting and tracking nanoparticles especially in human bodies. A recent publication showed that nanoscale metal barriers embedded in nanoslot antennas can be used to detect a single nanoparticle 101.
Safety issues and ethical considerations
Although THz imaging set-ups use non-ionising radiation, risk issues must be considered since hazards can arise from a variety of mechanisms other than ionisation 102. Taking into account that emerging modalities are so new, it is obvious that a number of phenomena remain unexplored and their possible effects are unknown. These concepts are becoming even more complicated when nanotechnology-based techniques are used, since this innovative field is still in its infancy. The possible biological effects when electromagnetic (EM) radiation reacts with tissue include: thermal, acoustic, optical and photochemical mechanisms and their combinations 25. The importance of the effects that EM radiation might have in humans is highlighted by the number of international and national bodies that are interested in the guidelines in relation to its effects 102. A full and detailed understanding of the optical properties of tissues at THz radiation is necessary in order to achieve a safe in vivo image with THz imaging. Early studies have shown that the adsorption relies on the hydration of each tissue and tissues that are characterised by low water content possess a lower attenuation coefficient 102, 103. These results suggest that clinical imaging could be feasible only for certain applications and appropriate clinical protocols must be developed.
Initial safety analysis, based on the available guidelines for skin exposure to radiation of 15–115 GHz, determined the maximum permissible exposure (MPE) 25, 103. The results showed that, according to the currently available guidelines, THz imaging set-ups are safe. But it must be noted that the majority of the published guidelines concerning THz radiation take into account only the heating effects and ignore other possibly damaging effects, e.g. thermo-mechanical damage. Furthermore, the guidelines were established for specific exposure durations and these are not always appropriate for THz imaging purposes 102.
Research in the area is still in progress and as new applications are emerging, a full understanding of the THz-tissue interaction mechanism is imperative. For example, communication systems have started using frequencies of 300 GHz and above, which are not yet regulated 104. In order for THz radiation to be used as a biomedical tool, researchers are trying to develop compact THz spectrometers that can be used for measuring optical properties of biological tissues 105 and further investigations on the biological effects of THz radiation have been performed. Recently it has been shown that the 2.52 THz radiation generated primarily thermal effects in cells (fibroblasts) and thermal damage models can be used to predict the THz bioeffects 106. A detailed review of the current state of the research on the biological effects of THz radiation can be found in a recent review paper, where projects, official regulations and publications are summarised 107.
On the other hand, all medical nanotechnology-based techniques require special attention since possible risk, toxicity, ethical and social considerations must be taken into account 2. This area cannot be ignored since nanotechnology is a new and still unknown field. Shape, size and morphology play a significant role in bio-toxicity, since at low dimensions, the surface increases and as a result has higher reactivity. The hazards due to nanoparticle toxicity are crucial 108 and our current knowledge of the toxicity of the chemicals and materials is not sufficient when materials have dimensions in the nanoscale 109. Moreover, due to their small size nanoparticles can penetrate humans through three paths: skin, breathing (mouth and/or nose) and digestive system (mouth) 110. None of these three possible ways has been well studied at the moment. The risk of nanoparticles does not appear only during their application but also in every stage of their cycle from their production to transfer 111, 112, while possible environment pollution cannot be ignored 113– 115. There is evidence that nanoparticles are responsible for unusual diseases 116 and what is more, the physical and biological mechanisms that are involved when nanoparticles are exposed to any kind of radiation within biological tissues remain unknown. Nonetheless, there is still a huge absence of clear regulatory guidelines, safety standards and MPE approvals for almost all the nanotechnology-related techniques 117. Concerning regulation, the history of previous technologies must be taken into account so as to avoid repeating past mistakes 118. Since nanotechnology is still in its infancy, new risks, ethical challenges and issues related with privacy and justice will arise while nanotechnology moves from research to clinical practice 117. Finally, a crucial point has to do with how society will react and how willing it is to accept an innovative technology that has not yet proved to be either effective or safe 119.
Conclusions
Throughout this review, we have shown that nanotechnology can support emerging THz imaging modality in order to overcome some of its limitations. This can be achieved in many nanotechnology-based techniques and several drawbacks can be overcome, from the low performance of the emitting source to the miniaturisation of the whole set-up. These research results indicate that nanotechnology could help in the development of high-resolution, sensitive and portable detectors and new efficient sources for THz imaging purposes. What is more, the use of nanoparticles as contrast agents can enhance THz signals and detection, not only from healthy areas but also from specific pathological areas such as tumours. Although the techniques are in their infancy, it seems possible that nanotechnology may be applied to help THz imaging modality find its way into real everyday clinical use.
Acknowledgments
AS would like to gratefully and sincerely thank Dr. Marck McJury who introduced AS to this topic as well as for his guidance, understanding and support during his graduate studies at The Open University, UK.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
v1; ref status: indexed
References
- 1.Feynman RP: There's plenty of room at the bottom: An invitation to enter a new field of physics. Eng Sci. 1960;23(5):22–36 Reference Source [Google Scholar]
- 2.Allhoff F: The coming era of nanomedicine. Am J Bioeth. 2009;9(10):3–11 10.1080/15265160902985027 [DOI] [PubMed] [Google Scholar]
- 3.Dupas C, Houdy P, Lahmani M: Nanoscience: Nanotechnologies and nanophysics. Springer, Berlin;2004. 10.1007/978-3-540-28617-2 [DOI] [Google Scholar]
- 4.Pifer LL, Kenwright K: Nanotechnology: A coming clinical laboratory revolution. Clin Lab Sci. 2010;23(2):107–11 [PubMed] [Google Scholar]
- 5.Roszek B, de Jong WH, Geertsma RE: Nanotechnology in medical applications: State-of-the-art in materials and devices. RIVM report 265001001;2005. Reference Source [Google Scholar]
- 6.Shah RR: Nano-technology: An emerging industrial revolution of 21st century. Electron Inf Plann. 2004;31(3–4):78–81 [Google Scholar]
- 7.Dempster T: Analysis: Regional strengths. Driving a new industrial revolution. Manuf Engin. 2006;85(2):8–9 10.1049/me:20060213 [DOI] [Google Scholar]
- 8.Boulaiz H, Alvarez PJ, Ramirez A, et al. : Nanomedicine: Application areas and development prospects. Int J Mol Sci. 2011;12(5):3303–21 10.3390/ijms12053303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García Jordá E: Fantastic voyage. Clin Transl Oncol. 2009;11(9):561–3 10.1007/s12094-009-0405-z [DOI] [PubMed] [Google Scholar]
- 10.Wolbarst AB, Hendee WR: Evolving and experimental technologies in medical imaging. Radiology. 2006;238(1):16–39 10.1148/radiol.2381041602 [DOI] [PubMed] [Google Scholar]
- 11.Humphreys K, Loughran JP, Gradziel M, et al. : Medical applications of terahertz imaging: A review of current technology and potential applications in biomedical engineering. Conf Proc IEEE Eng Med Biol Soc.EMBC 2004; 1–5 September 2004; San Francisco, CA,2004;2:1302–5 10.1109/IEMBS.2004.1403410 [DOI] [PubMed] [Google Scholar]
- 12.Wallace VP, Pickwell-MacPherson E, Reid C: The future of medical imaging. 35th international conference on infrared, millimeter, and terahertz waves, IRMMW-THz 2010; 5–10 September 2010; Rome;2010. 10.1109/ICIMW.2010.5612385 [DOI] [Google Scholar]
- 13.Tonouchi M: Cutting-edge terahertz technology. Nat Photon. 2007;1(2):97–105 10.1038/nphoton.2007.3 [DOI] [Google Scholar]
- 14.Arnone D, Ciesla C, Michael P: Terahertz imaging comes into view. Phys World. 2000;13(4):35–40 Reference Source [Google Scholar]
- 15.Chapter 7: Medical opportunities using THz radiation [Internet]. Opportunities in THz Science, Report of a DOE-NSF-NIH Workshop, Arligton VA;2004. Reference Source [Google Scholar]
- 16.Zhang C, Mu K: Applications of terahertz imaging. CLEO/Pacific rim 2009 - 8th pacific rim conference on lasers and electro-optics; 30 August–3 September 2009; Shanghai;2009. Reference Source [Google Scholar]
- 17.Davies AG, Linfield EH, Johnston MB: The development of terahertz sources and their applications. Phys Med Biol. 2002;47(21):3679–89 10.1088/0031-9155/47/21/302 [DOI] [PubMed] [Google Scholar]
- 18.Jansen C, Wietzke S, Peters O, et al. : Terahertz imaging: Applications and perspectives. Appl Opt. 2010;49(19):E48–57 10.1364/AO.49.000E48 [DOI] [PubMed] [Google Scholar]
- 19.Sakai K: A quest of a new eye for terahertz-waves region. Phys Procedia. 2010;3(2):1115–9 10.1016/j.phpro.2010.01.148 [DOI] [Google Scholar]
- 20.Balocco C, Kasjoo SR, Lu X, et al. : Novel terahertz nanodevices and circuits. 10th IEEE international conference on solid-state and integrated circuit technology; 1–4 November 2010; Shanghai;2010p. 1176–9 10.1109/ICSICT.2010.5667595 [DOI] [Google Scholar]
- 21.Pickwell E, Wallace VP: Biomedical applications of terahertz technology. J Phys D. 2006;39(17):R301–10 10.1088/0022-3727/39/17/R01 [DOI] [Google Scholar]
- 22.Hu BB, Nuss MC: Imaging with terahertz waves. Opt Lett. 1995;20(16):1716 [DOI] [PubMed] [Google Scholar]
- 23.Jepsen PU, Cooke DG, Koch M: Terahertz spectroscopy and imaging - modern techniques and applications. Laser Photon Rev. 2011;5(1):124–66 10.1002/lpor.201000011 [DOI] [Google Scholar]
- 24.Lee D, Kim H, Kim T, et al. : Characteristics of gadolinium oxide nanoparticles as contrast agents for terahertz imaging J Infrared Millim Terahertz Waves. 2011;32(4):506–12 10.1007/s10762-011-9776-7 [DOI] [Google Scholar]
- 25.Berry E, Walker GC, Fitzgerald AJ, et al. : Do in vivo terahertz imaging systems comply with safety guidelines? J Laser Appl. 2003;15(3):192–8 10.2351/1.1585079 [DOI] [Google Scholar]
- 26.Mueller ER: Terahertz radiation: Applications and sources. Ind Phys. 2003;9(4):27–9 Reference Source [Google Scholar]
- 27.Baxter JB, Guglietta GW: Terahertz spectroscopy. Anal Chem. 2011;83(12):4342–68 10.1021/ac200907z [DOI] [PubMed] [Google Scholar]
- 28.Wallace VP, Fitzgerald AJ, Shankar S, et al. : Terahertz pulsed imaging of basal cell carcinoma ex vivo and in vivo. Br J Dermatol. 2004;151(2):424–32 10.1111/j.1365-2133.2004.06129.x [DOI] [PubMed] [Google Scholar]
- 29.Woodward RM, Cole BE, Wallace VP, et al. : Terahertz pulse imaging in reflection geometry of human skin cancer and skin tissue. Phys Med Biol. 2002;47(21):3853–63 10.1088/0031-9155/47/21/325 [DOI] [PubMed] [Google Scholar]
- 30.Woodward RM, Wallace VP, Pye RJ, et al. : Terahertz pulse imaging of ex vivo basal cell carcinoma. J Invest Dermatol. 2003;120(1):72–8 10.1046/j.1523-1747.2003.12013.x [DOI] [PubMed] [Google Scholar]
- 31.Wallace VP, Fitzgerald AJ, Pickwell E, et al. : Terahertz pulsed spectroscopy of human Basal cell carcinoma. Appl Spectrosc. 2006;60(10):1127–33 10.1366/000370206778664635 [DOI] [PubMed] [Google Scholar]
- 32.Ashworth PC, Pickwell-MacPherson E, Provenzano E, et al. : Terahertz pulsed spectroscopy of freshly excised human breast cancer. Opt Express. 2009;17(15):12444–54 10.1364/OE.17.012444 [DOI] [PubMed] [Google Scholar]
- 33.Oh SJ, Maeng I, Hee JS, et al. : Nanoparticle contrast agents for terahertz medical imaging. 33rd international conference on infrared and millimeter waves and the 16th international conference on terahertz electronics, 2008, IRMMW-THz 2008; 15–19 September 2008; Pasadena, CA;2008. Reference Source [Google Scholar]
- 34.Abril J, Nova E, Capdevila S, et al. : Active and passive THz systems for short-range imaging applications. 4th european conference on antennas and propagation, EuCAP 2010; 12–16 April 2010; Barcelona;2010. Reference Source [Google Scholar]
- 35.Bogue R: Terahertz imaging: A report on progress. Sens Rev. 2009;29(1):6–12 10.1108/02602280910926706 [DOI] [Google Scholar]
- 36.Stanko S, Nötel D, Wahlen A, et al. : Active and passive mm-wave imaging for concealed weapon detection and surveillance. 33rd international conference on infrared and millimeter waves and the 16th international conference on terahertz electronics, 2008, IRMMW-THz 2008; 15–19 September 2008; Pasadena, CA;2008;1–2 10.1109/ICIMW.2008.4665741 [DOI] [Google Scholar]
- 37.Duncan WD, Schwall RE, Irwin KD, et al. : An optical system for body imaging from a distance using near-TeraHertz frequencies. J Low Temp Phys. 2008;151(3–4 PART 2):777–83 10.1007/s10909-008-9746-1 [DOI] [Google Scholar]
- 38.Bründermann E, Chamberlin DR, Haller EE: High duty cycle and continuous terahertz emission from germanium. Appl Phys Lett. 2000;76(21):2991–3 10.1063/1.126555 [DOI] [Google Scholar]
- 39.Bergner A, Heugen U, Bründermann E, et al. : New p-ge THz laser spectrometer for the study of solutions: THz absorption spectroscopy of water. Rev Sci Instrum. 2005;76(6):063110 10.1063/1.1928427 [DOI] [Google Scholar]
- 40.Hübers H, Pavlov SG, Shastin VN: Terahertz lasers based on germanium and silicon. Semicond Sci Technol. 2005;20(7):S211–21 10.1088/0268-1242/20/7/011 [DOI] [Google Scholar]
- 41.Ito H, Nakajima F, Furuta T, et al. : Continuous THz-wave generation using antenna-integrated uni-travelling-carrier photodiodes. Semicond Sci Technol. 2005;20(7):S191–8 10.1088/0268-1242/20/7/008 [DOI] [Google Scholar]
- 42.Sizov F, Rogalski A: THz detectors. Prog Quantum Electron. 2010;34(5):278–347 10.1016/j.pquantelec.2010.06.002 [DOI] [Google Scholar]
- 43.Nagatsuma T: Terahertz technologies: Present and future. IEICE Electron Express. 2011;8(14):1127–42 10.1587/elex.8.1127 [DOI] [Google Scholar]
- 44.Chattopadhyay G: Technology, capabilities, and performance of low power terahertz sources. IEEE Trans Terahertz Sci Technolog. 2011;1(1):33–53 10.1109/TTHZ.2011.2159561 [DOI] [Google Scholar]
- 45.Ji YB, Lee ES, Kim SH, et al. : A miniaturized fiber-coupled terahertz endoscope system. Opt Express. 2009;17(19):17082–7 10.1364/OE.17.017082 [DOI] [PubMed] [Google Scholar]
- 46.Zhang Xi, Xu J: Introduction to THz wave photonics. New York: Springer;2010. 10.1007/978-1-4419-0978-7 [DOI] [Google Scholar]
- 47.Johnston MB: Plasmonics: Superfocusing of terahertz waves. Nat Photon. 2007;1(1):14–15 10.1038/nphoton.2006.60 [DOI] [Google Scholar]
- 48.Joseph CS, Yaroslavsky AN, Neel VA, et al. : Continuous wave terahertz transmission imaging of nonmelanoma skin cancers. Lasers Surg Med. 2011;43(6):457–62 10.1002/lsm.21078 [DOI] [PubMed] [Google Scholar]
- 49.Taylor ZD, Singh RS, Bennett DB, et al. : THz medical imaging: In vivo hydration sensing. IEEE Trans Terahertz Sci Technolog. 2011;1(1):201–19 10.1109/TTHZ.2011.2159551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hailu DM, Ehtezazi IA, Safavi-Naeini S: Terahertz imaging of biological samples. 2010 IEEE international symposium on antennas and propagation and CNC-USNC/URSI radio science meeting - leading the wave, AP-S/URSI 2010; 11–17 July 2010; Toronto, ON;2010. 10.1109/APS.2010.5561667 [DOI] [Google Scholar]
- 51.Bennett DB, Taylor ZD, Tewari P, et al. : Terahertz sensing in corneal tissues. J Biomed Opt. 2011;16(5):057003 10.1117/1.3575168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shan L, Chopra A, Leung K, et al. : Characterization of nanoparticle-based contrast agents for molecular magnetic resonance imaging. J Nanopart Res. 2012;14(9):1122 10.1007/s11051-012-1122-z [DOI] [Google Scholar]
- 53.Rümenapp C, Gleich B, Haase A: Magnetic nanoparticles in magnetic resonance imaging and diagnostics. Pharm Res. 2012;29(5):1165–79 10.1007/s11095-012-0711-y [DOI] [PubMed] [Google Scholar]
- 54.Pan D, Caruthers SD, Senpan A, et al. : Revisiting an old friend: Manganese-based MRI contrast agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(2):162–73 10.1002/wnan.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sirotkina MA, Shirmanova MV, Bugrova ML, et al. : Continuous optical coherence tomography monitoring of nanoparticles accumulation in biological tissues. J Nanopart Res. 2011;13(1):283–91 10.1007/s11051-010-0028-x [DOI] [Google Scholar]
- 56.Tseng HY, Lee CK, Wu SY, et al. : Au nanorings for enhancing absorption and backscattering monitored with optical coherence tomography. Nanotechnology. 2010;21(29):295102 10.1088/0957-4484/21/29/295102 [DOI] [PubMed] [Google Scholar]
- 57.Son JH: Terahertz electromagnetic interactions with biological matter and their applications. J Appl Phys. 2009;105(10):102033 10.1063/1.3116140 [DOI] [Google Scholar]
- 58.Oh SJ, Kang J, Maeng I, et al. : Nanoparticle-enabled terahertz imaging for cancer diagnosis. Opt Express. 2009;17(5):3469–75 10.1364/OE.17.003469 [DOI] [PubMed] [Google Scholar]
- 59.Oh SJ, Choi J, Maeng I, et al. : High-sensitivity terahertz imaging technique using nanoparticle probes for medical applications. 2010 IEEE photonics society winter topicals meeting, WTM 2010; 11–13 January 2010; Majorca;2010;52–3 10.1109/PHOTWTM.2010.5421967 [DOI] [Google Scholar]
- 60.Oh SJ, Choi J, Maeng I, et al. : Molecular imaging with terahertz waves. Opt Express. 2011;19(5):4009–16 10.1364/OE.19.004009 [DOI] [PubMed] [Google Scholar]
- 61.Zibik EA, Grange T, Carpenter BA, et al. : Long lifetimes of quantum-dot intersublevel transitions in the terahertz range. Nat Mater. 2009;8(10):803–7 10.1038/nmat2511 [DOI] [PubMed] [Google Scholar]
- 62.Takatori S, Minh PH, Estacio E, et al. : Investigation of the terahertz emission characteristics of MBE-grown GaAs-based nanostructures. Optical Materials. 2010;32(7):776–9 10.1016/j.optmat.2010.02.014 [DOI] [Google Scholar]
- 63.Daghestani NS, Cataluna MA, Berry G, et al. : Terahertz emission from InAs/GaAs quantum dot based photoconductive devices. Appl Phys Lett. 2011;98(18):181107 10.1063/1.3586774 [DOI] [Google Scholar]
- 64.Ngo CY, Yoon SF, Teng JH: Bandgap engineering of 1.3 μm quantum dot structures for terahertz (THz) emission. J Cryst Growth. 2011;323(1):211–4 10.1016/j.jcrysgro.2010.11.132 [DOI] [Google Scholar]
- 65.Leyman R, Nikitichev DI, Bazieva N, et al. : Multimodal spectral control of a quantum-dot diode laser for THz difference frequency generation. Appl Phys Lett. 2011;99(17):171107 10.1063/1.3654154 [DOI] [Google Scholar]
- 66.Welsh GH, Hunt NT, Wynne K: Terahertz-pulse emission through laser excitation of surface plasmons in a metal grating. Phys Rev Lett. 2007;98(2):026803 10.1103/PhysRevLett.98.026803 [DOI] [PubMed] [Google Scholar]
- 67.Wu XJ, Chen XS, Zhao Y, et al. : Optical generation terahertz radiation from ZnSe surface nanoparticles. Joint 32nd international conference on infrared and millimetre waves, and 15th international conference on terahertz electronics, IRMMW-THz2007; 3–7 September 2007; Cardiff;2007p. 464–5 Reference Source [Google Scholar]
- 68.He S, Chen X, Wu X, et al. : Enhanced terahertz emission from ZnSe nano-grain surface. J Lightwave Technol. 2008;26(11):1519–23 10.1109/JLT.2008.923217 [DOI] [Google Scholar]
- 69.Gao Y, Chen MK, Yang CE, et al. : Analysis of terahertz generation via nanostructure enhanced plasmonic excitations. J Appl Phys. 2009;106(7):074302 10.1063/1.3236629 [DOI] [Google Scholar]
- 70.Iijima S: Helical microtubules of graphitic carbon. Nature. 1991;354(6348):56–8 10.1038/354056a0 [DOI] [Google Scholar]
- 71.Wang Y, Wu Q, Shi W, et al. : Radiation properties of carbon nanotubes antenna at terahertz/infrared range. Int J Infrared Millim Waves. 2008;29(1):35–42 10.1007/s10762-007-9306-9 [DOI] [Google Scholar]
- 72.Wang CY, Adhikari S: ZnO-CNT composite nanotubes as nanoresonators. Phys Lett Sect A Gen At Solid State Phys. 2011;375(22):2171–5 10.1016/j.physleta.2011.04.031 [DOI] [Google Scholar]
- 73.Manohara HM, Toda R, Lin RH, et al. : Carbon nanotube bundle array cold cathodes for THz vacuum tube sources. J Infrared Millim Terahertz Waves. 2009;30(12):1338–50 10.1007/s10762-009-9547-x [DOI] [Google Scholar]
- 74.Manohara HM, Siegel PH, Bronikowki MJ, et al. : Development of a micromachined THz nanoklystron: A status report. IEEE international conference on plasma science;2004;p. 421 10.1109/PLASMA.2004.1340211 [DOI] [Google Scholar]
- 75.Gamzina D, Barchfeld R, Barnett LR, et al. : Nano CNC milling technology for terahertz vacuum electronic devices. IEEE international vacuum electronics conference, IVEC-2011; 21–24 February 2011; Bangalore;2011p. 345–6 10.1109/IVEC.2011.5747017 [DOI] [Google Scholar]
- 76.Shin YM, Zhao J, Barnett LR, et al. : Investigation of terahertz sheet beam traveling wave tube amplifier with nanocomposite cathode. Phys Plasmas. 2010;17(12):123105 10.1063/1.3525098 [DOI] [Google Scholar]
- 77.Komiyama S, Astafiev O, Antonov V, et al. : Single-photon detection of THz-waves using quantum dots. Microelectron Eng. 2002;63(1–3):173–8 10.1016/S0167-9317(02)00608-1 [DOI] [Google Scholar]
- 78.Kawano Y, Fuse T, Toyokawa S, et al. : Terahertz photon-assisted tunneling in carbon nanotube quantum dots. J Appl Phys. 2008;103(3):034307 10.1063/1.2838237 [DOI] [Google Scholar]
- 79.Kawano Y, Fuse T, Toyokawa S, et al. : Highly sensitive and frequency-tunable THz detector using carbon nanotube quantum dots. 33rd international conference on infrared and millimeter waves and the 16th international conference on terahertz electronics, 2008, IRMMW-THz 2008; 15–19 September 2008; Pasadena, CA;2008. 10.1109/ICIMW.2008.4665488 [DOI] [Google Scholar]
- 80.Mahjoub AM, Motooka S, Aoki N, et al. : Towards graphene GHz/THz nanosensor. Jpn J Appl Phys. 2011;50(7):070119 10.1143/JJAP.50.070119 [DOI] [Google Scholar]
- 81.Kleinschmidt P, Giblin SP, Antonov V, et al. : A highly sensitive detector for radiation in the terahertz region. IEEE Trans Instrum Meas. 2007;56(2):463–7 10.1109/TIM.2007.891146 [DOI] [Google Scholar]
- 82.Hashiba H, Antonov V, Kulik L, et al. : Sensing individual terahertz photons. Nanotechnology. 2010;21(16):165203 10.1088/0957-4484/21/16/165203 [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Kempa K, Kimball B, et al. : Receiving and transmitting light-like radio waves: Antenna effect in arrays of aligned carbon nanotubes. Appl Phys Lett. 2004;85(13):2607–9 10.1063/1.1797559 [DOI] [Google Scholar]
- 84.Yngvesson KS, Fu K, Fu B, et al. : Experimental detection of terahertz radiation in bundles of single wall carbon nanotubes. 19 thInt. Symp. Space THz Techn.2008;304–13 Reference Source [Google Scholar]
- 85.Stebunov Y, Leiman V, Arsenin A, et al. : Detection of modulated terahertz radiation using combined plasma and mechanical resonances in double-carbon-nanotube device. Appl Phys Express. 2011;4(7):075101 10.1143/APEX.4.075101 [DOI] [Google Scholar]
- 86.Karasik BS, Pereverzev SV, Wei J, et al. : Ultrasensitive hot-electron nanobolometers for terahertz astrophysics. 33rd international conference on infrared and millimeter waves and the 16th international conference on terahertz electronics, 2008, IRMMW-THz 2008; Nat Nanotechnol. 2008;3(8):496–500 10.1038/nnano.2008.173 [DOI] [PubMed] [Google Scholar]
- 87.Wei J, Olaya D, Karasik BS, et al. : Ultrasensitive hot-electron nanobolometers for terahertz astrophysics. Nat Nanotechnol. 2008;3(8):496–500 10.1038/nnano.2008.173 [DOI] [PubMed] [Google Scholar]
- 88.Karasik BS, Sergeev AV, Prober DE: Nanobolometers for THz photon detection. IEEE Trans Terahertz Sci Technolog. 2011;1(1):97–111 10.1109/tthz.2011.2159560 [DOI] [Google Scholar]
- 89.Seliuta D, Kašalynas I, Tamošiunas V, et al. : Silicon lens-coupled bow-tie InGaAs-based broadband terahertz sensor operating at room temperature Electron Lett. 2006;42(14):825–7 10.1049/el:20061224 [DOI] [Google Scholar]
- 90.Kawano Y, Ishibashi K: An on-chip near-field terahertz probe and detector. Nat Photon. 2008;2(10):618–21 10.1038/nphoton.2008.157 [DOI] [Google Scholar]
- 91.Cha S, Choi JH, Baik CW, et al. : Perspectives on nanotechnology for RF and terahertz electronics. IEEE Trans Microwave Theory Tech. 2011;59(10):2709–2718 10.1109/tmtt.2011.2163728 [DOI] [Google Scholar]
- 92.Merbol H, Bitze A, Feure T: Second harmonic generation based on strong field enhancement in nanostructured THz materials. Opt Express. 2011;19(8):7262–73 10.1364/OE.19.007262 [DOI] [PubMed] [Google Scholar]
- 93.Maier SA, Andrews SR, Martín-Moreno L, et al. : Terahertz surface plasmon-polariton propagation and focusing on periodically corrugated metal wires. Phys Rev Lett. 2006;97(17):176805 10.1103/PhysRevLett.97.176805 [DOI] [PubMed] [Google Scholar]
- 94.Treizebré A, Bocquet B: Investigation on living cells with a THz BioMEMS. Joint 32nd international conference on infrared and millimetre waves, and 15th international conference on terahertz electronics, IRMMW-THz2007; 3–7 September 2007; Cardiff;2007;p. 82–3 Reference Source [Google Scholar]
- 95.Woolard DL, Zhao P: THz detection cell for sub-wavelength bio-molecular sensing. 2007 7th IEEE international conference on nanotechnology - IEEE-NANO 2007; 2–5 August 2007; Hong Kong;2007;p. 320–5 10.1109/NANO.2007.4601199 [DOI] [Google Scholar]
- 96.Huber AJ, Keilmann F, Wittborn J, et al. : Terahertz near-field nanoscopy of mobile carriers in single semiconductor nanodevices. Nano Lett. 2008;8(11):3766–70 10.1021/nl802086x [DOI] [PubMed] [Google Scholar]
- 97.Huber AJ, Ziegler A, Köck T, et al. : Infrared nanoscopy of strained semiconductors. Nat Nanotechnol. 2009;4(3):153–7 10.1038/nnano.2008.399 [DOI] [PubMed] [Google Scholar]
- 98.Hillenbrand R: Infrared and terahertz nanoscopy. 2010 IEEE photonics society summer topical meeting, PHOSST 2010; 19–21 July 2010; Playa del Carmen;2010;p. 58–9 10.1109/PHOSST.2010.5553706 [DOI] [Google Scholar]
- 99.Keilmann F: Viewing the nanoworld in infrared/THz light. 34th international conference on infrared, millimeter, and terahertz waves, IRMMW-THz 2009; 21–25 September 2009; Busan;2009. 10.1109/ICIMW.2009.5324706 [DOI] [Google Scholar]
- 100.Wittborn J, Weiland R, Huber AJ, et al. : Quantitative, nanoscale free-carrier concentration mapping using terahertz near-field nanoscopy. 49th international reliability physics symposium, IRPS 2011; 10–14 April 2011; Monterey, CA;2011;p. 5C.1.1–5C.1.7 10.1109/IRPS.2011.5784523 [DOI] [Google Scholar]
- 101.Park HR, Bahk YM, Ahn KJ, et al. : Controlling terahertz radiation with nanoscale metal barriers embedded in nano slot antennas. ACS Nano. 2011;5(10):8340–5 10.1021/nn2031885 [DOI] [PubMed] [Google Scholar]
- 102.Berry E: Risk perception and safety issues. J Biol Phys. 2003;29(2–3):263–7 10.1023/A:1024461313486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fitzgerald AJ, Berry E, Zinov'ev NN, et al. : Catalogue of human tissue optical properties at terahertz frequencies. J Biol Phys. 2003;29(2–3):123–8 10.1023/A:1024428406218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kleine-Ostmann T, Münter K, Spitzer M, et al. : The electromagnetic environment above 100 GHz Electromagnetic compatibility, personal safety and regulation issues. IRMMW-THz 2006 - 31st international conference on infrared and millimeter waves and 14th international conference on terahertz electronics; 18–22 September 2006; Shanghai;2006;p. 378 10.1109/icimw.2006.368586 [DOI] [Google Scholar]
- 105.Wilmink GJ, Ibey BL, Tongue T, et al. : Development of a compact terahertz time-domain spectrometer for the measurement of the optical properties of biological tissues. J Biomed Opt. 2011;16(4):047006 10.1117/1.3570648 [DOI] [PubMed] [Google Scholar]
- 106.Wilmink GJ, Rivest BD, Roth CC, et al. : In vitro investigation of the biological effects associated with human dermal fibroblasts exposed to 2.52 THz radiation. Lasers Surg Med. 2011;43(2):152–63 10.1002/lsm.20960 [DOI] [PubMed] [Google Scholar]
- 107.Wilmink GJ, Grundt JE: Invited review article: Current state of research on biological effects of terahertz radiation. J Infrared Millim Terahertz Waves. 2011;32(10):1074–1122 10.1007/s10762-011-9794-5 [DOI] [Google Scholar]
- 108.Oberdörster G, Stone V, Donaldson K: Toxicology of nanoparticles: A historical perspective. Nanotoxicology. 2007;1(1):2–25 10.1080/17435390701314761 [DOI] [Google Scholar]
- 109.de Jong WH, Roszek B, Geertsma RE: Nanotechnology in medical applications: Possible risks for human health. [Internet].: RIVM report 265001002;2005. Reference Source [Google Scholar]
- 110.Borm PJ, Robbins D, Haubold S, et al. : The potential risks of nanomaterials: A review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11 10.1186/1743-8977-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Puttagounder DS, Kalla DK, Zhang B, et al. : Sustainability in nanomanufacturing: Status and vision for the future. ASME 2011 international manufacturing science and engineering conference, MSEC 2011; 13–17 June 2011; Corvallis, OR;2011;p. 585–90 10.1115/MSEC2011-50271 [DOI] [Google Scholar]
- 112.Sarahan PC, Bushong H: Managing your environmental, health & safety risk: A guide for nano companies and their insurers. Electronics, devices, fabrication, MEMS, fluidics and computational - 2011 NSTI nanotechnology conference and expo, NSTI-nanotech 2011; 13–16 June 2011; Boston, MA; Nanotech. 2011;3:p. 561–4 Reference Source [Google Scholar]
- 113.Chen Z, Yadghar AM, Zhao L, et al. : A review of environmental effects and management of nanomaterials. Toxicol Environ Chem. 2011;93(6):1227–50 10.1080/02772248.2011.580579 [DOI] [Google Scholar]
- 114.Jiang G, Shen Z, Niu J, et al. : Nanotoxicity of engineered nanomaterials in the environment. Progr Chem. 2011;23(8):1769–81 Reference Source [Google Scholar]
- 115.Turco RF, Bischoff M, Tong ZH, et al. : Environmental implications of nanomaterials: Are we studying the right thing? Curr Opin Biotechnol. 2011;22(4):527–32 10.1016/j.copbio.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 116.Song Y, Tang S: Nanoexposure, unusual diseases, and new health and safety concerns. ScientificWorldJournal. 2011;11:1821–8 10.1100/2011/794801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bawa R: NanoBiotech 2008: Exploring global advances in nanomedicine. Nanomedicine. 2009;5(1):5–7 10.1016/j.nano.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 118.Marchant GE, Sylvester DJ, Abbott KW: What does the history of technology regulation teach us about nano oversight? J Law Med Ethics. 2009;37(4):724–31 10.1111/j.1748-720X.2009.00443.x [DOI] [PubMed] [Google Scholar]
- 119.Kuiken T: Nanomedicine and ethics: Is there anything new or unique? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;3(2):111–8 10.1002/wnan.90 [DOI] [PubMed] [Google Scholar]