Abstract
The B7 family member B7-H3 (CD276) plays a key role during an immune response but its function remains controversial. In this study, we found that murine B7-H3 up-regulated the proliferation and cytokine production of T cells. Our study suggested that there was no interaction of murine B7-H3 with a triggering receptor expressed on myeloid cells (TREM)-like transcript 2 (TLT-2). Further studies demonstrated that mouse B7-H3 specifically bound to T cells and its receptor was not murine TLT-2. Moreover, murine B7-H3 was a positive co-stimulatory molecule in the regulation of T cell-mediated immune responses.
Introduction
Anew member of the B7 superfamily, B7-H3 has been identified in both humans and mice by sharing ∼88% amino acid sequence identity. Mouse B7-H3 protein is a type I transmembrane glycoprotein containing two extracellular Ig domains.(1–3) The immunologic function of B7-H3 is controversial. It was originally identified as a co-stimulatory molecule that induces T cell proliferation and selectively stimulates IFN-γ production in humans.(4) However, some reports showed that both human and murine B7-H3 inhibited the T cell proliferation and cytokine production induced by anti-CD3 agnostic MAb.(5–7)
Since the triggering receptor expressed on myeloid cell like transcript 2 (TREM-like transcript 2, TREML2, TLT-2) has been recently described as a co-stimulatory receptor of murine B7-H3,(8,9) we obtained the mouse B7-H3 fusion protein and TLT-2 transfectant cells and analyzed the interaction of mouse B7-H3 with TLT-2. In these experiments we found no evidence for such an interaction. Furthermore we demonstrated that mouse B7-H3 binds to a putative receptor expressed on PHA- or anti-CD3 MAb-activated T cells. Thus mouse B7-H3 serves as a co-stimulatory regulator that preferentially affects T cell responses.
Materials and Methods
Cell lines and antibodies
Chinese hamster ovary (CHO) cell and human embryonic kidney cell 293 were originally obtained from American Type Culture Collection (Manassas, VA). These cells were cultured in RPMI 1640 or DMEM (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS, Hyclone, Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 25 mM HEPES buffer. Goat anti-mouse B7-H3 polyclonal antibody and agonistic anti-CD3 MAb were purchased from eBioscience (Woburn, MA). Mouse IL-2 and IFN-γ ELISA kits were purchased from Invitrogen (Carlsbad, CA). HRP-conjugate goat anti-rat IgG (H+L) and rat IgG2b were purchased from Immunotech (Marseille, France). All PCR reagents were purchased from TaKaRa (DaLian, China). All chemicals were obtained from Sigma (St. Louis, MO).
Construction of transfectants
The genes coding the extracellular domain of mouse B7-H3 and the Fc fragment of human IgG1 were amplified from pMD19-T/mouse B7-H3 and pMD19-T/human IgG1 vectors by PCR. The two genes were connected into mouse B7-H3-Fc fragment by overlap PCR. Then the target gene fragment was inserted into eukaryotic vector pIRES2-EGFP after being digested with EcoR I and Bgl II to construct the recombinant vector pIRES2-EGFP/B7-H3-Fc. The full-length cDNA encoding mouse TLT-2 was cloned from T cells by reverse transcription polymerase chain reaction (RT-PCR) with specific primers and was inserted into vector pIRES2-EGFP (Clontech, Mountain View, CA). The two recombinant vectors were transfected into CHO cells by Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The collected supernatant of mouse B7-H3-Fc transfected cell line after serum-free culture was ultra-filtrated and concentrated, then purified by Protein G column. The expression of mouse B7-H3-Fc fusion protein was confirmed by Western blot analysis. The mouse TLT-2 transfected cells was selected by G418 (Invitrogen) and confirmed by a combination of commercial anti-mouse TLT-2 MAb and GFP using flow cytometry (Beckman Coulter, Brea, CA). Empty vector-transfected CHO cell lines (CHO/mock) were obtained in the same way.
Cell proliferation assay
T cells were cultured in 96-well cell culture plate in triplicate (1×105 cells/well) in complete RPMI 1640 at 37°C, 5% CO2. The wells were coated with agonistic anti-CD3 MAb (0.5 μg/mL). Mouse T cells were incubated with various concentrations of B7-H3-Fc fusion protein for up to 3 days. Cell proliferation was analyzed by cell counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan) at 2, 6, 12, 24, 48, and 72 h, respectively. After incubation with MAb(10) to block the proliferation of T cells, cytokine assays followed. The cytokine levels in the supernatants of T cell cultures were assessed by commercial ELISA kits IFN-γ and IL-2 (Invitrogen/Biosource, Carlsbad, CA) according to the manufacturer's instructions.
Analysis of mouse B7-H3 binding to TLT-2 and T cells
To identify the counter-receptor for mouse B7-H3, the cDNA for TLT-2 was transfected into CHO cells. By flow cytometry we assessed the relative affinity of serially diluted B7-H3-Fc fusion protein (1 μg, 5 μg, 10 μg, 20 μg/mL) to the surface of TLT-2 transfected cells and T cells (stimulated by PHA at 0, 6, 12, 24, and 48 h, respectively). After incubation with mouse serum at 4°C for 30 min to block the FcR, cells were stained with B7-H3-Fc or human IgG as the control, followed by staining with PE-conjugated mouse anti-human IgG (Fc) for another 30 min. FACS analysis was performed to detect surface expression of a putative B7-H3 receptor.
Statistical analysis
The statistical analysis was performed using two-tailed t-test, and a p value of <0.05 was considered significant.
Results
B7-H3 stimulates T cell activation
In this study, the biological effect of B7-H3-Ig on T cell proliferation and cytokine production in vitro with plate-bound anti-CD3 antibody was performed by assays of CCK8 and ELISA. In this setting, we found that B7-H3-Ig could obviously promote the proliferation of T cells in a dose-dependent manner (Fig. 1A). Interestingly, the secretion of cytokines (IL-2 and IFN-γ stimulated by B7-H3-Fc reached maximum levels at 24 h (Fig. 1B). The levels of IL-2 and IFN-γ stimulated by B7-H3-Fc with agonistic anti-CD3 MAb in the supernatant were significantly reduced and were blocked by B7-H3 MAb (obtained in our laboratory). The above results showed that the mouse B7-H3 molecule is a positive regulator in immune response.
FIG. 1.
T cell proliferation and cytokine production stimulated by B7-H3-Fc. Purified T cells were activated with plate-bound agonistic anti-CD3 MAb (0.5 μg/mL). B7-H3-Fc was applied and cell proliferation (A) and cytokine production (B) were measured and then blocked by B7-H3 MAb (C). Human IgG was used as an isotype control. *p<0.01. Data shown are representative of three independent experiments with similar results.
Construction of mouse TLT-2 transfectants
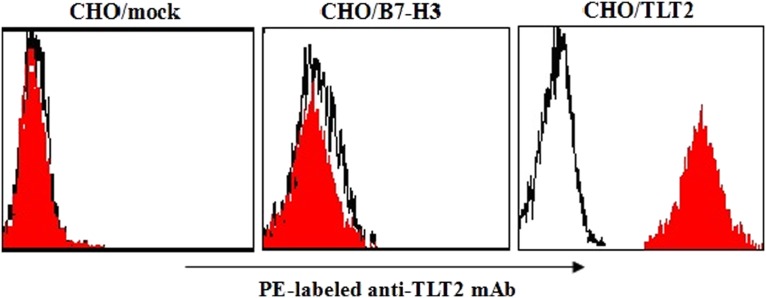
We cloned full-length mouse TLT-2 cDNA from mouse T cells and transfected it into CHO cells. FCM was performed to confirm the binding ability of the commercial MAb to recognize transfected cells. The MAb could recognize transfected cells CHO/TLT-2, but not CHO/mock or CHO/B7-H3 cells (Fig. 2).
FIG. 2.
Preparation of mouse TLT-2 transfectants. CHO/TLT-2 transfectants were selected and identified by flow cytometry using PE-labeled MAb (red histograms) against PE-conjugated IgG (open histograms). Reactivity of the commercial MAb with transfectants CHO/mock, CHO/B7-H3, and CHO/TLT-2. Cells were stained with MAb (red histograms) or negative control IgG (open histograms). Data shown are representative of four independent experiments.
B7-H3 binds to T cells
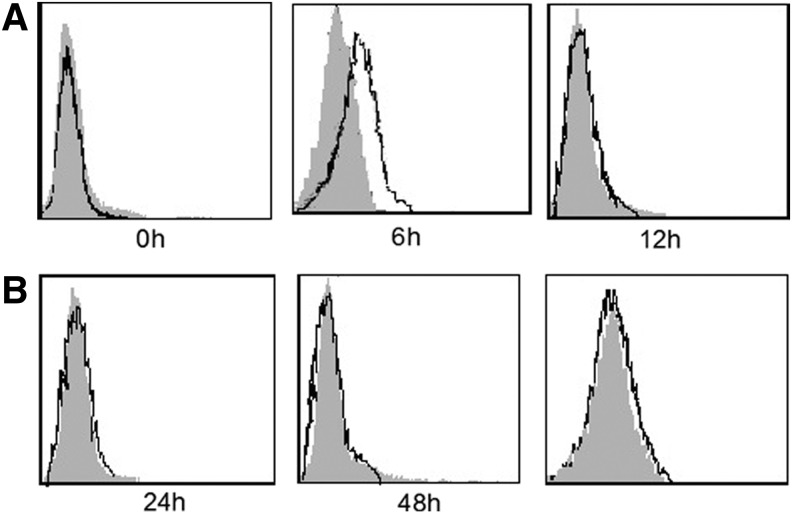
It is a fact that our fusion protein representing mouse B7-H3 bound to certain T cell receptors but not to cells expressing high levels of mouse TLT-2 (Fig. 3). Moreover in experiments we did not find any evidence for an interaction of mouse TLT-2 with mouse B7-H3. Finally we provide functional evidence that mouse TLT-2 is not a receptor for B7-H3.
FIG. 3.
Analysis of mouse B7-H3 binding to TLT-2 and T cells. B7-H3-Fc fusion protein to the surface of T cells (stimulated by PHA at 0, 6, 12, 24, and 48 h, respectively) (A) and TLT-2 transfected cells (B). Cells were stained with mouse B7-H3-Fc (open histograms) or negative control human IgG (grey histograms). Data shown are representative of three independent experiments.
Discussion
Murine B7-H3 mRNA is widely expressed in multiple tissues, but the B7-H3 protein is not detected in these tissues.(1–3) Recently, B7-H3 was shown to be uniformly aberrantly expressed in sera or tumor tissues of cancer patients.(11–15) Thus B7-H3 might be a promising target in diagnosis and therapy of malignancies. A recent study demonstrated that B7-H3 functions as a co-stimulator of innate immunity by augmenting pro-inflammatory cytokine release from bacterial cell wall product-stimulated monocytes/macrophages and may contribute positively to the development of sepsis.(16)
The functional role of B7-H3 is still controversial. It was originally described as a potent co-stimulatory molecule and inducer of IFN-γ in human T cells.(4) In contrast, others found human B7-H3 to strongly down-regulate T cell proliferation and cytokine production.
In a previous study, murine TLT-2 was a demonstrated receptor for B7-H3.(8) To investigate the functional roles of B7-H3 and TLT-2, we established a fusion protein against B7-H3 and TLT-2 transfectant cells and analyzed a potential interaction of B7-H3 with TLT-2. Moreover, we used a mouse MAb against B7-H3 for flow cytometric staining and the functional blocking analyses. In this study, we did not find that mouse B7-H3-Fc fusion protein binds to the surface of TLT-2 transfectants. Thus murine TLT-2 was not a receptor of B7-H3. Furthermore, we also found that mouse B7-H3 could stimulate the proliferation and enhance the cytokine secretion of T cells. Thus mouse B7-H3 is a co-stimulatory regulator in immune responses.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (81202348 and 81301494) and Jiangsu Province National Natural Science Funds (BK2012171).
Author Disclosure Statement
The authors have no financial interests to disclose. The generated antibody has been solely distributed to non-profit research organizations for research purposes only.
References
- 1.Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, and Dong C: Characterization of mouse and human B7-H3 genes. J Immunol 2002;168:6294–6297 [DOI] [PubMed] [Google Scholar]
- 2.Ling V, Wu PW, Spaulding V, Kieleczawa J, Luxenberg D, Carreno BM, and Collins M: Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics 2003;82:365–377 [DOI] [PubMed] [Google Scholar]
- 3.Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stöckl J, and Knapp W: Molecular characterization of human 4Ig-B7-H3, a member of B7 family with four Ig-like domains. J Immunol 2004;172:2352–2359 [DOI] [PubMed] [Google Scholar]
- 4.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, and Chen L: B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol 2001;2:269–274 [DOI] [PubMed] [Google Scholar]
- 5.Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, and Dong C: Murine B7-H3 is a negative regulator of T cells. J Immunol 2004;173:2500–2506 [DOI] [PubMed] [Google Scholar]
- 6.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da Costa J, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, and Mak TW: The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol 2003;4:899–906 [DOI] [PubMed] [Google Scholar]
- 7.Luo L, Chapoval AI, Files DB, Zhu G, Hirano F, Wang S, Lau JS, Dong H, Tamada K, Files AS, Liu Y, and Chen L: B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+cytolytic T cells. J Immunol 2004;173:5445–5450 [DOI] [PubMed] [Google Scholar]
- 8.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, and Azuma M: Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci USA 2008;105:10495–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, Kreil DP, Dong C, Yamazaki T, Zlabinger G, Pfistershammer K, and Steinberger P: B7-H3 is a potent inhibitor of human T-cell activation: no evidence for B7-H3 and TREML2 interaction. Eur J Immunol 2009;39:1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan R, Yang S, Sun J, Chen X, Zhang G, Feng P, and Zhang X: A novel monoclonal antibody against mouse B7-H3 developed in rats. Hybridoma 2012;31:267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang GB, Zhou H, Chen Y-J, Ge Y, Xie F, Shi Q, Ma H-B, Fei M, and Zhang X-G: Characterization and application of two novel monoclonal antibodies against 2IgB7-H3: expression analysis of 2IgB7-H3 on dendritic cells and tumor cells. Tissue Antigens 2005;66:83–92 [DOI] [PubMed] [Google Scholar]
- 12.Zhang G, Hou J, Shi J, Yu G, Lu B, and Zhang X: Soluble CD276 (B7-H3) is released from monocytes, dendritic cells, and activated T cells and is detectable in normal human serum. Immunology 2008;123:538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich BC, Blute ML, and Kwon ED: Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res 2008;14:5150–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B, and Zhang X: Diagnosis value of serum B7-H3 expression in non-small cell lung cancer. Lung Cancer 2009;66:245–249 [DOI] [PubMed] [Google Scholar]
- 15.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML, Cheville JC, Sebo TJ, and Kwon ED: B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res 2007;67:7893–7900 [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Wang J, Kelly J, Gu G, Hou J, Zhou Y, Redmond HP, Wang JH, and Zhang X: B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol 2010;185:3677–3684 [DOI] [PubMed] [Google Scholar]