Abstract
We recently demonstrated that daily whole egg consumption during moderate carbohydrate restriction leads to greater increases in plasma HDL-cholesterol (HDL-C) and improvements in HDL profiles in metabolic syndrome (MetS) when compared to intake of a yolk-free egg substitute. We further investigated the effects of this intervention on HDL composition and function, hypothesizing that the phospholipid species present in egg yolk modulate HDL lipid composition to increase the cholesterol-accepting capacity of subject serum. Men and women classified with MetS were randomly assigned to consume either three whole eggs (EGG, n = 20) per day or the equivalent amount of egg substitute (SUB, n = 17) throughout a 12-week moderate carbohydrate-restricted (25–30 % of energy) diet. Relative to other HDL lipids, HDL-cholesteryl ester content increased in all subjects, with greater increases in the SUB group. Further, HDL-triacylglycerol content was reduced in EGG group subjects with normal baseline plasma HDL-C, resulting in increases in HDL-CE/TAG ratios in both groups. Phospholipid analysis by mass spectrometry revealed that HDL became enriched in phosphatidylethanolamine in the EGG group, and that EGG group HDL better reflected sphingomyelin species present in the whole egg product at week 12 compared to baseline. Further, macrophage cholesterol efflux to EGG subject serum increased from baseline to week 12, whereas no changes were observed in the SUB group. Together, these findings suggest that daily egg consumption promotes favorable shifts in HDL lipid composition and function beyond increasing plasma HDL-C in MetS.
Keywords: HDL, Phospholipids, Cholesterol efflux, Metabolic syndrome, Eggs, Carbohydrate-restricted diet
Introduction
HDL is a key cardioprotective biomarker in obesity-related metabolic diseases, including cardiovascular disease (CVD) and metabolic syndrome (MetS) [1]. The primary mechanism by which HDL promotes cardiovascular health is thought to be through mediating the acquisition of lipids from macrophage foam cells within the arterial wall for participation in reverse cholesterol transport (RCT) [2], while antithrombotic, anti-inflammatory, and antioxidant properties of HDL confer further protection against atherosclerosis [3]. It is well recognized, however, that HDL function is impaired under inflammatory conditions associated with obesity, and that standard clinical measures of steady-state plasma HDL-cholesterol (HDL-C) often fail to capture the true atheroprotective—or even atherogenic—potential of circulating HDL particles [3, 4]. Therefore, therapeutic strategies aimed at enhancing HDL-mediated cardioprotection should target mechanisms that go beyond simply increasing HDL-C, and rather improve the quality of HDL as a lipid acceptor [5, 6].
The capacity of HDL to facilitate cellular cholesterol efflux is differentially affected by the diverse, heterogeneous nature of HDL size and structure, as well as protein and lipid composition [6]. While various HDL components and pathways have been targeted, modulation of HDL lipid composition—particularly the HDL-phospholipid (HDL-PL) fraction—is an emerging and promising approach to improving HDL function [7, 8]. In the majority of circulating HDL, phospholipids represent ~45–50 % of total lipids [9], with predominant phospholipid classes including phosphatidylcholine (PtdCho; ~80 %), sphingomyelin (CerPCho; ~10 %), phosphatidylethanolamine (PtdEtn; ~3 %), lysophosphatidylcholine (LysoPtdCho; ~1–2 %) and phosphatidylinositol (PtdIns; ~1–2 %) [10, 11].
Total HDL-PL, as well as the distribution of HDL phospholipid classes, has been related to CVD risk and severity [12, 13], and can differentially affect the lipid-accepting capacity of HDL [8, 14]. Greater enrichment of HDL in phospholipids—such as PtdCho and CerPCho—has also been associated with a greater lipid-accepting capacity of HDL and/or human serum; however, the extent by which different phospholipid classes can promote efflux may be variable across cellular models and efflux pathways mediated by ATP-binding cassette transporter A I (ABCA1), ATP-binding cassette transporter G I (ABCG1), scavenger receptor B I (SR-BI), or aqueous diffusion [7, 8, 15].
Dietary intake of phospholipids derived from soy, safflower, dairy, marine, and egg sources are known to favorably modulate plasma lipid levels in both humans and animal models [16-20]; however, few studies have investigated how intake of phospholipid-rich foods or supplements affect the relative distribution HDL-PL classes or lipid-accepting functions. Dietary phospholipids are known to be highly bioavailable (>90 %), can be partially absorbed intact in the intestine, and are preferentially incorporated into HDL following ingestion [21, 22]. Of all food sources to favorably modulate HDL-PL distribution and particle function, eggs represent one of the most promising candidates, since egg yolk is one of the richest dietary sources of PtdCho, PtdEtn, and CerPCho [23, 24]. Previous studies in our laboratory have demonstrated that consumption of three eggs per day as part of a carbohydrate-restricted diet improves atherogenic lipoprotein profiles and increases plasma HDL-C in over-weight men [25] and men and women with MetS [26, 27] to a greater extent when compared to intake of a yolk-free egg substitute. In MetS, beneficial changes from whole egg intake included greater increases in large HDL particles, HDL and LDL diameters, and lecithin–cholesterol acyltransferase (LCAT) activity, as well as greater reductions in atherogenic large and medium VLDL particles when compared to the intake of egg substitute [26]. These findings are significant since individuals with MetS can have dysfunctional HDL [28] and a twofold increased risk of developing CVD [29]. Therefore, the aim of this study was to determine whether improvements in HDL profiles from egg intake correspond to changes in HDL lipid composition and the cholesterol-accepting capacity of HDL. We hypothesized that daily consumption of phospholipid-rich egg yolk for 12 weeks would modulate HDL-PL distribution to reflect egg-derived phospholipid species, corresponding to greater cholesterol efflux to subject serum.
Materials and Methods
Study Design and Subjects
Experimental design and subject characteristics have previously been described [26, 27]. Briefly, 37 subjects (25 women; 12 men) classified with MetS were recruited to participate in a 12-week parallel, randomized, single-blind diet intervention. Eligibility for participation was dependent upon subjects falling within the age range of 30–70 years old, meeting the National Cholesterol Education Program Adult Treatment Panel III (NCEP:ATP III) revised criteria for MetS [30], and having no clinical diagnosis of chronic/metabolic disease [26, 27]. During the 12-week study, subjects were instructed to follow an ad libitum moderate carbohydrate-restricted diet (25–30 % of energy from carbohydrates) in addition to consuming either three whole eggs (EGG group) or the equivalent amount of egg yolk-free egg substitute (SUB group) each day. The egg substitute product consisted of egg whites (99 %), <1 % xanthan and guar gums, beta-carotene for color, and provided 0 mg of cholesterol, whereas the daily serving of whole egg contained 534 mg of cholesterol. Dietary analysis and nutrient composition of both egg products have been reported in greater detail elsewhere [26, 27]. This study was approved by the University of Connecticut Institutional Review Board; Protocol #: H10-173.
Plasma and Serum Collection
Fasting plasma and serum samples were collected at baseline and week 12. Plasma was obtained following blood collection into EDTA-coated tubes and centrifugation at 2,200×g for 20 min at 4 °C. A preservative cocktail (1 mL/L sodium azide, 1 mL/L phenylmethylsulfonyl fluoride, and 5 mL/L aprotinin) was added to plasma prior to storage. Serum was obtained following collection of blood into anticoagulant-free tubes and processed under sterile conditions. Both plasma and serum were aliquoted and stored at −80 °C until analysis.
Plasma Lipids and Body Weight
Fasting plasma HDL-C, total cholesterol (TC) and triacylglycerol (TAG) were measured by enzymatic methods using a Cobas c 111 clinical analyzer (Roche Diagnostics, Indianapolis, IN), as previously described [26]. LDL-C was estimated by the Friedewald equation [31]. Body weight was assessed biweekly from baseline to week 12.
Isolation of HDL Subfractions from Plasma
HDL subfractions (d = 1.063–1.21) were isolated from EGG (n = 19) and SUB (n = 15) subject plasma at baseline and week 12 by sequential ultracentrifugation as previously described [27]. Plasma yields from three subjects were not adequate to perform isolation. Following removal of VLDL, IDL, and LDL fractions (d < 1.063 g/mL), the remaining plasma was adjusted to d = 1.21 g/mL with KBr, and layered beneath a density solution (3 M KBr, 2.6 M NaCl, 2.7 mM EDTA; pH = 7.4; d = 1.21) in QuickSeal tubes (Beckman Coulter). Using an Optima LE-80 K ultracentrifuge, HDL subfractions were isolated by centrifugation for 3 h at 60,000 rpm in a vertical vTi-65 rotor. The HDL samples were then dialyzed for 24 h (0.9 % NaCl, 0.01 % Na2EDTA, pH 7.4), aliquoted, and stored at −80 °C for further analysis.
Analysis of HDL Lipid Components
Phospholipid (PL), free cholesterol (FC) (Wako Chemicals, Richmond, VA), TC and TAG (Pointe Scientific, Canton, MI) content of HDL subfractions (d = 1.063–1.21) was determined by commercially available reagent kits according to the manufacturer’s instructions. Cholesteryl ester (CE) content was calculated as (TC–FC) × 1.67.
Analysis of HDL and Egg Product Phospholipid Classes and Associated Species
Quantification of PtdCho, LysoPtdCho, PtdEtn, PtdIns, and CerPCho species in HDL samples and egg products was performed as described by Sorci-Thomas et al. [32]. Briefly, egg product and HDL lipids were extracted by the Bligh–Dyer method [33] containing internal standards, evaporated under an argon stream, and dissolved in chloroform/methanol (2:1). Lipid extracts were injected onto a 3.9 × 200 mm Waters μPorasil column (10 μm particle size) and analyzed by tandem mass spectrometry utilizing a Finnigan TSQ Quantum Discovery Max mass spectrometer (MS/MS). Data for each phospholipid class in egg products are presented mg/g of egg protein or mole-percent (mol%) of total phospholipids. Data for HDL phospholipid classes are presented as mol%. Percent homology of phospholipid molecular species for the EGG group was calculated as: [HDL molecular species (nmol) identified in both HDL and egg product/Total HDL molecular species (nmol)] × 100. Phospholipid species homology of the SUB group was similarly calculated by identifying the percentage of total HDL species (nmol) that were identified in the egg substitute product.
LDL Acetylation
LDL was isolated from combined fasting plasma samples from two healthy donors by sequential ultracentrifugation, as described previously [27]. Following isolation, LDL was dialyzed (0.15 M NaCL) for 24 h at 4 °C. Protein content was measured by bicinchoninic acid (BCA) assay (Thermo Scientific) modified to contain 0.2 % sodium dodecyl sulfate (SDS) as recommended by the manufacturer for measurement of lipoprotein samples. The LDL sample was added in equal parts to a saturated sodium acetate solution with continuous stirring in an ice water bath while protected from light. Acetic anhydride was then slowly added over 1 h (1.5 μL/mg LDL protein). The acetylated-LDL (acLDL) solution was dialyzed (0.15 M NaCl, 0.3 mM EDTA, pH 7.4) for 24 h at 4 °C then filter-sterilized for use in cholesterol efflux assays.
Cholesterol Efflux Assays
RAW 264.7 macrophages were used to conduct cholesterol efflux assays for a subset of subjects. Twenty-four hours after seeding on 12-well plates, cells were loaded with 100 μg/mL acLDL and 0.5 μCi [1,2-3H(N)]cholesterol (American Radiolabeled Chemicals, Inc., St. Louis, MO) for 24 h. Cells were then washed and treated with 10 μM T0901317 for 24 h to stimulate liver X receptor (LXR)-mediated expression of lipid transporters, ABCA1 and ABCG1. Efflux media was then added containing 20 % subject serum and 0.2 % BSA in RPMI, with each serum sample ran in triplicate. Efflux was performed for 3 h at 37 °C, followed by collection of cell media and cell lysates. Cell lysates were obtained by washing cells with 0.1 N NaOH and collection of the supernatant. Aliquots of cell media and lysates were added to a liquid scintillation cocktail and radioactivity was measured with a Beckman LS 6500 Scintillation Counter (Beckman Coulter Inc., Indianapolis, IN). Percent cholesterol efflux was calculated as [(3H-cholesterol radioactivity in media) × 100]/[(3H-cholesterol radioactivity in media) + (3H-cholesterol radioactivity in cell lysate)].
Statistical Analysis
All statistical analyses were performed using SPSS version 18. Repeated measures ANOVA was used to test the overall effects of the intervention between EGG vs. SUB groups (the between-subjects factor) and over time (the within-subjects factor). Paired t tests were used to test differences between baseline vs. week 12 values within EGG or SUB groups. Independent t tests were used to compare the differences in absolute or percent change in variables between groups. Bivariate Pearson correlations were used to determine relationships between parameters. Data are reported as means ± SD unless noted otherwise. P < 0.05 was considered significant.
Results
Egg Product Phospholipid Analysis
Whole egg and yolk-free egg substitute products were analyzed by mass spectrometry to identify the predominant phospholipid classes and associated molecular species (Table 1). The relative distribution of phospholipid classes was similar between the two egg products, with PtdCho representing the most abundant phospholipid class, followed by PtdEtn, and smaller amounts of CerPCho, LysoPtdCho, and PtdIns. As expected, the whole egg product provided significantly greater amounts of phospholipid within each class, since the majority of egg phospholipids are found in the yolk [23]. Additionally, a greater number of molecular species within each class—with the exception of CerPCho—were identified in the whole egg product. The percentage similarity of molecular species detected within each phospholipid class (i.e. species homology) between whole egg and egg substitute products ranged from 62.5 to 100 %.
Table 1.
Phospholipid composition of whole egg and egg substitute products
| Nutrient | Whole egg | Egg substitute | Species homology (%) |
|---|---|---|---|
| Total phospholipids (mg/serving) | 413.6 | 18.9 | |
| Molecular species (#) | 65 | 57 | 82.1 |
| PtdCho (mg/serving) | 308.8 (72.1 %) | 14.3 (73.2 %) | |
| Molecular species (#) | 21 | 19 | 82.0 |
| PtdEtn (mg/serving) | 86.1 (20.5 %) | 3.4 (17.9 %) | |
| Molecular species (#) | 19 | 17 | 89.5 |
| CerPCho (mg/serving) | 10.4 (2.6 %) | 0.6 (3.3 %) | |
| Molecular species (#) | 5 | 5 | 100.0 |
| LysoPtdCho (mg/serving) | 8.2 (2.9 %) | 0.3 (2.6 %) | |
| Molecular species (#) | 7 | 6 | 62.5 |
| PtdIns (mg/serving) | 9.4 (1.9 %) | 0.7 (3.0 %) | |
| Molecular species (#) | 13 | 10 | 69.2 |
Total phospholipids and individual phospholipid classes are reported as mg/daily serving (1/2 cup) of whole egg or egg substitute products. Values in parentheses represent the contribution of each phospholipid class to total phospholipids, reported as mole percent (mol%). The total number of individual molecular species identified within each phospholipid class is shown for both egg products. Species homology represents the (%) similarity of individual molecular species within each phospholipid class between whole egg and egg substitute products
PtdCho phosphatidylcholine, PtdEtn phosphatidylethanolamine, CerPCho sphingomyelin, LysoPtdCho lysophosphatidylcholine, PtdIns phosphatidylinositol
Baseline Characteristics and Changes in Body Weight
We have previously reported that there were no differences in plasma TC, LDL-C, HDL-C, weight, BMI, age, or the additional MetS parameters between EGG and SUB groups at baseline [26, 27]. As expected with moderate carbohydrate restriction, subjects in both groups significantly decreased body weight over the course of the 12-week intervention, with no differences between groups (EGG: −3.6 ± 2.6 kg vs. SUB: −3.4 ± 2.3 kg; P = 0.78).
Plasma HDL-C Classification and Response
Changes in plasma lipids and lipoprotein particle profiles have been reported elsewhere [26, 27]. Upon admission into the study, 55 % of EGG subjects met the MetS criteria for low plasma HDL-C (men: <40 mg/dL (1.03 mmol/L); women: <50 mg/dL (1.29 mmol/L)), similar to 53 % of SUB subjects enrolled. While no changes were observed in either TC or LDL-C throughout the 12-week intervention, plasma HDL-C increased in all subjects, with greater increases in the EGG group (+19.1 %) when compared to the SUB group (+9.9 %) (P < 0.05) (data not shown) [26, 27]. Interestingly, the difference in HDL-C response between groups was primarily driven by subjects who entered the study with normal HDL-C levels (EGG (n = 9): +11.56 ± 8.0 mg/dL vs. SUB (n = 8): +4.0 ± 3.5 mg/dL; P = 0.026), rather than those with low baseline HDL-C (EGG (n = 11): +6.1 ± 6.3 mg/dL vs. SUB (n = 9): +5.3 ± 6.9 mg/dL; P = 0.79). By the end of the intervention, only 15 % of subjects in the EGG group still met the MetS criteria for low plasma HDL-C, compared to 23.5 % of SUB subjects.
HDL Lipid Composition
HDL subfractions (d = 1.063–1.21) were analyzed to assess whether egg feeding could impact the relative distribution of HDL lipid fractions (Table 2). While there were no changes in HDL-FC or -PL content from baseline to week 12, HDL became relatively more enriched in CE in all subjects, with slightly greater increases in the SUB group. Reductions in HDL-TAG (P = 0.078) and HDL-PL (P = 0.082) content from baseline to week 12 were observed in EGG and SUB groups, respectively, although these changes failed to reach statistical significance. Interestingly, however, comparison of subjects with normal baseline HDL-C revealed that HDL-TAG content was reduced in the EGG group only (EGG: −2.2 ± 2.4 % vs. SUB: 0.0 ± 0.9 %; P = 0.03). Together, these changes led to increases in HDL-CE/TAG ratios in all subjects, whereas the slightly greater increases in the EGG group failed to reach significance when compared to the changes in the SUB group (P = 0.071). Baseline plasma HDL-C levels and gender did not appear to have an effect on changes in HDL lipid composition in either EGG or SUB group.
Table 2.
Effects of egg feeding on HDL lipid composition during moderate carbohydrate restriction
| EGG
|
SUB
|
P value
|
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Change | Baseline | Week 12 | Change | Time | Time × group | |
| CE (mass%) | 40.4 ± 3.4a | 41.3 ± 4.2a | +1.00 | 40.7 ± 3.4a | 42.1 ± 2.2b | +1.40 | 0.037 | 0.72 |
| FC (mass%) | 5.1 ± 1.5 | 5.6 ± 1.3 | +0.51 | 5.6 ± 1.0 | 5.5 ± 1.0 | −0.13 | 0.36 | 0.13 |
| PL (mass%) | 46.0 ± 3.8 | 45.4 ± 4.2 | −0.60 | 45.0 ± 3.8 | 43.9 ± 3.6 | −1.16 | 0.14 | 0.64 |
| TAG (mass%) | 8.5 ± 2.7 | 7.6 ± 2.4 | −0.91 | 8.6 ± 2.7 | 8.5 ± 2.7 | −0.11 | 0.11 | 0.21 |
| CE/TAG (mass ratio) | 5.5 ± 2.9 | 6.1 ± 2.6 | + 0.6 | 5.2 ± 1.8 | 5.4 ± 1.7 | +0.2 | 0.048 | 0.32 |
Individual lipid components of HDL subfractions (d = 1.063–1.21) from EGG (n = 19) and SUB (n = 15) groups are presented as percentage of the total HDL lipid mass (mass%). CE/TAG ratios are presented as mass ratios
Data are reported as means ± SD
Different letters within the same row indicate significantly different mean values (P < 0.05)
CE cholesteryl ester, FC free cholesterol, PL phospholipid, TAG triacylglycerol
Distribution of HDL Phospholipid Classes
HDL subfractions (d = 1.063–1.21) were further analyzed by mass spectrometry to identify the distribution of the predominant PL classes (Table 3). While the relative distribution of HDL-PtdCho, -LysoPtdCho, -PtdIns, or -CerPCho did not change throughout the intervention, enrichment of HDL in PtdEtn was observed in the EGG group only (EGG: +40.8 ± 16.3 % vs. SUB: −3.6 ± 25.5 %; P = 0.026). Interestingly, changes in HDL-PtdEtn content were positively associated with increases in plasma HDL-C (r = 0.74; P = 0.038), and negatively associated with changes in HDL-TAG content (r = −0.833; P = 0.010).
Table 3.
Effects of egg feeding on HDL-PL class distribution during moderate carbohydrate restriction
| mol% | EGG
|
SUB
|
P value
|
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Change | Baseline | Week 12 | Change | Time | Time × group | |
| PtdCho | 79.1 ± 3.4 | 76.1 ± 4.3 | −3.0 | 78.6 ± 1.4 | 77.3 ± 2.8 | −1.3 | 0.070 | 0.40 |
| PtdEtn | 3.1 ± 0.9a | 4.3 ± 1.0b | +1.2* | 3.2 ± 0.4a | 3.1 ± 0.7a | −0.2 | 0.085 | 0.031 |
| CerPCho | 11.8 ± 1.4 | 13.5 ± 3.6 | +1.7 | 12.1 ± 0.4 | 12.5 ± 0.6 | +0.5 | 0.28 | 0.54 |
| LysoPtdCho | 3.2 ± 2.3 | 3.9 ± 1.7 | +0.7 | 3.7 ± 1.7 | 4.4 ± 2.1 | +0.7 | 0.15 | 1.00 |
| PtdIns | 2.9 ± 0.5 | 2.3 ± 0.6 | −0.5 | 2.3 ± 0.4 | 2.7 ± 0.7 | +0.5 | 0.87 | 0.10 |
Phospholipid classes identified in HDL subfractions (d = 1.063–1.21) from EGG (n = 4) and SUB (n = 4) groups are presented as percent of total HDL-PL (mol%). Data are reported as means ± SD
Different letters within the same row indicate significantly different mean values (P < 0.05)
PtdCho phosphatidylcholine, PtdEtn phosphatidylethanolamine, CerPCho sphingomyelin, LysoPtdCho lysophosphatidylcholine, PtdIns phosphatidylinositol
P < 0.05; independent t test comparing changes in %HDL-PtdEtn from baseline and week 12 between EGG vs. SUB
Phospholipid Species Homology Between HDL and Egg Products
We further aimed to identify whether HDL subfractions became relatively more enriched in phospholipid molecular species present in the egg products throughout the intervention. In calculating the homology between HDL and egg product phospholipids, we found that molecular species identified in egg products represented an average 85, 84, 95, and 90 % of total HDL species of PtdCho, PtdEtn, LysoPtdCho, and PtdIns classes, respectively. This trend remained constant from baseline to week 12. The primary differences between PtdCho and PtdEtn from egg and human HDL were the concentrations of 1-O-alkyl- or -alk-1-enyl species. On average, human HDL contained three-fold more 1-O-alkyl- or -alk-1-enyl-phosphocholine and ninefold more 1-O-alkyl- or -alk-1-enyl-phosphoethanolamine (data not shown). In contrast, the CerPCho species identified in whole egg and egg substitute products represented only 23.2 and 24.5 % of HDL-CerPCho species in EGG and SUB groups at baseline, respectively. By the end of the 12-week intervention, HDL subfractions from the EGG group shared greater CerPCho species homology with the whole egg product (Fig. 1), whereas no changes in homology between SUB group HDL and the egg substitute product were observed. Further, changes in CerPCho species homology were positively correlated with changes in HDL-C (r = 0.80, P = 0.017). Increases in CerPCho species homology in the EGG group were due to increases in the appearance of egg product CerPCho species in HDL subfractions rather than non-specific reductions in non-egg product-derived CerPCho species (data not shown). These findings suggest that EGG-derived CerPCho species may be incorporated into HDL in a manner that can significantly alter HDL phospholipid species distribution.
Fig. 1.
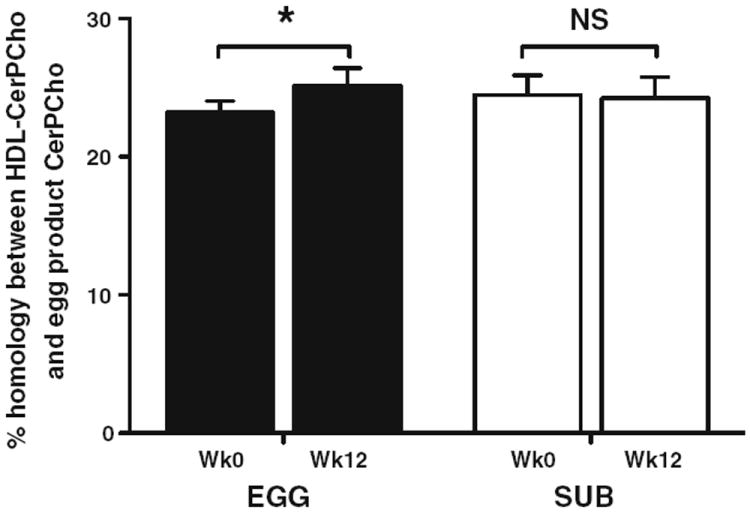
Percent homology of CerPCho species in HDL and egg products. CerPCho species homology represents the percentage of total HDL-CerPCho species (nmol) that were similarly identified in the whole egg (EGG group) or egg substitute (SUB group) products. n = 4 per group; data are represented as means ± SEM. *P < 0.05; NS non-significant
Cholesterol Efflux
Cholesterol efflux to subject serum was measured to assess whether changes in HDL lipid composition from egg feeding affected the cholesterol-accepting capacity of HDL. While no changes in cholesterol efflux were observed in the SUB group, cholesterol efflux significantly increased in the EGG group (+2.4 %) from baseline to week 12 (Fig. 2). There was a trend toward a positive association between changes in cholesterol efflux and changes in CerPCho homology between HDL and egg product CerPCho species (r = 0.69; P = 0.060), as well as a trend toward a negative correlation between changes in cholesterol efflux and changes in fasting plasma glucose (reported in [26]) (r = − 0.69; P = 0.060). No correlations were found between changes in cholesterol efflux and other HDL lipids.
Fig. 2.
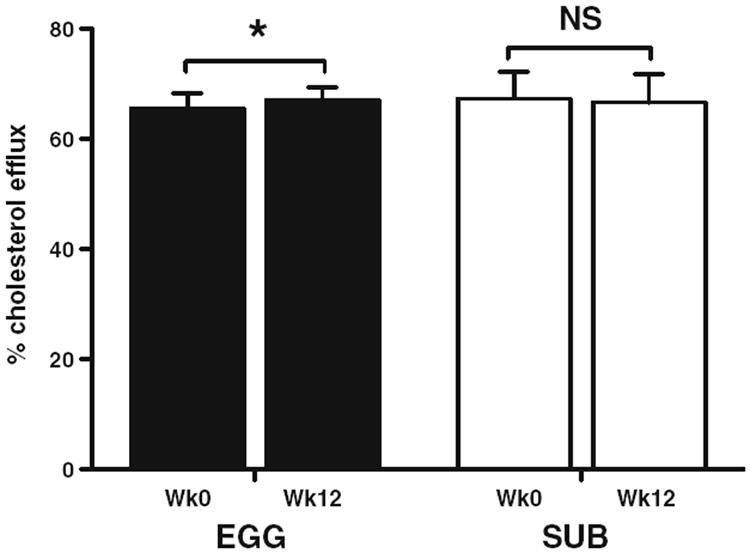
Percent cholesterol efflux from macrophage foam cells to subject serum. Cholesterol efflux was performed over a 3-h period to assess the total cholesterol-accepting capacity of serum obtained from EGG (n = 4) and SUB (n = 4) subjects. Data are represented as means ± SEM. *P < 0.05; NS non-significant
Discussion
While previous studies have found that egg consumption increases HDL-C and improves HDL particle profiles [25-27, 34], it has yet to be determined whether these changes correspond to alterations in HDL composition or HDL function. Since eggs are a rich source of dietary phospholipids which are known to exert plasma lipid-modulating effects, we hypothesized that daily egg consumption may additionally alter HDL lipid composition and PL class distribution to improve the ability of HDL to function as a lipid acceptor in MetS—individuals often characterized with low HDL-C, dysfunctional HDL [6], and an overall twofold increased risk of developing CVD [29]. Compared to intake of a yolk-free egg substitute, consumption of three eggs per day for 12 weeks resulted in greater enrichment of HDL in CE, PtdEtn, and CerPCho species present in whole eggs, as well as greater cholesterol efflux from cholesterol-loaded macrophages to subject serum. These changes correspond to previously reported improvements in HDL particle profiles in this population, along with beneficial effects on additional markers of atherogenic dyslipidemia and insulin resistance [26]. To our knowledge, this is the first study to demonstrate that increases in HDL-C from egg consumption during carbohydrate restriction correspond to modulation of HDL lipid composition and increase in the overall lipid-accepting capacity of serum in MetS.
The classification of MetS by the NCEP:ATP III criteria is diverse in nature due to the requirement of any 3 or more qualifying parameters [29], thereby creating a spectrum of MetS severity. Severity of MetS may be related to the degree of metabolic disturbances that affect lipoprotein metabolism, including insulin resistance and low-grade systemic inflammation [1]. Prospective cohort studies have further demonstrated that individuals who meet a greater number of MetS parameters have an increased risk of CVD [35]—particularly when low plasma HDL-C is one of the factors [36]. In this population, over half of the subjects randomized to each group had low plasma HDL-C levels. While we have observed greater increases in HDL-C from whole egg feeding in previous studies [18, 26, 27], it is interesting to note that the greater HDL-C response to whole egg feeding in MetS was driven by subjects who began the intervention with normal HDL-C levels. As we did not identify any differences in HDL lipid composition between subjects with low and normal baseline plasma HDL-C levels, this observation may be reflective of a greater responsiveness to the intervention in subjects falling within the healthier end of the MetS spectrum, as 7 out of 8 EGG group subjects with normal baseline HDL-C met only three MetS parameters, whereas EGG group subjects with low baseline HDL-C more commonly met 3, 4, and 5 MetS parameters (data not shown). Although the pattern of MetS parameters between subjects with low and normal baseline HDL-C were similarly observed in the SUB group, these factors did not appear to impact the overall reduced HDL-C response in the SUB group when compared to the EGG group [26, 27]. While it is important to note that the dietary cholesterol provided by whole eggs may play a role in HDL-C responses [18], the overall pattern of plasma lipid changes in the EGG group (i.e. increases in HDL-C without adversely affecting TC or LDL-C levels [26, 27]) is similar to findings reported from dietary phospholipid supplementation studies, suggesting that the yolk-derived phospholipids are responsible for the observed effects [16, 17]. Although further investigation is warranted, it is reasonable to hypothesize that consumption of phospholipid-rich foods other than egg may confer benefits to HDL lipid composition and function similarly to those observed in the current study.
HDL lipid composition has been shown to be an important factor in determining HDL function, metabolism, and CVD risk beyond plasma HDL-C levels. Individuals with low HDL-C [37] and MetS [38] often have greater enrichment in HDL-TAG and reduction in HDL-CE and HDL-PL. Enrichment of HDL in TAG has been associated with greater cholesteryl ester transfer protein (CETP) activity [39], enhanced clearance of apoA-I from circulation [40], and impaired LCAT-mediated esterification of free cholesterol and HepG2 cell uptake of HDL-CE [41]. Conversely, CE enrichment of HDL promotes greater SR-BI-mediated HDL-CE uptake [42], and is indicative of larger, more buoyant HDL particles that often correlate with reduced risk of CVD [43]. We observed relative increases in HDL-CE content in all subjects, concurrent with the previously described increases in HDL particle size in this population [26]. Although there were slightly greater relative increases in HDL-CE in the SUB group, this is most likely reflective of greater non-significant reductions in HDL-FC and HDL-PL compared to the EGG group. Interestingly, whole egg feeding led to greater reductions in HDL-TAG content in subjects who entered the study with normal plasma HDL-C levels, further suggesting that these subjects were metabolically healthier and more responsive to the dietary intervention. HDL-CE/TAG ratios increased all subjects, suggesting an overall improvement in HDL lipid composition regardless of group assignment, as would be expected due to the hypotriglyceridemic effects of carbohydrate-restricted diets [44].
Although changes in total HDL-PL were not found as we hypothesized, we did observe changes in the distribution of HDL phospholipids classes and CerPCho species, including an increase in relative HDL-PtdEtn content and CerPCho species homology to the whole egg product in the EGG group only. Alterations in HDL-PtdEtn content may affect the cholesterol-accepting capacity of patient serum, as well as pathways of HDL metabolism and the anti-thrombotic properties of HDL [6, 8, 45]. In hypercholesterolemic men with exceptionally high plasma HDL-C (>1.75 mmol/L, or 68 mg/dL), HDL was found to be enriched in PtdEtn, yet HDL-PtdEtn content was negatively correlated with cholesterol efflux from rat Fu5AH hepatoma cells [8]. Conversely, the relationship between HDL-PtdEtn and cholesterol efflux was positive in men with normal HDL (1.10–<1.50 mmol/L, or 43–58 mg/dL), although this pattern did not reach statistical significance. Our findings suggest that greater HDL-PtdEtn content may be associated with greater cholesterol efflux in a macrophage cell model in men and women with MetS—a population predominantly with low plasma HDL-C.
Enrichment in HDL with specific phospholipids may also confer atheroprotective benefits beyond that of modulating cholesterol efflux capacity. PtdEtn is also known to promote activated protein C-mediated anticoagulant activity [45]; therefore, PtdEtn-enriched HDL may exert greater antithrombotic activity [6, 46]. Oxidation of HDL has also been shown to promote depletion of PtdEtn [47], suggesting that enrichment of HDL in PtdEtn may be indicative of a less oxidized HDL profile.
In addition to increases in HDL-PtdEtn content, we observed that daily whole egg consumption for 12 weeks led to the enrichment of HDL in CerPCho species present in the egg product, potentially reflecting egg-derived CerPCho that became incorporated into HDL. Egg yolk serves as a uniquely rich and highly bioavailable (>90 %) source of dietary phospholipids, which can be absorbed partially intact in the intestine and have been shown to be preferentially incorporated into HDL particles following ingestion [21, 22]. Further, in various animal models, feeding of natural PtdCho and synthetic dimyristoylphosphatidylcholine (DMPC) has been shown to increase jejunal apoA-I synthesis [48, 49], which may promote intestinal secretion of HDL—a process which is thought to contribute ~30 % of total circulating HDL [50, 51]. These concepts suggest that consumption of egg-derived phospholipids may serve as a relatively direct mechanism to modulate HDL lipid composition and intestinal HDL production.
We further demonstrated that cholesterol efflux to subject serum increased from baseline to week 12 in the EGG group only (+2.4 %). Due to the variability in experimental methodology for efflux assays and the relatively recent application of this approach to assessing CVD risk in response to therapeutic intervention, it is yet to be determined how the observed changes in efflux affect long-term CVD risk and development. However, through implementation of statistical modeling that adjusted for standard cardiovascular disease risk factors, Khera et al. [5] found that coronary artery disease (CAD) risk (as measured by carotid intima-media thickness) was reduced by 30 % for every 1-SD increase in cholesterol efflux capacity. These findings suggest that CAD risk could be significantly reduced with relatively small increases in cholesterol efflux. Following this logic, we believe that the +2.4 % (or +0.5 SD) increase in efflux observed in the EGG group is a clinically relevant improvement in CVD status.
The increases in cholesterol efflux associated with whole egg consumption may be attributed to an increased lipid-accepting capacity of HDL, as well as changes in other serum components. Various factors related to MetS have been associated with impaired cellular cholesterol efflux, including hypertension [52], overweight/obesity [53], low HDL-C [54], elevated blood glucose [55], and markers of inflammation [56]; however, it remains unclear whether cholesterol efflux is impaired in MetS. Conflicting results may be due to variability of different cellular efflux models [15], as well as the innate diversity of MetS populations in regard to qualifying parameters [57]. Cholesterol and phospholipid efflux from fibroblasts to subject plasma was shown to be similar between healthy individuals and those classified as non-diabetic MetS [58, 59]; however, fibroblasts express minimal SR-BI—a pathway that is more responsive to shifts in HDL-PL, larger HDL particles, and a greater contributor to total efflux in macrophages [60, 61]. Although few clinical trials have examined the effects of diet on cholesterol efflux, weight loss has been shown to increase cholesterol efflux to plasma via SR-BI- and ABCG1-mediated pathways, whereas ABCA1-mediated cholesterol efflux was reduced—presumably due to a reduction in more nascent preb HDL [62]. These findings corresponded to increases in the larger HDL2 subfractions, which additionally become more phospholipid rich [62]. Cholesterol efflux was also increased in MetS subjects following a 12-week treatment of insulin-sensitizing drug pioglitazone [5]. Accordingly, we observed a trend toward a negative correlation between changes in fasting plasma glucose and cholesterol efflux, suggesting that improvements in insulin resistance promote greater efflux in MetS.
In summary, we have demonstrated that increases in HDL-C from daily whole egg intake during moderate carbohydrate restriction coincide with clinically relevant changes in HDL lipid composition and the lipid-accepting capacity of subject serum in MetS. Further, it appears that changes in HDL-C and lipid composition in response to egg feeding vary within the MetS classification, whereas subjects with normal plasma HDL-C levels at baseline displayed more favorable increases in HDL-C and a reduction in HDL-TAG content. Similar to the changes in cholesterol efflux, these observations may be related to variation in insulin sensitivity within this MetS population. Overall, these findings indicate that eggs may serve as a functional food to promote beneficial shifts in HDL composition, metabolism, and function in MetS.
Acknowledgments
This study was supported in part by the Egg Nutrition Center from funds received by MLF, and by the Agriculture and Food Research Initiative Competitive Grant No. 2012-67011-19914 from the USDA National Institute of Food and Agriculture to CJA. Mass spectrometric analyses were performed in the Mass Spectrometer Facility of CCCWF School of Medicine and supported in part by NCI Center Grant 5P30CA12197. The Finnigan TSQ Quantum XLS GC/MS/MS was purchased with funds from NIH Shared Instrumentation Grant 1S10RR027940MS to MJT.
Abbreviations
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- BCA
Bicinchoninic acid
- CAD
Coronary artery disease
- CE
Cholesteryl ester
- CerPCho
Sphingomyelin
- CETP
Cholesteryl ester transfer protein
- CVD
Cardiovascular disease
- DMPC
Dimyristoylphosphatidylcholine
- EGG
Whole egg group
- FC
Free cholesterol
- HDL-C
Plasma HDL-cholesterol
- HDL-PL
HDL-phospholipids
- LCAT
Lecithin-cholesterol acyltransferase
- LysoPtdCho
Lysophosphatidylcholine
- NCEP ATP III
National Cholesterol Education Program Adult Treatment Panel III
- MetS
Metabolic syndrome
- PL
Phospholipid
- PtdCho
Phosphatidylcholine
- PtdEtn
Phosphatidylethanolamine
- PtdIns
Phosphatidylinositol
- RCT
Reverse cholesterol transport
- SDS
Sodium dodecyl sulfate
- SR-BI
Scavenger receptor class B I
- SUB
Egg yolk-free egg substitute group
- TAG
Triacylglycerol
Footnotes
Conflict of Interest MLF received funds from the Egg Nutrition Center to perform the study. CJA, CNB, JL, JB, DS, and MJT declare that they have no conflicts of interest.
Contributor Information
Catherine J. Andersen, Department of Nutritional Sciences, University of Connecticut, 3624 Horsebarn Road Ext., Unit 4017, Storrs, CT 06269-4017, USA
Christopher N. Blesso, Department of Nutritional Sciences, University of Connecticut, 3624 Horsebarn Road Ext., Unit 4017, Storrs, CT 06269-4017, USA
Jiyoung Lee, Department of Nutritional Sciences, University of Connecticut, 3624 Horsebarn Road Ext., Unit 4017, Storrs, CT 06269-4017, USA.
Jacqueline Barona, Department of Nutritional Sciences, University of Connecticut, 3624 Horsebarn Road Ext., Unit 4017, Storrs, CT 06269-4017, USA.
Dharika Shah, Biochemistry, Wake Forest School of Medicine, Winston-Salem, NC 27157, USA.
Michael J. Thomas, Biochemistry, Wake Forest School of Medicine, Winston-Salem, NC 27157, USA
Maria Luz Fernandez, Email: maria-luz.fernandez@uconn.edu, Department of Nutritional Sciences, University of Connecticut, 3624 Horsebarn Road Ext., Unit 4017, Storrs, CT 06269-4017, USA.
References
- 1.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asztalos BF, Tani M, Schaefer EJ. Metabolic and functional relevance of HDL subspecies. Curr Opin Lipidol. 2011;22:176–185. doi: 10.1097/MOL.0b013e3283468061. [DOI] [PubMed] [Google Scholar]
- 4.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 5.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 7.Tchoua U, Gillard BK, Pownall HJ. HDL superphospholipidation enhances key steps in reverse cholesterol transport. Atherosclerosis. 2010;209:430–435. doi: 10.1016/j.atherosclerosis.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fournier N, Paul JL, Atger V, Cogny A, Soni T, de la Llera-Moya M, Rothblat G, Moatti N. HDL phospholipid content and composition as a major factor determining cholesterol efflux capacity from Fu5AH cells to human serum. Arterioscler Thromb Vasc Biol. 1997;17:2685–2691. doi: 10.1161/01.atv.17.11.2685. [DOI] [PubMed] [Google Scholar]
- 9.Rosenson RS, Brewer HB, Jr, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011;57:392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- 10.Dashti M, Kulik W, Hoek F, Veerman EC, Peppelenbosch MP, Rezaee F. A phospholipidomic analysis of all defined human plasma lipoproteins. Sci Rep. 2011;1:139. doi: 10.1038/srep00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Asztalos B, Roheim PS, Wong L. Characterization of phospholipids in pre-alpha HDL: selective phospholipid efflux with apolipoprotein A-I. J Lipid Res. 1998;39:1601–1607. [PubMed] [Google Scholar]
- 12.Piperi C, Kalofoutis C, Papaevaggeliou D, Papapanagiotou A, Lekakis J, Kalofoutis A. The significance of serum HDL phospholipid levels in angiographically defined coronary artery disease. Clin Biochem. 2004;37:377–381. doi: 10.1016/j.clinbiochem.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Bovet P, Darioli R, Essinger A, Golay A, Sigwart U, Kappen-berger L. Phospholipids and other lipids in angiographically assessed coronary artery disease. Atherosclerosis. 1989;80:41–47. doi: 10.1016/0021-9150(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 14.Fournier N, de la Llera Moya M, Burkey BF, Swaney JB, Paterniti J, Jr, Moatti N, Atger V, Rothblat GH. Role of HDL phospholipid in efflux of cell cholesterol to whole serum: studies with human apoA-I transgenic rats. J Lipid Res. 1996;37:1704–1711. [PubMed] [Google Scholar]
- 15.Mweva S, Paul JL, Cambillau M, Goudouneche D, Beaune P, Simon A, Fournier N. Comparison of different cellular models measuring in vitro the whole human serum cholesterol efflux capacity. Eur J Clin Invest. 2006;36:552–559. doi: 10.1111/j.1365-2362.2006.01673.x. [DOI] [PubMed] [Google Scholar]
- 16.Klimov AN, Konstantinov VO, Lipovetsky BM, Kuznetsov AS, Lozovsky VT, Trufanov VF, Plavinsky SL, Gundermann KJ, Schumacher R. “Essential” phospholipids versus nicotinic acid in the treatment of patients with type IIb hyperlipoproteinemia and ischemic heart disease. Cardiovasc Drugs Ther. 1995;9:779–784. doi: 10.1007/BF00879871. [DOI] [PubMed] [Google Scholar]
- 17.Bunea R, El Farrah K, Deutsch L. Evaluation of the effects of Neptune Krill Oil on the clinical course of hyperlipidemia. Altern Med Rev. 2004;9:420–428. [PubMed] [Google Scholar]
- 18.Mutungi G, Ratliff J, Puglisi M, Torres-Gonzalez M, Vaishnav U, Leite JO, Quann E, Volek JS, Fernandez ML. Dietary cholesterol from eggs increases plasma HDL cholesterol in overweight men consuming a carbohydrate-restricted diet. J Nutr. 2008;138:272–276. doi: 10.1093/jn/138.2.272. [DOI] [PubMed] [Google Scholar]
- 19.Iwata T, Hoshi S, Takehisa F, Tsutsumi K, Furukawa Y, Kimura S. The effect of dietary safflower phospholipid and soybean phospholipid on plasma and liver lipids in rats fed a hypercholesterolemic diet. J Nutr Sci Vitaminol (Tokyo) 1992;38:471–479. doi: 10.3177/jnsv.38.471. [DOI] [PubMed] [Google Scholar]
- 20.Wat E, Tandy S, Kapera E, Kamili A, Chung RW, Brown A, Rowney M, Cohn JS. Dietary phospholipid-rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high-fat diet. Atherosclerosis. 2009;205:144–150. doi: 10.1016/j.atherosclerosis.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Kullenberg D, Taylor LA, Schneider M, Massing U. Health effects of dietary phospholipids. Lipids Health Dis. 2012;11:3. doi: 10.1186/1476-511X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zierenberg O, Grundy SM. Intestinal absorption of polyenephosphatidylcholine in man. J Lipid Res. 1982;23:1136–1142. [PubMed] [Google Scholar]
- 23.Weihrauch JL, Son Y-S. The phospholipid content of foods. J Am Oil Chem Soc. 1983;60:1971–1978. [Google Scholar]
- 24.Cohn JS, Kamili A, Wat E, Chung RW, Tandy S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients. 2010;2:116–127. doi: 10.3390/nu2020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutungi G, Waters D, Ratliff J, Puglisi M, Clark RM, Volek JS, Fernandez ML. Eggs distinctly modulate plasma carotenoid and lipoprotein subclasses in adult men following a carbohydrate-restricted diet. J Nutr Biochem. 2010;21:261–267. doi: 10.1016/j.jnutbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Blesso CN, Andersen CJ, Barona J, Volek JS, Fernandez ML. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism. 2013;62:400–410. doi: 10.1016/j.metabol.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Blesso CN, Andersen CJ, Bolling BW, Fernandez ML. Egg intake improves carotenoid status by increasing plasma HDL cholesterol in adults with metabolic syndrome. Food Funct. 2013;31:213–221. doi: 10.1039/c2fo30154g. [DOI] [PubMed] [Google Scholar]
- 28.Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, Kontush A. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89:4963–4971. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- 29.Executive Summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–e18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 32.Sorci-Thomas MG, Owen JS, Fulp B, Bhat S, Zhu X, Parks JS, Shah D, Jerome WG, Gerelus M, Zabalawi M, Thomas MJ. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J Lipid Res. 2012;53:1890–1909. doi: 10.1194/jlr.M026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 34.Mayurasakorn K, Srisura W, Sitphahul P, Hongto PO. High-density lipoprotein cholesterol changes after continuous egg consumption in healthy adults. J Med Assoc Thai. 2008;91:400–407. [PubMed] [Google Scholar]
- 35.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care. 2002;25:1790–1794. doi: 10.2337/diacare.25.10.1790. [DOI] [PubMed] [Google Scholar]
- 36.Hong Y, Jin X, Mo J, Lin HM, Duan Y, Pu M, Wolbrette DL, Liao D. Metabolic syndrome, its preeminent clusters, incident coronary heart disease and all—cause mortality-results of prospective analysis for the atherosclerosis risk in communities study. J Intern Med. 2007;262:113–122. doi: 10.1111/j.1365-2796.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 37.Shuhei N, Soderlund S, Jauhiainen M, Taskinen MR. Effect of HDL composition and particle size on the resistance of HDL to the oxidation. Lipids Health Dis. 2010;9:104. doi: 10.1186/1476-511X-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park KH, Shin DG, Kim JR, Hong JH, Cho KH. The functional and compositional properties of lipoproteins are altered in patients with metabolic syndrome with increased cholesteryl ester transfer protein activity. Int J Mol Med. 2010;25:129–136. [PubMed] [Google Scholar]
- 39.Parra ES, Urban A, Panzoldo NB, Nakamura RT, Oliveira R, de Faria EC. A reduction of CETP activity, not an increase, is associated with modestly impaired postprandial lipemia and increased HDL-cholesterol in adult asymptomatic women. Lipids Health Dis. 2011;10:87. doi: 10.1186/1476-511X-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamarche B, Uffelman KD, Carpentier A, Cohn JS, Steiner G, Barrett PH, Lewis GF. Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J Clin Invest. 1999;103:1191–1199. doi: 10.1172/JCI5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skeggs JW, Morton RE. LDL and HDL enriched in triglyceride promote abnormal cholesterol transport. J Lipid Res. 2002;43:1264–1274. [PubMed] [Google Scholar]
- 42.Kinoshita M, Fujita M, Usui S, Maeda Y, Kudo M, Hirota D, Suda T, Taki M, Okazaki M, Teramoto T. Scavenger receptor type BI potentiates reverse cholesterol transport system by removing cholesterol ester from HDL. Atherosclerosis. 2004;173:197–202. doi: 10.1016/j.atherosclerosis.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Arsenault BJ, Lemieux I, Despres JP, Gagnon P, Wareham NJ, Stroes ES, Kastelein JJ, Khaw KT, Boekholdt SM. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Atherosclerosis. 2009;206:276–281. doi: 10.1016/j.atherosclerosis.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 44.Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ, Kraemer WJ, Bibus DM, Fernandez ML, Feinman RD. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids. 2009;44:297–309. doi: 10.1007/s11745-008-3274-2. [DOI] [PubMed] [Google Scholar]
- 45.Smirnov MD, Esmon CT. Phosphatidylethanolamine incorporation into vesicles selectively enhances factor Va inactivation by activated protein C. J Biol Chem. 1994;269:816–819. [PubMed] [Google Scholar]
- 46.Griffin JH, Kojima K, Banka CL, Curtiss LK, Fernandez JA. High-density lipoprotein enhancement of anticoagulant activities of plasma protein S and activated protein C. J Clin Invest. 1999;103:219–227. doi: 10.1172/JCI5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradamante S, Barenghi L, Giudici GA, Vergani C. Free radicals promote modifications in plasma high-density lipoprotein: nuclear magnetic resonance analysis. Free Radic Biol Med. 1992;12:193–203. doi: 10.1016/0891-5849(92)90027-e. [DOI] [PubMed] [Google Scholar]
- 48.Navab M, Hama S, Hough G, Fogelman AM. Oral synthetic phospholipid (DMPC) raises high-density lipoprotein cholesterol levels, improves high-density lipoprotein function, and markedly reduces atherosclerosis in apolipoprotein E-null mice. Circulation. 2003;108:1735–1739. doi: 10.1161/01.CIR.0000089375.60050.35. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Du J, Lu S, Yao Y, Hunter F, Black DD. Regulation of intestinal apolipoprotein A-I synthesis by dietary phosphatidylcholine in newborn swine. Lipids. 2001;36:683–687. doi: 10.1007/s11745-001-0773-x. [DOI] [PubMed] [Google Scholar]
- 50.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rachmilewitz D, Fainaru M. Apolipoprotein A-I synthesis and secretion by cultured human intestinal mucosa. Metabolism. 1979;28:739–743. doi: 10.1016/0026-0495(79)90179-3. [DOI] [PubMed] [Google Scholar]
- 52.Xu M, Zhou H, Gu Q, Li C. The expression of ATP-binding cassette transporters in hypertensive patients. Hypertens Res. 2009;32:455–461. doi: 10.1038/hr.2009.46. [DOI] [PubMed] [Google Scholar]
- 53.Xu M, Zhou H, Wang J, Li C, Yu Y. The expression of ATP-binding cassette transporter A1 in Chinese overweight and obese patients. Int J Obes (Lond) 2009;33:851–856. doi: 10.1038/ijo.2009.120. [DOI] [PubMed] [Google Scholar]
- 54.Nakanishi S, Vikstedt R, Soderlund S, Lee-Rueckert M, Hiukka A, Ehnholm C, Muilu M, Metso J, Naukkarinen J, Palotie L, Kovanen PT, Jauhiainen M, Taskinen MR. Serum, but not monocyte macrophage foam cells derived from low HDL-C subjects, displays reduced cholesterol efflux capacity. J Lipid Res. 2009;50:183–192. doi: 10.1194/jlr.M800196-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Gantman A, Fuhrman B, Aviram M, Hayek T. High glucose stimulates macrophage SR-BI expression and induces a switch in its activity from cholesterol efflux to cholesterol influx. Biochem Biophys Res Commun. 2010;391:523–528. doi: 10.1016/j.bbrc.2009.11.091. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Liao D, Bharadwaj U, Li M, Yao Q, Chen C. C-reactive protein inhibits cholesterol efflux from human macrophage-derived foam cells. Arterioscler Thromb Vasc Biol. 2008;28:519–526. doi: 10.1161/ATVBAHA.107.159467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report. 2009:1–7. [PubMed] [Google Scholar]
- 58.Dullaart RP, Groen AK, Dallinga-Thie GM, de Vries R, Sluiter WJ, van Tol A. Fibroblast cholesterol efflux to plasma from metabolic syndrome subjects is not defective despite low high-density lipoprotein cholesterol. Eur J Endocrinol. 2008;158:53–60. doi: 10.1530/EJE-07-0451. [DOI] [PubMed] [Google Scholar]
- 59.Alenezi MY, Marcil M, Blank D, Sherman M, Genest J., Jr Is the decreased high-density lipoprotein cholesterol in the metabolic syndrome due to cellular lipid efflux defect? J Clin Endocrinol Metab. 2004;89:761–764. doi: 10.1210/jc.2003-031213. [DOI] [PubMed] [Google Scholar]
- 60.Duong M, Collins HL, Jin W, Zanotti I, Favari E, Rothblat GH. Relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arterioscler Thromb Vasc Biol. 2006;26:541–547. doi: 10.1161/01.ATV.0000203515.25574.19. [DOI] [PubMed] [Google Scholar]
- 61.Jian B, de la Llera-Moya M, Ji Y, Wang N, Phillips MC, Swaney JB, Tall AR, Rothblat GH. Scavenger receptor class B type I as a mediator of cellular cholesterol efflux to lipoproteins and phospholipid acceptors. J Biol Chem. 1998;273:5599–5606. doi: 10.1074/jbc.273.10.5599. [DOI] [PubMed] [Google Scholar]
- 62.Aron-Wisnewsky J, Julia Z, Poitou C, Bouillot JL, Basdevant A, Chapman MJ, Clement K, Guerin M. Effect of bariatric surgery-induced weight loss on SR-BI-, ABCG1-, and ABCA1-mediated cellular cholesterol efflux in obese women. J Clin Endocrinol Metab. 2011;96:1151–1159. doi: 10.1210/jc.2010-2378. [DOI] [PubMed] [Google Scholar]


