Abstract
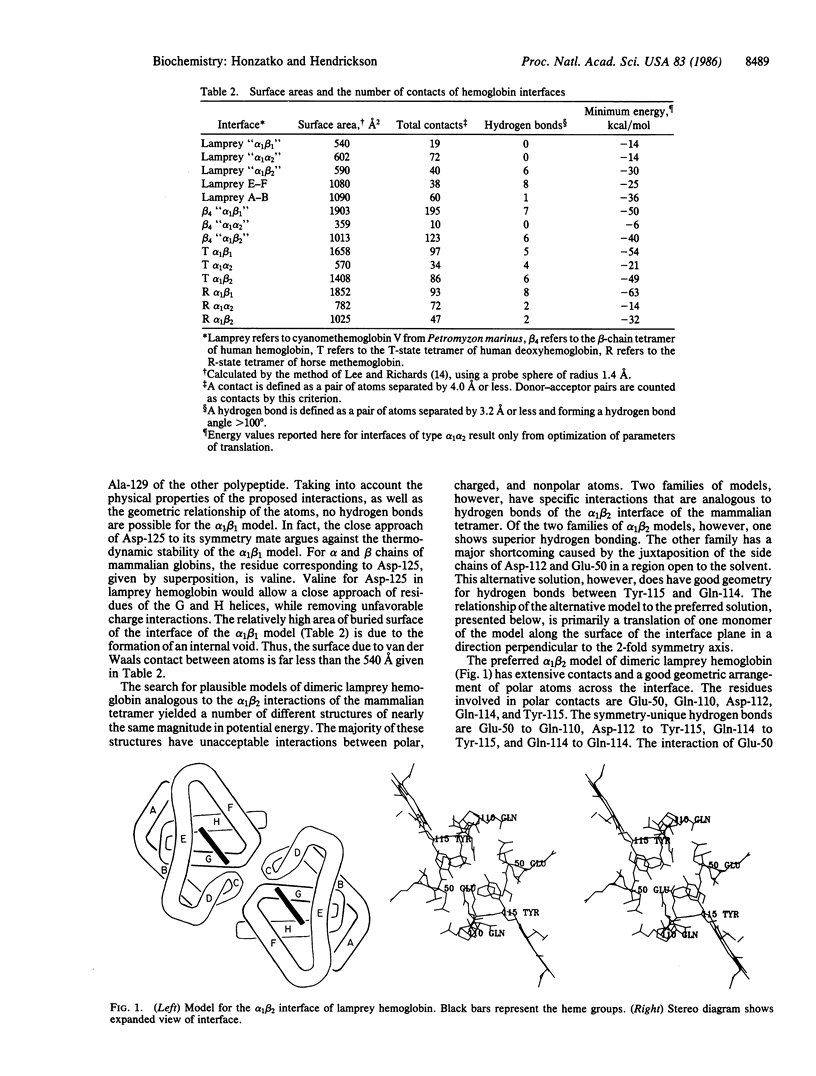
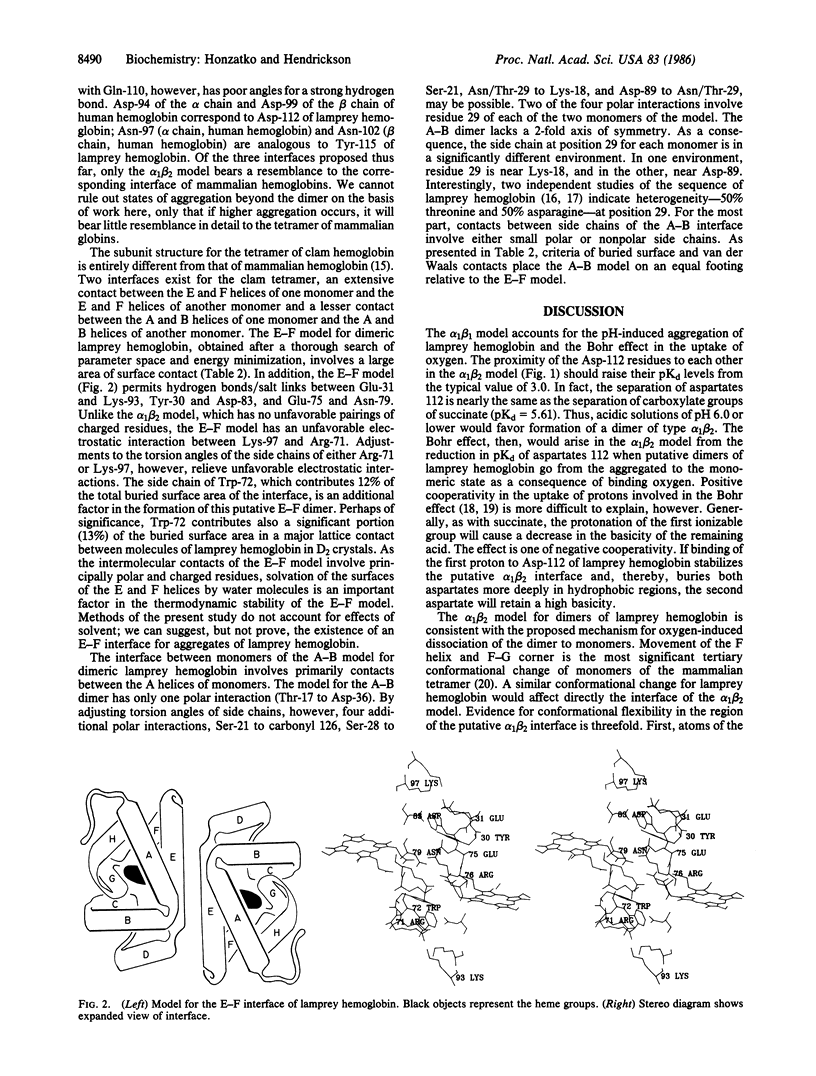
The known structures for the tetramers of mammalian and clam hemoglobins provide a point of departure for the modeling of putative dimers of lamprey hemoglobin. The association of subunits is dissimilar for the clam and mammalian tetramers; the superposition of the molecular model for lamprey methemoglobin onto the mammalian and clam tetramers gives five distinct dimers. After energy minimization of the interface regions of the five models, three models afford promising interactions between side chains. One model is analogous to the alpha 1 beta 2 pairing of subunits of mammalian hemoglobins. The other two models are similar to the interfaces between the E and F helices and between the A and B helices of clam hemoglobin. Although the model based on the alpha 1 beta 2 mode of association provides the best explanation of biochemical properties of lamprey hemoglobin, such as the Bohr effect and the dependency of dimer formation on pH, interfaces between the E and F and the A and B helices could be important in the aggregation of monomers of lamprey hemoglobin beyond the level of the dimer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., BELLELLI L., RUMEN N., SINISCALCO M. THE OXYGEN EQUILIBRIUM OF SOME LAMPREY HEMOGLOBINS. Arch Biochem Biophys. 1964 May;105:404–408. doi: 10.1016/0003-9861(64)90024-4. [DOI] [PubMed] [Google Scholar]
- Andersen M. E. Sedimentation equilibriujm experiments on the self-assocation of hemoglobin from the lamprey Petromyzon marinus. A model for oxygen transport in the lamprey. J Biol Chem. 1971 Aug 10;246(15):4800–4806. [PubMed] [Google Scholar]
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol. 1979 Apr 5;129(2):175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Bardack D., Zangerl R. First fossil lamprey: a record from the Pennsylvanian of Illinois. Science. 1968 Dec 13;162(3859):1265–1267. doi: 10.1126/science.162.3859.1265. [DOI] [PubMed] [Google Scholar]
- Behlke J., Scheler W. Dder einfluss von liganden auf den assoziationsgrad des desoxy-hämoglobins der flussneunaugen (lampetra fluviatilis L.). FEBS Lett. 1970 Apr 2;7(2):177–179. doi: 10.1016/0014-5793(70)80150-8. [DOI] [PubMed] [Google Scholar]
- Behlke J., Scheler W. Zur Wirkung von Liganden auf das Assoziations-Dissoziations-Gleichgewicht des Methämoglobins der Flussneunaugen (Lampetra fluviatilis L.) Eur J Biochem. 1970 Aug;15(2):245–249. doi: 10.1111/j.1432-1033.1970.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Furuta H., Kajita A. Dimeric hemoglobin of the bivalve mollusc Anadara broughtonii: complete amino acid sequence of the globin chain. Biochemistry. 1983 Feb 15;22(4):917–922. doi: 10.1021/bi00273a032. [DOI] [PubMed] [Google Scholar]
- Goodman M., Moore G. W., Matsuda G. Darwinian evolution in the genealogy of haemoglobin. Nature. 1975 Feb 20;253(5493):603–608. doi: 10.1038/253603a0. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A. Structural effects accompanying ligand change in crystalline lamprey hemoglobin. Biochim Biophys Acta. 1973 May 17;310(1):32–38. doi: 10.1016/0005-2795(73)90005-6. [DOI] [PubMed] [Google Scholar]
- Hombrados I., Rodewald K., Neuzil E., Braunitzer G. Haemoglobins, LX. Primary structure of the major haemoglobin of the sea lamprey Petromyzon marinus (var. Garonne, Loire). Biochimie. 1983 Apr-May;65(4-5):247–257. doi: 10.1016/s0300-9084(83)80276-4. [DOI] [PubMed] [Google Scholar]
- Honzatko R. B., Hendrickson W. A., Love W. E. Refinement of a molecular model for lamprey hemoglobin from Petromyzon marinus. J Mol Biol. 1985 Jul 5;184(1):147–164. doi: 10.1016/0022-2836(85)90049-x. [DOI] [PubMed] [Google Scholar]
- Ladner R. C., Heidner E. J., Perutz M. F. The structure of horse methaemoglobin at 2-0 A resolution. J Mol Biol. 1977 Aug 15;114(3):385–414. doi: 10.1016/0022-2836(77)90256-x. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Levitt M. Energy refinement of hen egg-white lysozyme. J Mol Biol. 1974 Jan 25;82(3):393–420. doi: 10.1016/0022-2836(74)90599-3. [DOI] [PubMed] [Google Scholar]
- Li S. L., Riggs A. The amino acid sequence of hemoglobin V from the lamprey, Petromyzon marinus. J Biol Chem. 1970 Nov 25;245(22):6149–6169. [PubMed] [Google Scholar]
- Phillips S. E. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980 Oct 5;142(4):531–554. doi: 10.1016/0022-2836(80)90262-4. [DOI] [PubMed] [Google Scholar]
- Royer W. E., Jr, Love W. E., Fenderson F. F. Cooperative dimeric and tetrameric clam haemoglobins are novel assemblages of myoglobin folds. Nature. 1985 Jul 18;316(6025):277–280. doi: 10.1038/316277a0. [DOI] [PubMed] [Google Scholar]
- Tentori L., Vivaldi G., Carta S., Marinucci M., Massa A., Antonini E., Brunori M. The amino acid sequence of myoglobin from the mollusc Aplysia limacina. Int J Pept Protein Res. 1973;5(4):187–200. doi: 10.1111/j.1399-3011.1973.tb03452.x. [DOI] [PubMed] [Google Scholar]
- Weber E., Steigemann W., Jones T. A., Huber R. The structure of oxy-erythrocruorin at 1.4 X resolution. J Mol Biol. 1978 Apr 5;120(2):327–336. doi: 10.1016/0022-2836(78)90071-2. [DOI] [PubMed] [Google Scholar]



