Abstract
Nasopharyngeal colonization precedes invasive pneumococcal disease. HIV infection increases rates of invasive disease; its effect on colonization is unknown. In a longitudinal cohort of HIV-positive/negative Zambian mothers, HIV increased the risk of colonization (RR 1.9, 95% CI 1.3–2.8) and repeated colonizations (RR 2.4, 95% CI 1.1–5.3), and reduced time to new colonization (p=0.01). Repeated colonizations with homologous sero/factor-types occurred only among HIV-positive mothers. Pediatric serotypes 6, 19 and 23 accounted for the excess colonization in the HIV-positive mothers. HIV significantly increases the risk of pneumococcal colonization. Increased colonization by pediatric serotypes suggests a potential role for the 7-valent pneumococcal vaccine.
INTRODUCTION
HIV/AIDS significantly increases the risk of invasive disease with Streptococcus pneumoniae[1–7] Nasopharyngeal and/or oropharyngyeal colonization is considered a pre-condition for invasive pneumococcal disease [8, 9]. Whether HIV increases the risk of nasopharyngeal colonization in children or adults is unclear.[10, 11] Recent studies suggest that HIV-positive adults tend to be colonized by pneumococcal serotypes more commonly seen in children.[12–14]
The ‘co-Trimoxazole in Zambian Infants’ study’ (TZI) was a prospective cohort study primarily focused on the effect of cotrimoxazole (trimethoprim-sulfamethoxazole) prophylaxis on pneumococcal colonization and drug resistance among infants with perinatal HIV exposure, but also looked at colonization in the mothers. This study provided a means to address two fundamental questions about the relationship between HIV and S. pneumoniae colonization in adults: 1.) Does HIV increase rates of pneumococcal colonization?; and 2.) Is the distribution of pneumococcal serotypes influenced by HIV?
METHODS
Study overview
Because the principal focus of TZI was on the impact of cotrimoxazole prophylaxis[15] among infants, CD4 counts were only obtained on 25 of the mothers, and not based on any a priori sampling scheme. HIV-positive mothers were not provided with prophylactic cotrimoxazole, though infants of HIV positive mothers did receive cotrimoxazole through at least 12 months of age. Antiretroviral medications were virtually unavailable during the study period.
Laboratory procedures
Mothers attending three antenatal clinics in Ndola, Zambia underwent HIV voluntary counseling and testing. Screening was via Determine 1+2 tests™ (Abbott Laboratories, Abbott Park, IL), confirmed using the Capillus test™ (Cambridge Biotech Ltd., Galway, Ireland).[16] with Bioline™ testing (Bionor AS, Skien, Norway) for indeterminate/discrepant results.[17]
Our target sample size was 260 mother/infant pairs, balanced equally between HIV-positive and negative mothers. HIV-positive mothers were approached sequentially following HIV testing. All mothers provided written informed consent prior to enrollment. Whenever an HIV-positive mother was enrolled, we subsequently enrolled an HIV-negative mother/infant pair from the same clinic. Hence, the cohort was matched by infant age, maternal HIV status, clinic, and time of year. Each mother was seen according to a 7-visit schedule, dictated by the age of their infants starting at 6 weeks through 18 months.
We screened for S. pneumoniae colonization at each visit using posterior nasopharyngeal samples using sterile calcium-alginate tipped aluminum swabs.[18] To maximize yields, swabs were plated immediately onto room temperature soy-trypticase agar plates with 5% sheep’s blood/5% gentamicin (gent/BAP) and streaked later for colony separation at our study laboratory.[18–20] Screening plates were incubated at 37°C under 5% CO2 atmosphere for 48 hours. Colonies were presumptively identified as S. pneumoniae by colony morphology, and typical diplococcoid morphology on gram stain.[21] For confirmation, colonies from the screening gent/BAP plates were subcultured onto gentamicin-free BAP, and defined as S. pneumoniae if they had ≥ 14 MM optochin (ethylhydrocupreine, Difco, Detroit) inhibition zones, or via bile-solubility for <14 MM optochin inhibition.[22] Sub-cultured isolates were characterized to the serogroup and serotype level using the Statens Serum Institute (SSI) (Copenhagen, Denmark) latex slide agglutination system, with subsequent factor typing of the dominant serotypes.
Statistical analyses
We constructed Kaplan-Meier survival curves for time to first colonization using visit numbers (baseline = visit 1; 3 months = visit 2; etc…) as the time variable, and bivariate estimates of risk ratios (RR), hazard ratios (HR) with 95% confidence intervals (95% CI), and/or tests of proportions using maternal HIV status as the grouping variable. Due to the longitudinal structure of the data set with multiple observations on individuals we estimated relative risks (RR) using a log-linear model using robust standard errors to adjust for repeated measures.[23]
RESULTS
Between December 2003 and November 2005, we enrolled 132 HIV-positive and 128 HIV-negative mother/infant pairs (260 total). Baseline demographics were similar between the two groups (see ‘’) except that a slightly higher proportion of the HIV-positive mothers were widowed (4/129 vs. 0/128, p=0.04). Study attrition was more common among the HIV-negative mothers (see ‘’). No mothers died.
We obtained 1402 nasopharyngeal swabs from the mothers. Streptococcus pneumoniae were identified in 124 samples (8.8%) (86/756 samples from HIV-positive others were positive (11.4%) vs. only 38/646 (5.9%) samples from HIV-negative mothers (p<0.001)). Maternal HIV seropositivity nearly doubled the risk of a sample testing positive (RR 1.9, 95% CI 1.3–2.8). The risk of colonization did not change significantly after multivariate adjustments for any baseline variables (data not shown).
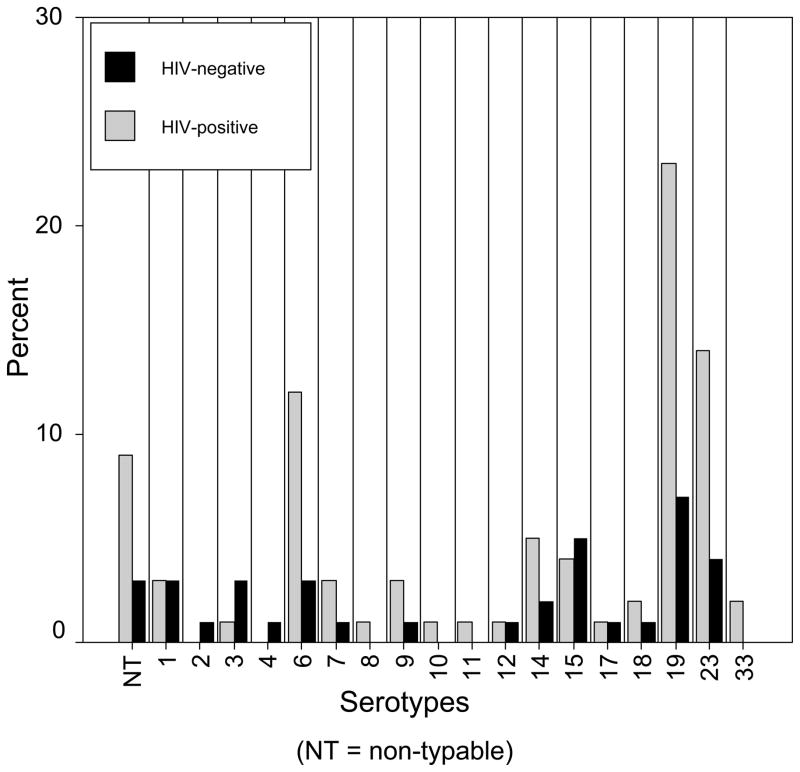
Pneumococcal serotype distribution differed between the two study arms (Figure 1), reflected as increases in serotypes 6, 19, and 23 among the HIV-positive mothers. These serotypes accounted for 59% of serotypes among the HIV-positive mothers vs. 37% serotypes among the HIV-negatives (RR 1.5, 95% CI 0.96–2.4). Serotypes 7, 8, 9, 10, 11, 14 and 33, occurred predominantly or exclusively among the HIV-positive mothers (RR 1.8, 95% CI 0.7–5.0). Among the dominant serotypes the predominant sero/factor types were: 6b, 19f, and 23f. HIV-positive mothers were significantly more likely to be colonized with a serotype that was represented by the 7-valent conjugate vaccine (for colonization with serotypes 4, 6b, 9, 14, 18, 19f, or 23f: RR 1.4, 95% CI 1.1–1.7).
Figure 1.
Distribution of colonizing pneumococcal serotypes among HIV-positive and HIV-negative mothers.
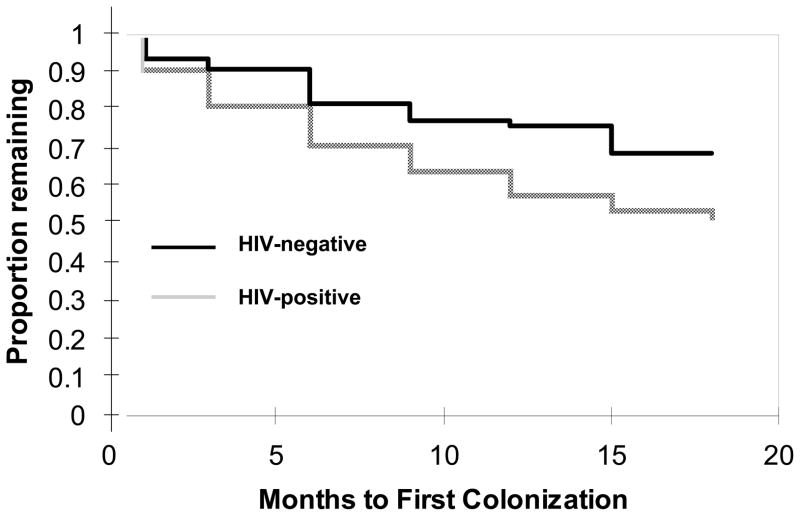
More HIV-positive mothers were colonized at baseline (Figure 2). Thereafter, new colonizations occurred significantly earlier among the HIV-positive mothers (log rank, p=0.01). HIV-positive mothers had increased risk for ever becoming colonized vs. never being colonized (56/131 (42%) HIV-positive vs. 30/128 (23%) HIV-negative mothers: HR 1.8, 95% CI 1.3–2.7).
Figure 2.
Time to first colonization by HIV status
HIV positivity significantly increased the risk that a mother would be colonized repeatedly (Table 1). Twenty HIV-positive mothers were colonized on ≥ 2 visits vs. 8 HIV-negative mothers (RR 2.4, 95% CI 1.1–5.3). Six HIV-positive mothers were colonized ≥ 3 times; 1 HIV-positive mother who was colonized at five of her seven visits, each with a different serotype. No HIV-negative mothers were colonized more than twice and never more than once with the same serotype.
Table 1.
Frequency of colonization with S. pneumoniae (any serotype) among HIV-positive and negative mothers
| Colonized on this many visits | Number (%) of HIV-positives (N = 131*) | Number (%) of HIV-negatives (N = 128) |
|---|---|---|
| 0 | 75 (57.3%) | 98 (76.6%) |
| 1 | 36 (27.5%) | 22 (17.2%) |
| 2 | 14 (10.7%) | 8 (6.3%) |
| 3 | 3 (2.3%) | 0 (0.0%) |
| 4 | 2 (1.5%) | 0 (0.0%) |
| 5 | 1 (0.8%) | 0 (0.0%) |
| ≥6 | 0 (0.0%) | 0 (0.0%) |
One subject refused all nasopharyngeal swabbing.
Repeat colonizations with a homologous sero/factor type at >1 visit was uncommon, and only occurred among HIV-positive mothers. Six of 132 HIV-positive mothers had repeated colonizations with a homologous strain vs. zero of 128 HIV-negative mothers (p=0.01). Among these, repeat colonizations occurred for 19a, 19c, 19f, 23f, and 33f (Table 2). For cases 2, 3, 4, and 5, repeat colonizations occurred at contiguous visits.
Table 2.
Homologous pneumococcal sero/factor types repeatedly colonizing individual mothers
| Case No. | Mother’s HIV status* | Visit | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 9 months | 12 months | 15 months | 18 months | ||
| 1 | Positive | 19a | 19a | |||||
| 2 | Positive | 19c | 19c | |||||
| 3 | Positive | 19f | 19f | |||||
| 4 | Positive | 19f | 19f | 19f | ||||
| 5 | Positive | 23f | 23f | 7 | 23a | |||
| 6 | Positive | 33f | 6b | 33f | ||||
No repeat colonizations with a homologous strain of S. pneumoniae occurred among HIV-negative mothers
DISCUSSION
HIV significantly increased the risk of pneumococcal colonization. This conclusion rests on the following observations: 1.) A significantly higher proportion of nasopharyngeal swabs yielded pneumococci from HIV-positive mothers than HIV-negative mothers; 2.) HIV-positive mothers became newly colonized earlier than HIV-negative mothers; 3.) HIV-positive mothers were more likely to be colonized repeatedly with different strains of pneumococci over time; and 4.) repeat colonization with a homologous pneumococcal strain occurred only among the HIV-positive mothers, with most of these occurring at sequential visits. Thus, not only are HIV-positive mothers more likely to be colonized, to become newly colonized, and to be repeatedly colonized, but when they are colonized, colonization appears to persist longer than in HIV-negative mothers.
HIV infection strongly influenced the dominant serotypes of the colonizing pneumococci. HIV-positive mothers were far more likely to be colonized with the pediatric serotypes 6, 19, and 23 (and in particular 6b, 19f and 23f) than the HIV-negative mothers. This is consistent with other reports [14, 24] and has significant implications, given that these same serotypes are represented by the 7-valent protein conjugate vaccine. While the TZI study focused only on colonization rather than invasive disease, it remains true that the most common serotypes found colonizing our patients happen to be the same as those identified time and again in the context of invasive pneumococcal disease surveys.[25–27]
It is worth considering why previous reports reached different conclusions about the effect of HIV on colonization. Nicolletti et al found no association between HIV viral loads and CD4 counts and the risk of pneumococcal colonization. However, this conclusion rested on only 64 isolates and used historical controls, with no internal HIV-negative control arm to serve as a true reference group.[12] Leibowitz actually found lower rates of pneumococcal colonization in a cross-sectional study of HIV-positive and negative orphans in Romania.[11] However, only a minority of the children had HIV, and the HIV-positive and HIV-negative orphans were housed separately, thereby introducing a significant cluster effect not accounted for in their analysis. Several other studies finding no association between HIV and pneumococcal carriage rates were conducted cross-sectionally in the context of patients either under evaluation for or being treated for acute respiratory infections.[28, 29] However, whether HIV affects colonization rates in patients who may already have invasive disease is a very different question from whether HIV alters the colonization burden and the risk for future invasive disease. Because the sample collection for TZI was done systematically according to a pre-defined schedule, regardless of HIV status or of intercurrent clinical events, it should be free of such ascertainment biases.
The 23-valent polysaccharide vaccine is currently recommended for HIV-positive patients by the US Centers for Disease Control and Prevention,[30] despite evidence of reduced efficacy among patients with HIV.[31] Prior research suggest that this reflects attenuated immune responses among HIV-positive patients from failure to develop therapeutic level of capsule-specific IgG[32] and more rapid declines in antibody titres over time.[33] Quite troubling are data from the only randomized controlled trial specifically designed to test the efficacy of the 23-valent vaccine among HIV-positive patients, which failed to show benefit to the vaccine and suggested potential harm. This study showed a statistically significant increase in all-cause pneumonia among HIV-positive Ugandan adult vaccinees, with a seemingly paradoxical increased risk of invasive disease due to serotypes represented by the 23-valent vaccine.[34] This highlights the need for more immunogenic and efficacious vaccines suitable for use in HIV-positive adults. The 7-valent conjugate protein vaccine is licensed for pediatric use. However, the increase in colonization with pediatric serotypes seen here supports a possible role for the 7-valent or similarly constructed vaccine among HIV-positive adults. Our finding that HIV increased the probability that colonization would be due to a serotype covered partially or specifically by the vaccine strengthens this argument.
One of the principal limitations of this analysis is that we had very little data on the immune status of the mothers beyond their HIV status. Only 25 of the HIV-positive women underwent CD4 testing, among whom the mean CD4 count was nearly 500. If this sub-sample is representative of the larger group of HIV-positive women in our cohort, then it is likely that most were at a relatively early stage in HIV/AIDS. Similarly, we did not have detailed information about intercurrent antibiotic use among the mothers, so were unable to determine whether the rate of colonization among HIV-positive mothers using cotrimoxazole prophylaxis might have been suppressed compared with HIV-positive non-users. Similarly, the infants of the HIV-positive mothers used cotrimoxazole prophylaxis for most of the study period. However, both of these would likely bias the effect of HIV on pneumococcal colonization towards the null. Our study participants were all post-partum Zambian women who were breastfeeding, and this could theoretically affect the generalizability of these findings.
A methodological limitation is that we were unable to determine whether a given isolate that occurred repeatedly in a given individual, consecutively or in a punctuated pattern, was the same isolate persisting over time or genetically unique isolates that happened to share the same serotype. Given that serial colonizations only occurred in the HIV-positive population, and then most often across contiguous visits, the former explanation appears more plausible. Nevertheless, the latter explanation would still suggest that HIV-positive patients were failing to develop adequate mucosal immunity to prevent re-colonization with a genetically distinct but serologically homologous serotype.
In conclusion, our analysis supports a causal association between HIV infection and increased colonization with S. pneumoniae, an observation that we hypothesize may partially explain the increased rates of invasive pneumococcal disease in patients with HIV/AIDS. The increased prevalence of pediatric serotypes among the HIV-positive patients, all of which are targeted by existing 7-valent conjugate pneumococcal vaccines should encourage further research into whether these vaccines might be used to protect HIV-positive adults, either by increasing pediatric vaccination coverage to reduce spread to the adults, or by vaccinating the adults themselves.
Acknowledgments
We wish to thank the following individuals: Ms. Anne Bolmström, president and CEO of AB-Biodisk, for donating a portion of the E-tests used in this study; Mr. Theo Leuenberger of Roche Pharmaceuticals for donating the cotrimoxazole; Dr. Anne von Gottberg for her invaluable advice on all microbiological aspects of this study; Ms. Christine Ayash and Ms. Anna Knapp who served as project managers for TZI; ans Ms. Sushma Hyoju who cleaned the TZI data set. We would particularly like to thank the study nurses who worked on TZI: Victoria Luo, Joyce M. Mulenga, Joyce W. Mulenga, Rosaline Kapupa, Edna Mungwa, and sister Kasongo. The nevirapine and Determine 1+2 tests were donated by Boehringer Ingelheim and Abbott laboratories through the Axios Corporation. The TZI study was supported by NIH/NIAID K23 AI 62208 and a cooperative agreement between Boston University and the Office of Health and Nutrition of the United States Agency for International Development: GHS-A-00-03-00020-00. The funders and commercial donors listed above played no role in the design, implementation, analysis/interpretation of the data, or writing of the manuscript. Some of these data were presented in abstract for at the 2005 Infectious Disease Society of America Conference.
References
- 1.Janoff EN, Breiman RF, Daley CL, Hopewell PC. Pneumococcal disease during HIV infection. Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992;117:314–24. doi: 10.7326/0003-4819-117-4-314. [DOI] [PubMed] [Google Scholar]
- 2.Jones N, Huebner R, Khoosal M, Crewe-Brown H, Klugman K. The impact of HIV on Streptococcus pneumoniae bacteraemia in a South African population. Aids. 1998;12:2177–84. doi: 10.1097/00002030-199816000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Gilks CF, Ojoo SA, Ojoo JC, et al. Invasive pneumococcal disease in a cohort of predominantly HIV-1 infected female sex-workers in Nairobi, Kenya. Lancet. 1996;347:718–23. doi: 10.1016/s0140-6736(96)90076-8. [DOI] [PubMed] [Google Scholar]
- 4.Madhi SA, Petersen K, Madhi A, Wasas A, Klugman KP. Impact of human immunodeficiency virus type 1 on the disease spectrum of Streptococcus pneumoniae in South African children. Pediatr Infect Dis J. 2000;19:1141–7. doi: 10.1097/00006454-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis. 2000;31:170–6. doi: 10.1086/313925. [DOI] [PubMed] [Google Scholar]
- 6.Karstaedt AS, Khoosal M, Crewe-Brown HH. Pneumococcal bacteremia in adults in Soweto, South Africa, during the course of a decade. Clin Infect Dis. 2001;33:610–4. doi: 10.1086/322589. [DOI] [PubMed] [Google Scholar]
- 7.Gesner M, Desiderio D, Kim M, et al. Streptococcus pneumoniae in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J. 1994;13:697–703. doi: 10.1097/00006454-199408000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Gray BM, Converse GM, 3rd, Dillon HC., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–33. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 9.Gray BM, Dillon HC., Jr Clinical and epidemiologic studies of pneumococcal infection in children. Pediatr Infect Dis. 1986;5:201–7. doi: 10.1097/00006454-198603000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso VC, Cervi MC, Cintra OA, Salathiel AS, Gomes AC. Nasopharyngeal colonization with Streptococcus pneumoniae in children infected with human immunodeficiency virus. J Pediatr (Rio J) 2006;82:51–7. doi: 10.2223/JPED.1437. [DOI] [PubMed] [Google Scholar]
- 11.Leibovitz E, Dragomir C, Sfartz S, et al. Nasopharyngeal carriage of multidrug-resistant Streptococcus pneumoniae in institutionalized HIV-infected and HIV-negative children in northeastern Romania. Int J Infect Dis. 1999;3:211–5. doi: 10.1016/s1201-9712(99)90027-9. [DOI] [PubMed] [Google Scholar]
- 12.Nicoletti C, Brandileone MC, Guerra ML, Levin AS. Prevalence, serotypes, and risk factors for pneumococcal carriage among HIV-infected adults. Diagn Microbiol Infect Dis. 2007 doi: 10.1016/j.diagmicrobio.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Frankel RE, Virata M, Hardalo C, Altice FL, Friedland G. Invasive pneumococcal disease: clinical features, serotypes, and antimicrobial resistance patterns in cases involving patients with and without human immunodeficiency virus infection. Clin Infect Dis. 1996;23:577–84. doi: 10.1093/clinids/23.3.577. [DOI] [PubMed] [Google Scholar]
- 14.Crewe-Brown HH, Karstaedt AS, Saunders GL, et al. Streptococcus pneumoniae blood culture isolates from patients with and without human immunodeficiency virus infection: alterations in penicillin susceptibilities and in serogroups or serotypes. Clin Infect Dis. 1997;25:1165–72. doi: 10.1086/516104. [DOI] [PubMed] [Google Scholar]
- 15.UNAIDS. UNAIDS/WHO hail consensus on use of cotrimoxazole for prevention of HIV-related infections in Africa. 2000. [PubMed] [Google Scholar]
- 16.McKenna SL, Muyinda GK, Roth D, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. Aids. 1997;11 (Suppl 1):S103–10. [PubMed] [Google Scholar]
- 17.Phili R, Vardas E. Evaluation of a rapid human immunodeficiency virus test at two community clinics in Kwazulu-Natal. S Afr Med J. 2002;92:818–21. [PubMed] [Google Scholar]
- 18.O’Brien KL, Nohynek H. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:e1–11. doi: 10.1097/01.inf.0000049347.42983.77. [DOI] [PubMed] [Google Scholar]
- 19.Converse GM, 3rd, Dillon HC., Jr Epidemiological studies of Streptococcus pneumoniae in infants: methods of isolating pneumococci. J Clin Microbiol. 1977;5:293–6. doi: 10.1128/jcm.5.3.293-296.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sondag JE, Morgens RK, Hoppe JE, Marr JJ. Detection of pneumococci in respiratory secretions: clinical evaluation of gentamicin blood agar. J Clin Microbiol. 1977;5:397–400. doi: 10.1128/jcm.5.4.397-400.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NCCLS. NCCLS document M100-S9. Villanova, PA: National Committee for Clinical Laboratory Standards; 1991. Performance standards for antimicrobial susceptibility testing: 9th informational supplement. [Google Scholar]
- 22.Pikis A, Campos JM, Rodriguez WJ, Keith JM. Optochin resistance in Streptococcus pneumoniae: mechanism, significance, and clinical implications. J Infect Dis. 2001;184:582–90. doi: 10.1086/322803. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey FL, Schafer DW. The Statistical Sleuth - a Course in Methods of Data Analysis. 1. Belmont, CA: Duxbury Press; 1997. [Google Scholar]
- 24.Blossom DB, Namayanja-Kaye G, Nankya-Mutyoba J, et al. Oropharyngeal colonization by Streptococcus pneumoniae among HIV-infected adults in Uganda: assessing prevalence and antimicrobial susceptibility. Int J Infect Dis. 2006;10:458–64. doi: 10.1016/j.ijid.2006.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huebner RE, Wasas AD, Klugman KP. Trends in antimicrobial resistance and serotype distribution of blood and cerebrospinal fluid isolates of Streptococcus pneumoniae in South Africa, 1991–1998. Int J Infect Dis. 2000;4:214–8. doi: 10.1016/s1201-9712(00)90112-7. [DOI] [PubMed] [Google Scholar]
- 26.Feikin DR, Davis M, Nwanyanwu OC, et al. Antibiotic resistance and serotype distribution of Streptococcus pneumoniae colonizing rural Malawian children. Pediatr Infect Dis J. 2003;22:564–7. [PubMed] [Google Scholar]
- 27.Klugman KP, Koornhof HJ. Drug resistance patterns and serogroups or serotypes of pneumococcal isolates from cerebrospinal fluid or blood, 1979–1986. J Infect Dis. 1988;158:956–64. doi: 10.1093/infdis/158.5.956. [DOI] [PubMed] [Google Scholar]
- 28.Polack FP, Flayhart DC, Zahurak ML, Dick JD, Willoughby RE. Colonization by Streptococcus pneumoniae in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2000;19:608–12. doi: 10.1097/00006454-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Rusen ID, Fraser-Roberts L, Slaney L, et al. Nasopharyngeal pneumococcal colonization among Kenyan children: antibiotic resistance, strain types and associations with human immunodeficiency virus type 1 infection. Pediatr Infect Dis J. 1997;16:656–62. doi: 10.1097/00006454-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 30.CDC updates opportunistic disease prevention guidelines. AIDS Policy Law. 2002;17:3. [PubMed] [Google Scholar]
- 31.Mangtani P, Cutts F, Hall AJ. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infect Dis. 2003;3:71–8. doi: 10.1016/s1473-3099(03)00514-0. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed F, Steinhoff MC, Rodriguez-Barradas MC, Hamilton RG, Musher DM, Nelson KE. Effect of human immunodeficiency virus type 1 infection on the antibody response to a glycoprotein conjugate pneumococcal vaccine: results from a randomized trial. J Infect Dis. 1996;173:83–90. doi: 10.1093/infdis/173.1.83. [DOI] [PubMed] [Google Scholar]
- 33.Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Antibodies against pneumococcal polysaccharides after vaccination in HIV-infected individuals: 5-year follow-up of antibody concentrations. Vaccine. 1999;18:524–30. doi: 10.1016/s0264-410x(99)00240-6. [DOI] [PubMed] [Google Scholar]
- 34.French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355:2106–11. doi: 10.1016/s0140-6736(00)02377-1. [DOI] [PubMed] [Google Scholar]