Abstract
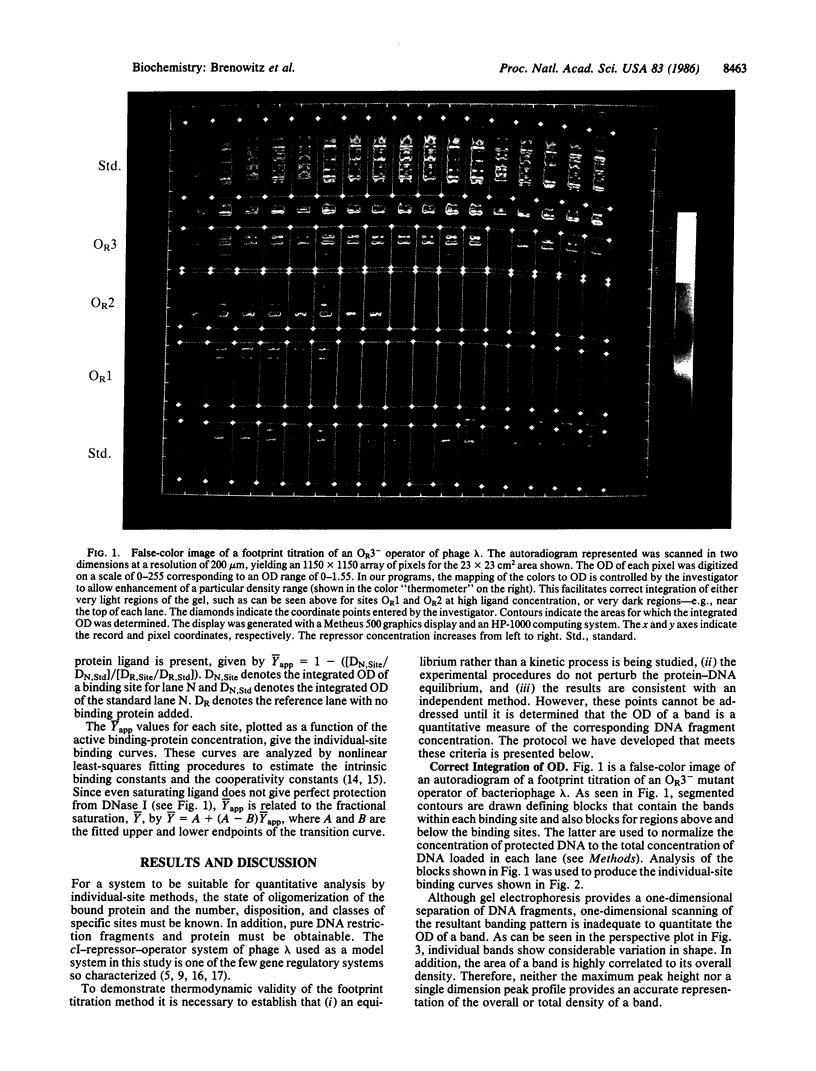
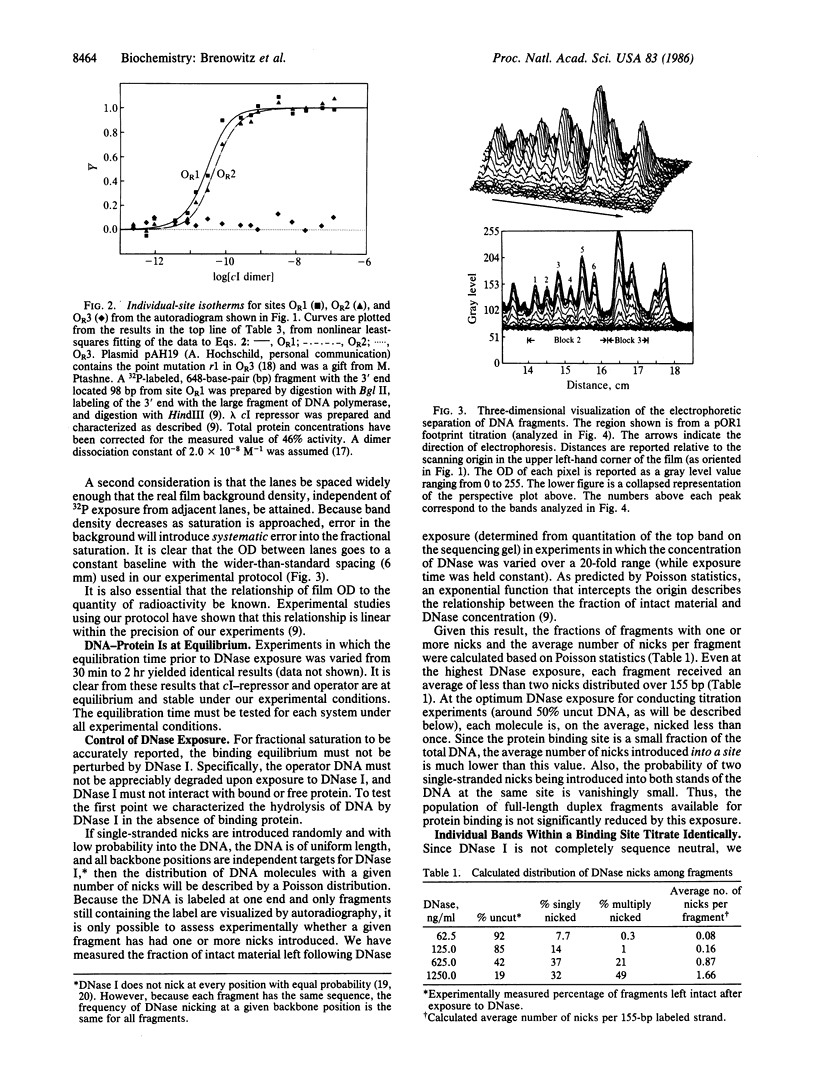
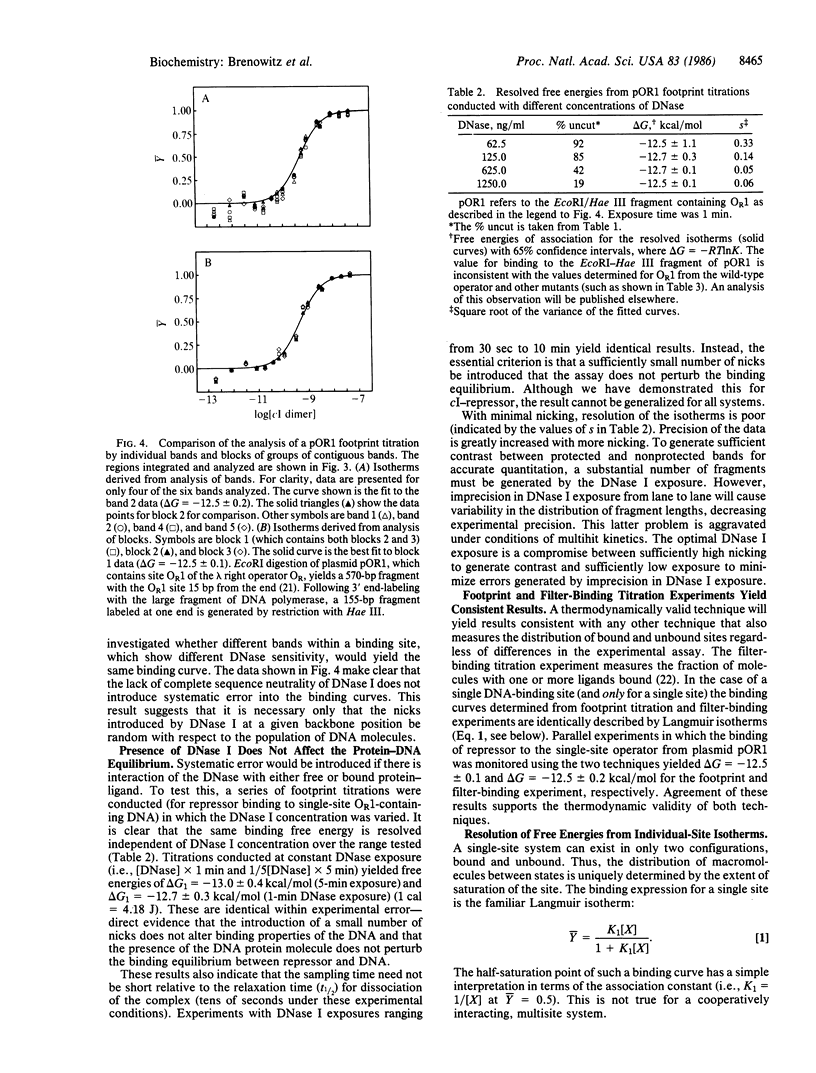
A central issue in gene regulation is the mechanism, and biological function, of the cooperative binding of regulatory protein ligands to specific sites on DNA. To elucidate the physical-chemical basis of these interactions we have developed a thermodynamically rigorous method for conducting DNase I "footprint" (protection) titration experiments. The intrinsic binding constants and also those for cooperative interactions between various sites can be resolved from the individual-site binding curves determined by this technique. Experimental studies of cI-repressor-operator binding have demonstrated that the method provides an accurate representation of the fractional saturation of a binding site. We present individual-site binding curves for a lambda operator with two competent sites that demonstrate the presence of cooperative interactions between the sites. These curves set a lower limit to the magnitude of the cooperative free energy without comparison to single-site mutant operators.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Johnson A. D., Shea M. A. Quantitative model for gene regulation by lambda phage repressor. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1129–1133. doi: 10.1073/pnas.79.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackers G. K., Shea M. A., Smith F. R. Free energy coupling within macromolecules. The chemical work of ligand binding at the individual sites in co-operative systems. J Mol Biol. 1983 Oct 15;170(1):223–242. doi: 10.1016/s0022-2836(83)80234-4. [DOI] [PubMed] [Google Scholar]
- Bossinger J., Miller M. J., Vo K. P., Geiduschek E. P., Xuong N. H. Quantitative analysis of two-dimensional electrophoretograms. J Biol Chem. 1979 Aug 25;254(16):7986–7998. [PubMed] [Google Scholar]
- Brenowitz M., Senear D. F., Shea M. A., Ackers G. K. Quantitative DNase footprint titration: a method for studying protein-DNA interactions. Methods Enzymol. 1986;130:132–181. doi: 10.1016/0076-6879(86)30011-9. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Gronenborn A. M., Davies R. W. Theoretical aspects of specific and non-specific equilibrium binding of proteins to DNA as studied by the nitrocellulose filter binding assay. Co-operative and non-co-operative binding to a one-dimensional lattice. J Mol Biol. 1982 Mar 15;155(4):447–466. doi: 10.1016/0022-2836(82)90481-8. [DOI] [PubMed] [Google Scholar]
- Dervan P. B. Design of sequence-specific DNA-binding molecules. Science. 1986 Apr 25;232(4749):464–471. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber P. W., Wool I. G. Identification of the binding site on 5S rRNA for the transcription factor IIIA: proposed structure of a common binding site on 5S rRNA and on the gene. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1593–1597. doi: 10.1073/pnas.83.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayun Z., Jeffrey A., Ptashne M. Completed DNA sequences and organization of repressor-binding sites in the operators of phage lambda. J Mol Biol. 1977 May 15;112(2):265–277. doi: 10.1016/s0022-2836(77)80143-5. [DOI] [PubMed] [Google Scholar]
- Johnson A. D., Meyer B. J., Ptashne M. Interactions between DNA-bound repressors govern regulation by the lambda phage repressor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5061–5065. doi: 10.1073/pnas.76.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. L., Halvorson H. R., Ackers G. K. Oxygenation-linked subunit interactions in human hemoglobin: analysis of linkage functions for constituent energy terms. Biochemistry. 1976 Nov 30;15(24):5363–5371. doi: 10.1021/bi00669a024. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lomonossoff G. P., Butler P. J., Klug A. Sequence-dependent variation in the conformation of DNA. J Mol Biol. 1981 Jul 15;149(4):745–760. doi: 10.1016/0022-2836(81)90356-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meyer B. J., Maurer R., Ptashne M. Gene regulation at the right operator (OR) of bacteriophage lambda. II. OR1, OR2, and OR3: their roles in mediating the effects of repressor and cro. J Mol Biol. 1980 May 15;139(2):163–194. doi: 10.1016/0022-2836(80)90303-4. [DOI] [PubMed] [Google Scholar]
- Shea M. A., Ackers G. K. The OR control system of bacteriophage lambda. A physical-chemical model for gene regulation. J Mol Biol. 1985 Jan 20;181(2):211–230. doi: 10.1016/0022-2836(85)90086-5. [DOI] [PubMed] [Google Scholar]
- Tullius T. D., Dombroski B. A. Hydroxyl radical "footprinting": high-resolution information about DNA-protein contacts and application to lambda repressor and Cro protein. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5469–5473. doi: 10.1073/pnas.83.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. W., Pettigrew D. W., Ackers G. K. Measurement and analysis of ligand-linked subunit dissociation equilibria in human hemoglobins. Methods Enzymol. 1981;76:596–628. doi: 10.1016/0076-6879(81)76147-0. [DOI] [PubMed] [Google Scholar]




