Abstract
Purpose
To evaluate the efficacy and safety of intravitreal bevacizumab (IVB) injections for the treatment of proliferative diabetic retinopathy (PDR) with new dense vitreous hemorrhage (VH) after previous full panretinal photocoagulation (PRP).
Methods
Prospective study of consecutive PDR with prior complete PRP patients, who presented with new dense VH, were treated with IVB injection. Complete ophthalmic examination and/or ocular ultrasonography were performed at baseline and 1, 6, and 12 weeks and 6, 9, and 12 months after the first injection. Reinjection was done in non-clearing and recurrent VH.
Results
Eighteen eyes of 18 patients, mean age 47.7±12.69 years were included. In all, 14 (77.78%) patients had type 2 diabetes mellitus. Systemic hypertension and dyslipidemia were the most common systemic diseases. All cases were phakic eye with previous complete PRP. Patients received 1.6±0.42 intravitreal injections over a 12-month period. VH cleared completely in 7 (38.89%), 9 (50%), and 13 (72.22%) eyes after 6 weeks, 6 months, and 12 months, respectively. Re-bleeding, however, occurred in 10 (56%) eyes during the follow-up period, and 5 (28%) eyes still had residual VH at the last visit. Statistically significant visual gain was observed in 9 (50%) eyes. Unfortunately, 2 (11%) eyes had severe visual loss because of the tractional retinal detachment (TRD). Mild ocular complication was detected in one patient.
Conclusion
IVB injection had good efficacy and safety for treatment of new VH in patients with PDR and prior complete PRP. This procedure may be especially relevant for diabetic patients at high-risk for surgical intervention.
Keywords: bevacizumab, Avastin, proliferative diabetic retinopathy, vitreous hemorrhage, panretinal photocoagulation
Introduction
Diabetic retinopathy (DR) is the leading cause of vision loss in working-age populations worldwide.1, 2 Although the diabetic macular edema (DME) is the most common cause of vision impairment, advanced proliferative diabetic retinopathy (PDR) with vitreous hemorrhage (VH) or tractional retinal detachment (TRD) is an important cause of severe visual loss in diabetic patients. At present, the gold standard treatment for PDR is panretinal photocoagulation (PRP), which can reduce the risk of severe visual loss by 50–60%.3 Notwithstanding, many patients still need supplemental laser treatment and nearly 4.5% show disease progression that ultimately requires pars plana vitrectomy (PPV), although the PRP was considered adequate.4 Alternative and adjunctive treatment options have been attempted in order to provide better outcomes and/or reduce side-effects.
Bevacizumab (Avastin, Genetech Inc., South San Francisco, CA, USA) is a full-length humanized recombinant antiVEGF. Bevacizumab is currently used on an off-label basis for a variety of ophthalmic conditions. In a systematic review, bevacizumab is likely cost effective compared with laser treatment in DME.5 However, the study of bevacizumab for PDR with VH after previous PRP is limited. In this prospective interventional case series, we evaluated the efficacy and safety of IVB injection in clearing of new dense VH in PDR with previous complete PRP.
Materials and methods
Patients with PDR and prior complete PRP who presented with new dense VH were recruited at the Ophthalmology Department at Srinagarind Hospital, Khon Kaen University, Khon Kaen, Thailand, between December 2006 and December 2010. After institutional review board approval, the study was registered with ClinicalTrials.gov, an approved ICMJE clinical trial registry (NCT01724385). The study was approved by the Khon Kaen University Ethics Committee. All patients provided written informed consent before entering the study. Emphasis was placed on the off-label use and the potential risk of intravitreal bevacizumab (IVB) injection. The inclusion criteria were as follows: (1) total dense VH presenting as an obscured fundus or invisible retinal details in 1–3 quadrants indicating partial dense VH; and (2) previous complete laser treatment according to the Diabetic Retinopathy Study Research Group Guidelines.
The exclusion criteria were as follows: (1) one-eyed patients; (2) a previous intraocular surgery; (3) severe lens opacity precluding fundus examination; (4) advanced glaucoma; (5) history of thromboembolic events such as myocardial infarction and cerebrovascular accident; (6) uncontrolled systemic hypertension, systolic blood pressure>180 mm Hg or diastolic blood pressure>110 mm Hg; (7) known coagulation abnormalities or current use of anticoagulant medications other than aspirin; (8) known allergies to any relevant drugs being used in this study; or (9) evidence of external ocular infection such as conjunctivitis and significant blepharitis.
Demographics, detailed medical history, and clinical findings were taken at the outset for baseline data. The best-corrected visual acuity (BCVA) was measured using the Snellen chart and recorded in logarithm of minimum angle of resolution (log MAR). Complete ocular examination was performed including slit-lamp biomicroscopy, applanation tonometry, and indirect ophthalmoscopy at the outset and at the follow-up visits.
Approximately 1.25 mg in 0.05 ml bevacizumab was injected intravitreally through a 30-gauge needle via the inferotemporal pars plana. A prophylactic topical antibiotic was prescribed for 1 week after the procedure. Follow-up was scheduled for 1 and 4 weeks afterwards and then every 4–6 weeks for the next 12 months. At each visit, a complete ocular examination was performed. Evidence of fibrovascular proliferation was to be noted during the follow-up. Ocular and systemic adverse events were monitored. In the event of any fibrovascular proliferation or if TRD was suspected, ocular ultrasonography was provided to evaluate its severity and progression. Reinjection was considered if there were non-clearing VH within 3 months or if VH recurred during the follow-up period. Then the same follow-up schedule and treatment was arranged. The primary outcome measures were the clearing of VH and the regression of fibrovascular traction (FVT). Secondary outcomes were any changes in the BCVA and adverse events.
Statistical method
Number and percentage were used to describe the qualitative data and mean±SD to describe the quantitative data. The paired sample t-test and Wilcoxon signed ranks test were used to compare the outcome data with the baseline values. All statistical analyses were performed using the SPSS 11.0 statistical software (IBM Inc., Chicago, IL, USA).
Results
The study was conducted on 18 eyes of 18 patients with new dense VH (severity grade 3–4), refractory to previous complete PRP (9 males and 9 females), with a mean age of 47.7±12.69 years. The majority of cases (14 eyes, 77.78%) had type 2 diabetes mellitus. The mean diabetic duration was 9.7±7.7 years. Most patients had poor glycemic control with a mean HbA1C of 10±2.32%, whereas only one patient had fair glycemic control (HbA1C 7.1%). All of the patients had additional systemic diseases of which systemic hypertension was the most common. (Table 1)
Table 1. Demographic data.
| Eyes/patients | 18/18 |
|---|---|
| Ages (years) | 47.7±12.69 (23–59) |
| Sex | |
| Female | 9 (50%) |
| Male | 9 (50%) |
| Type of Diabetes Mellitus | |
| I | 4 (22.22%) |
| II | 14 (77.78%) |
| Diabetic duration (years) | 9.7±7.7 |
| HbA1C (%) | 10±2.32 (7.1–14) |
| Systemic diseases | |
| Hypertension | 13 (72.22%) |
| Dyslipidemia | 10 (55.55%) |
| Renal failure | 6 (33.33%) |
Seven eyes had total dense VH, whereas 11 had partial dense VH involving 2–3 quadrants. The mean laser burns were 2323±661 spots, and the mean duration of the prior complete PRP before IVB injection was 11.60±11.98 months. The mean duration of VH before treatment was 2±1.02 months. Reinjection was required in 10 (55.56%) patients because of recurrence of VH in three cases and non-clearing VH in seven. Mild anterior uveitis and secondary glaucoma were revealed in one patient (5.56%); however, these events were transient and resolved within 1 week of topical medication (Table 2). No serious ocular and/or systemic side-effects were detected.
Table 2. Ocular manifestations and treatment.
| Eyes/patients | |
| Total dense vitreous hemorrhage | 7 |
| Partial dense vitreous hemorrhage (2-3 quadrants) | 11 |
| Previous complete PRP (spots) | 2323±661 (1500–3553) |
| Duration of prior complete PRP before IVB injection (months) | 11.60±11.98 |
| Duration of VH before treatment (months) | 2±1.02 |
| Intravitreal bevacizumab injection | |
| Single injection | 8 |
| 2 injections | 9 |
| 3 injections | 1 |
| Indication of reinjection | |
| Recurrent VH | 3 |
| Non-clearing VH | 7 |
| Frequency of IVB reinjection (months) | 1.6±0.42 |
| Pre-injection visual acuity (logMAR) | 1.32±1.03 |
| Post-injection visual acuity (logMAR) | 1.09±1.10 |
| Follow-up period (months) | 14.50±2.56 |
| Complication | |
| Mild anterior uveitis and secondary glaucoma | 1 |
Anatomical outcome
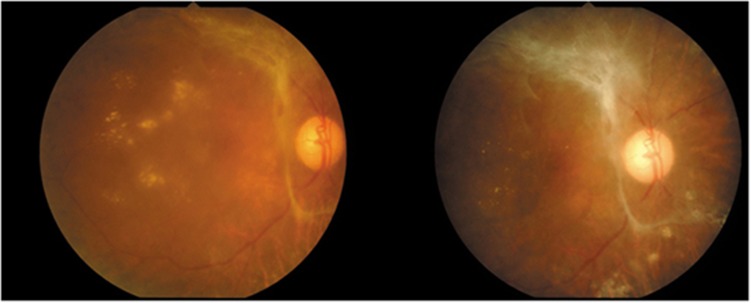
VH cleared completely in a respective 7 (38.89%), 9 (50%), and 13 (72.22%) eyes at 6 weeks, 6 months, and 12 months. At the last follow-up, there were five eyes with residual VH: three with VH grade 2, one accompanied with tractional retinal detachment (TRD), and two with VH grade 4. Although five eyes had indications for surgery—due to TRD in three eyes and non-clearing VH in two eyes—pars plana vitrectomy (PPV) was performed in four patients, as a patient was a high-risk candidate for surgical intervention. After VH was decreased with time, neovascular glaucoma also developed later in the non-operated, high operation risk case. All three cases of TRD were managed by PPV. Two of three eyes demonstrated localized TRD outside the arcade by ocular ultrasonography at baseline; however, progression of TRD to the macular area was observed within 4–8 weeks after the first IVB injection and severe visual loss (≥ 3 lines) still occurred in these cases despite PPV having been achieved. Only one (5.56%) eye developed TRD after the second IVB injection. (Figure 1)
Figure 1.
Progression of TRD to the macular area was observed at 8 weeks after first IVB injection.
Visual outcome
At the baseline examination, the mean BCVA was 1.32±1.03 logMAR. Half of patients experienced visual improvement by week 4, as the mean BCVA had improved to 1.05±0.97 logMAR. In all, 9 (50%) patients experienced visual improvement by 12 months whereas 7 (38.89) patients had visual improvement >three lines. Stable vision was found in five patients with good VA after the IVB injection, and mild-to-moderate visual loss was observed in two patients with residual VH. In all, 2 (11.11%) patients with previous TRD suffered from severe visual loss (≥ three lines) despite having had a successful surgery. At the last follow-up visit, the mean BCVA was 1.09±1.10 logMAR: the mean change from baseline BCVA was −0.24±1.24 logMAR—a statistically significant increase (P=0.433, 95%CI: −0.85, 0.38).
Discussion
Numerous studies point to the role of vascular endothelial growth factor (VEGF) in the pathogenesis of DR; therefore, antiangiogenic drugs have been extensively used for the treatment of DR.6, 7, 8, 9, 10, 11, 12 However, there have not been many reports on pegaptanib/ranibizumab for the treatment of PDR.13, 14, 15, 16, 17, 18, 19 The majority of studies on antiVEGF agents for PDR have been trials of bevacizumab. In Thailand, the National List of Essential Medicine Committee recently announced the inclusion of IVB injection for the treatment of age-related macular degeneration and diabetic macular edema.20 Most of research evaluated the benefit of IVB in adjunctive therapy to PPV. The systematic review and meta-analysis of IVB pretreatment for severe PDR confirmed both intra- and postoperative advantages.9, 12 IVB after a vitrectomy also appears safe and effective for reducing postoperative VH, albeit without the benefit on final visual acuity.21, 22 In a randomized clinical trial, 25.7% of patients avoided the need for vitrectomy after IVB injection.23
Although PRP is the principal therapy for PDR, substantial regression of NV may take weeks after completion of laser treatment, and it is not always effective. Patients who already have extensive VH, which would preclude the possibility of laser photocoagulation, may increase the incidence of complications due to the progression of PDR. Severe visual loss may, however, occur despite maximal laser treatment. Many researchers have evaluated the efficacy of combined IVB injection and PRP for treatment of high-risk PDR, but most did not conduct large-scale trials.24, 25, 26, 27, 28 The advantage on visual acuity, central macular thickness, reduction in NV size, reduction in FLA, and development of vitreous hemorrhage was shown in the PRP plus IVB group. An angiographic study demonstrated that leakage had diminished as early as 24 h after IVB injection, and NV of the disc was more responsive than NV elsewhere in the retina.29
Published research comparing antiVEGF agents for PDR and prior complete PRP is scarce especially that accompanied with recurrent vitreous hemorrhage.29, 30, 31, 32 Previous studies had several limitations including a relatively small sample size and a relatively short duration of follow-up. In addition, many trials mentioned the prior PRP in the material and methods, but no details of any previous laser treatment were shown in the results.33, 34 IVB injection, nevertheless, seems to be a promising treatment for active PDR with previous adequate PRP. The most comparable study to ours was conducted by Jorge et al29 who assessed IVB in persistent leaking NV, unresponsive to complete PRP, performed at least four months. Their study included 15 consecutive patients who completed the 12-week follow-up evaluation. No leakage was observed at week 6 and visual improvement was revealed, but a very high recurrence of leakage (93%) was detected at 12 weeks after IVB injection. Similarly, Avery et al30 evaluated the efficacy of IVB in treatment of PDR with retinal and/or iris NV. Thirty-nine of 45 (87%) of the eyes had received prior PRP, performed between 7 and 588 days before IVB injection. Complete resolution of angiographic leakage of NV of the disc was observed and leakage of iris NV was completely resolved in 82%. There was only one patient with VH. Compared with our study, the patients in both these studies had PDR without VH and a shorter follow-up period. Furthermore, our patients seem to have had a more severe PDR than the first comparative study. Moreover, the primary outcome measurement between our study and these two similar studies was different.
El-Batarny31 reported the short-term anatomical and visual response of IVB injection in 10 eyes with PDR. Unfortunately, the four eyes in which retinal NV regression failed to be induced after performing complete PRP were not described thoroughly. Meanwhile Moradian et al32 assessed the efficacy of IVB injection on VH in patients with active PDR refractory to prior complete PRP, which they described as the first group. As with El-Batarny, they did not provide detailed separate results of the IVB on active PDR with VH refractory to a previous complete PRP.
On the basis of histologic studies, IVB may induce changes in immature NV leading to endothelial apoptosis with vascular regression, while inducing normalization of premature vessels by increasing the pericyte coverage and reducing vessel fenestration.35 IVB does not, therefore, induce complete regression of the vascular endothelium of the fibrovascular membranes, and the blood flow of newly formed vessels is easily reperfused when the effect of bevacizumab diminishes. Because of the temporary nature of the effect of bevacizumab, many eyes from previous publications revealed the recurrences of NV and multiple injections were needed. The recurrence of retinal NV varies from a few days to a few months depending on the severity of PDR and previous treatment in each case.29, 30, 31, 32 The balance between the connective tissue growth factor and VEGF may be the strongest predictor of the degree of fibrosis; a shift toward connective tissue growth factor can underlie the aggravation of fibrosis after IVB injection. Previous publications reported exacerbation and subsequent contracture of fibrous tissue leading to TRD in 2.2–5.26%.32, 33, 36 A longer interval between IVB and PPV was among the main risk factors for the development or progression of TRD. Oshima et al37 reported on the progression of pre-existing TRD after IVB occurred in seven eyes (18%). They proposed that the absence of previous laser photocoagulation and the presence of a ring-shaped fibrovascular membrane were relevant findings in eyes with this complication. In our study, new TRD was detected in one eye (5.56%) and progressed TRD was observed in two eyes (11.11%).
There are not any clinical trials that can support the efficacy and safety of IVB injection in case of active PDR with previous complete PRP who presented with VH. So, our study may be the first trial of IVB injection in this aspect of PDR. The main limitation of our study is the relative small sample size because of the unusual clinical manifestation. There was also a lack of a control group for comparison; nevertheless, our study had a relative long follow-up period. Further studies to investigate the role of antiVEGF therapy for the management of PDR refractory to laser treatment with or without VH are warranted. Although the safety data are favorable, the use of antiVEGF medications for any indications of PDR is off-label. We are therefore cautious before the results of large, controlled trials to substantiate the efficacy and safety of anti-VEGF drugs for PDR.
Conclusion
IVB injection was safe and effective treatment for dense VH in PDR with previous complete PRP. In all, 72.22% of patients could avoid the PPV, and hence the benefit of IVB was significant in patients who were a high-risk candidate for surgery.

Acknowledgments
We thank the patients and their families for their participation, Dr Oracha Teerakapong, Dr Tidarat Prechanond, and Dr Wipada Laovirojjanakul for data collection, Dr Ratchaneewan Poomsa-ad for artworks, and Mr Bryan Roderick Hamman and Mrs Janice Loewen Hamman for assistance with the English-language presentation of the manuscript.
The authors declare no conflict of interest.
Footnotes
Presentation: The 28th Asia-Pacific Academy of Ophthalmology Congress, January 17-20, 2013, India
References
- Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP. Global estimates of visual impairment. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- The Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of diabetic retinopathy study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- Flynn HW, Jr, Chew EY, Simons BD, Barton FB, Remaley NA, Ferris FL., 3rd Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. The Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1992;99:1351–1357. doi: 10.1016/s0161-6420(92)31779-8. [DOI] [PubMed] [Google Scholar]
- Ford JA, Elders A, Shyangdan D, Royle P, Waugh N.The relative clinical effectiveness of ranibizumab and bevacizumab in diabetic macular oedema: an indirect comparison in a systematic review BMJ 201213345e5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson BP, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:915–930. doi: 10.1007/s00417-010-1315-z. [DOI] [PubMed] [Google Scholar]
- Salam A, Mathew R, Sivaprasad S. Treatment of proliferative diabetic retinopathy with anti-VEGF agents. Acta Ophthalmol. 2011;89:405–411. doi: 10.1111/j.1755-3768.2010.02079.x. [DOI] [PubMed] [Google Scholar]
- Montero JA, Ruiz-Moreno JM, Correa ME. Intravitreal anti-VEGF drugs as adjuvant therapy in diabetic retinopathy surgery. Curr Diabetes Rev. 2011;7:176–184. doi: 10.2174/157339911795843104. [DOI] [PubMed] [Google Scholar]
- Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2011;11:CD008214. doi: 10.1002/14651858.CD008214.pub2. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun X, Liu K, Xu X. Intravitreal ranibizumab (lucentis) for the treatment of diabetic macular edema: a systematic review and meta-analysis of randomized clinical control trials. Curr Eye Res. 2012;37:661–670. doi: 10.3109/02713683.2012.675616. [DOI] [PubMed] [Google Scholar]
- Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabeticmacular oedema. Cochrane Database Syst Rev. 2012;12:CD007419. doi: 10.1002/14651858.CD007419.pub3. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Liu HY, Hernandez-Da Mota SE, Romano MR, Falavarjani KG, Ahmadieh H, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol. 2013;156:106–115. doi: 10.1016/j.ajo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- González VH, Giuliari GP, Banda RM, Guel DA. Intravitreal injection of pegaptanib sodium for proliferative diabetic retinopathy. Br J Ophthalmol. 2009;93:1474–1478. doi: 10.1136/bjo.2008.155663. [DOI] [PubMed] [Google Scholar]
- Hornan D, Edmeades N, Krishnan R, Khan J, Lochhead J. Use of pegaptanib for recurrent and non-clearing vitreous haemorrhage in proliferative diabetic retinopathy. Eye (Lond) 2010;24:1315–1319. doi: 10.1038/eye.2010.14. [DOI] [PubMed] [Google Scholar]
- Mendrios E, Donati G, Pournaras CJ. Rapid and persistent regression of severe new vessels on the disc in proliferative diabetic retinopathy after a single intravitreal injection of pegaptanib. Acta ophthalmol. 2009;87:683–684. doi: 10.1111/j.1755-3768.2008.01391.x. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Goverdhan S, Lochhead J. Intravitreal pegaptanib in severe proliferative diabetic retinopathy leading to the progression of tractional retinal detachment. Eye (Lond) 2009;23:1238–1239. doi: 10.1038/eye.2008.179. [DOI] [PubMed] [Google Scholar]
- Filho JA, Messias A, Almeida FP, Ribeiro JA, Costa RA, Scott IU, et al. Panretinal photocoagulation (PRP) versus PRP plus intravitreal ranibizumab for high-risk proliferative diabetic retinopathy. Acta Ophthalmol. 2011;89:e567–e572. doi: 10.1111/j.1755-3768.2011.02184.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Messias A, de Almeida FP, Costa RA, Scott IU, de Figueiredo-Pontes LL, et al. The effect of intravitreal ranibizumab on intraoperative bleeding during pars plana vitrectomy for diabetic traction retinal detachment. Br J Ophthalmol. 2011;95:1337–1339. doi: 10.1136/bjo.2010.195693. [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research Network Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous from proliferative diabetic retinopathy. JAMA Ophthalmol. 2013;131:283–293. doi: 10.1001/jamaophthalmol.2013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anothaisintawee T, Leelahavarong P, Ratanapakorn T, Teerawattananon Y. The use of comparative effectiveness research to inform policy decisions on the inclusion of bevacizumab for the treatment of macular diseases in Thailand's pharmaceutical benefit package. Clinicoecon Outcomes Res. 2012;4:361–374. doi: 10.2147/CEOR.S37458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema RA, Mushtaq J, Al-Khars W, Al-Askar E, Cheema MA. Role of intravitreal bevacizumab (Avastin) injected at the end of diabetic vitrectomy in preventing postoperative recurrent vitreous hemorrhage. Retina. 2010;30:1646–1650. doi: 10.1097/IAE.0b013e3181d6def0. [DOI] [PubMed] [Google Scholar]
- Park DH, Shin JP, Kim SY. Intravitreal injection of bevacizumab and triamcinolone acetonide at the end of vitrectomy for diabetic vitreous hemorrhage: a comparative study. Graefes Arch Clin Exp Ophthalmol. 2010;248:641–650. doi: 10.1007/s00417-009-1247-7. [DOI] [PubMed] [Google Scholar]
- Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology. 2009;116:1943–1948. doi: 10.1016/j.ophtha.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Mirshahi A, Roohipoor R, Lashay A, Mohammadi SF, Abdoallahi A, Faghihi H. Bevacizumab-augmented retinal laser photocoagulation in proliferative diabetic retinopathy: a randomized double-masked clinical trial. Eur J Ophthalmol. 2008;18:263–269. doi: 10.1177/112067210801800215. [DOI] [PubMed] [Google Scholar]
- Tonello M, Costa RA, Almeida FP, Barbosa JC, Scott IU, Jorge R. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study) Acta Ophthalmol. 2008;86:385–389. doi: 10.1111/j.1600-0420.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- Shin YW, Lee YJ, Lee BR, Cho HY. Effects of an intravitreal bevacizumab injection combined with panretinal photocoagulation on high-risk proliferative diabetic retinopathy. Korean J Ophthalmol. 2009;23:266–272. doi: 10.3341/kjo.2009.23.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WB, Oh SB, Moon JW, Kim HC. Panretinal photocoagulation combined with intravitreal bevacizumab in high-risk proliferative diabetic retinopathy. Retina. 2009;29:516–522. doi: 10.1097/IAE.0b013e31819a5fc2. [DOI] [PubMed] [Google Scholar]
- Cho WB, Moon JW, Kim HC. Intravitreal triamcinolone and bevacizumab as adjunctive treatments to panretinal photocoagulation in diabetic retinopathy. Br J Ophthalmol. 2010;94:858–863. doi: 10.1136/bjo.2009.168997. [DOI] [PubMed] [Google Scholar]
- Jorge R, Costa RA, Calucci D, Cintra LP, Scott IU. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study) Retina. 2006;26:1006–1013. doi: 10.1097/01.iae.0000246884.76018.63. [DOI] [PubMed] [Google Scholar]
- Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695–1705. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- El-Batarny AM. Intravitreal bevacizumab treatment for retinal neovascularization and vitreous hemorrhage in proliferative diabetic retinopathy. Clin Ophthalmol. 2007;1:149–155. [PMC free article] [PubMed] [Google Scholar]
- Moradian S, Ahmadieh H, Malihi M, Soheilian M, Dehghan MH, Azarmina M. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1699–1705. doi: 10.1007/s00417-008-0914-4. [DOI] [PubMed] [Google Scholar]
- Arevalo JF, Wu L, Sanchez JG, Maia M, Saravia MJ, Fernandez CF, et al. Intravitreal bevacizumab (Avastin) for proliferative diabetic retinopathy: 6-months follow-up. Eye (Lond) 2009;23:117–123. doi: 10.1038/sj.eye.6702980. [DOI] [PubMed] [Google Scholar]
- Huang YH, Yeh PT, Chen MS, Yang CH, Yang CM. Intravitreal bevacizumab and panretinal photocoagulation for proliferative diabetic retinopathy associated with vitreous hemorrhage. Retina. 2009;29:1134–1140. doi: 10.1097/IAE.0b013e3181b094b7. [DOI] [PubMed] [Google Scholar]
- Kimoto K, Kubota T. Anti-VEGF agents for ocular angiogenesis and vascular permeability. J Ophthalmol. 2012;2012:852183. doi: 10.1155/2012/852183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo JF, Maia M, Flynn HW, Jr, Saravia M, Avery RL, Wu L, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:213–216. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009;116:927–938. doi: 10.1016/j.ophtha.2008.11.005. [DOI] [PubMed] [Google Scholar]