Abstract
Slowed information processing is a prominent deficit in late-life depression (LLD). To better differentiate processing speed components in LLD, we examined characteristics of visual search performance in 32 LLD and 32 control participants. Data showed specific slowing in the comparison stage of visual search in LLD, rather than in encoding/response stages, but also greater overall slowing in LLD during inefficient versus efficient search. We found no group differences on traditional neuropsychological measures of processing speed. Slowed processing speed in LLD may be specific rather than general, which underscores the need to link components of processing speed to underlying neural circuitry.
Keywords: Depression, Aging, Cognition, Visual Search, Reaction Time
Cognitive deficits are a prominent feature of late-life depression (LLD) and are a risk factor for adverse outcomes, including reduced treatment response (Alexopoulos et al., 2000; Potter, Kittinger, Wagner, Steffens, & Krishnan, 2004; Sheline et al., 2012), greater limitation in functional activities (Kiosses, Alexopoulos, & Murphy, 2000), increased risk of dementia (Alexopoulos, Young, & Meyers, 1993; Potter et al., 2012), and increased mortality (Mehta et al., 2003). Cognitive deficits in LLD often persist after remission of the depressive episode (Bhalla et al., 2006; Kohler, Thomas, Barnett, & O’Brien, 2010; J. S. Lee, Potter, Wagner, Welsh-Bohmer, & Steffens, 2007), which suggests that this clinical feature of LLD may be less amenable to current treatments than other clinical features. The many negative outcomes associated with cognitive deficits in LLD highlight the need for a better characterization of these deficits, which would help inform cognitively oriented treatments.
Research on cognitive deficits in major depressive disorder has shown that while depressed individuals perform broadly worse than nondepressed comparison groups (R. S. Lee, Hermens, Porter, & Redoblado-Hodge, 2012; Zakzanis, Leach, & Kaplan, 1998), the most consistent deficits occur in tasks involving executive functions and episodic memory (Beats, Sahakian, & Levy, 1996; Snyder, 2013; Thomas & O’Brien, 2008), and appear to become more prominent with age (Boone et al., 1995; Elliott, 1998; Schweitzer, Tuckwell, O’Brien, & Ames, 2002). Many of the LLD-related differences appear to be due to an underlying deficit in basic information processing speed (Sheline et al., 2012). In this context, decreased speed in stimulus perception, elementary cognitive processing, and motor response can occupy processing resources in a manner that compromises performance of higher order cognitive operations (Nebes et al., 2000). This perspective is supported by evidence from several studies showing that performances on neuropsychological measures of processing speed mediate the differences between depressed and nondepressed older adults on other neuropsychological measures, such as executive functions, language, and episodic memory (Butters et al., 2004; Nebes et al., 2000; Sheline et al., 2006). One study, however, identified processing speed as only a partial mediator of cognitive deficits and found this construct insufficient to explain the full range of neurocognitive deficits observed in LLD (Kohler et al., 2010). Even if processing speed does not fully mediate other cognitive deficits associated with LLD, it nonetheless appears to be an important contributor to performance.
While the previously referenced studies have identified a broad contribution of processing speed to the neuropsychological deficits of LLD, important questions remain about specific relationships between processing speed and cognition in LLD. Many of the prior studies defined processing speed based on an aggregation of traditional paper-and-pencil neuropsychological measures in which speed of performance is intertwined with the unique stimulus characteristics and demands of the individual tasks. The task demands of these theoretically identified measures of processing speed were as varied as color naming, symbol encoding, visual scanning, motor praxis, and even incidental memory (e.g., Symbol Digit Modalities Test; Demakis, Sawyer, Fritz, & Sweet, 2001).Tests such as these assess the time it takes to complete a complex, multi-domain cognitive operation, and not processing speed per se. A resulting limitation of this approach is difficulty estimating the extent to which the processing speed differences between depressed and nondepressed elders are disproportionately influenced by the unique task characteristics of any individual neuropsychological measure. Another limitation is difficultly identifying whether speed-related differences are due to a specific information processing stage, such as perceptual encoding, comparison, motor speed, or decisional processes. This is important because different stages of information processing may be associated with integrity of different types of brain tissue (e.g., grey matter versus white matter), or with different brain systems. In order to better understand the nature and substrates of processing speed deficits in LLD, it is important to complement traditional neuropsychological tasks with laboratory-based measures that allow a systematic examination of processing speed across different stages of task performance (Deluca & Kalmar, 2007).
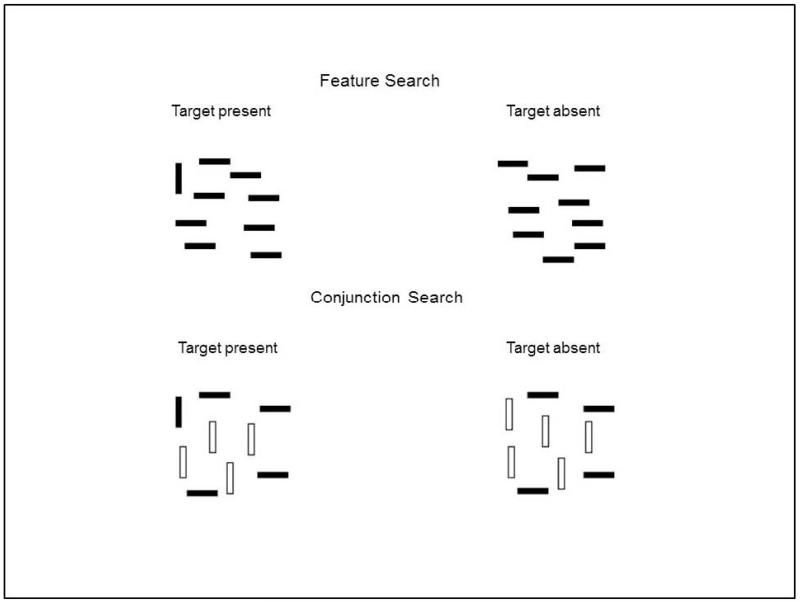
One relevant experimental paradigm for studying processing speed in LLD is visual search, which is an established model for studying how manipulation of task attributes affects speed of performance (Duncan & Humphreys, 1989; Eckstein, 2011; Schneider & Shiffrin, 1977; Wolfe, 1998a). For instance, searching for a target that differs from nontarget (distractor) display items in a salient feature is typically highly efficient (e.g., feature search: a black vertical bar among black horizontal bars); in contrast, search for a target item that is a conjunction of features in the distractor items is typically less efficient (e.g., conjunction search: a black vertical bar among black horizontal bars and white vertical bars). Feature search performance is considered relatively automatic, and is defined by a reaction time that is minimally affected by an increased number of items to be searched. Conjunction search, on the other hand, is considered more cognitively demanding, with a reaction time (RT) that increases in a roughly linear manner with each additional display item to search. Further, RT slopes in conjunction search are typically twice as high for target-absent trials than for target-present trials, reflecting a serial search process that terminates when the target is found (Wolfe, 1998a, 1998b). In analyzing differences among search conditions, the slope function represents a comparison component that increases in RT as a function of increasing display size, while the accompanying intercept of the RT-display size function represents the combined durations of perceptual encoding and response-related components of search. Thus, the slope and intercept of RT-display size functions each provide differentiated information about cognitive processes that contribute to overall reaction time.
Research on visual search performance in depression is limited to a few studies in non-elderly populations, but extant data suggest consistency in basic visual search findings relative to nonpsychiatric populations. Hammar and colleagues (Hammar, Lund, & Hugdahl, 2003a) used a search task with a single display size (14 items) and found that depressed and nondepressed younger adults were comparable in a feature search condition, whereas a significant increase in RT among depressed individuals was evident in a conjunction search condition, which presumably required a less efficient and more attention-demanding comparison process. These authors also found that depression-related deficits in conjunction search persisted 6 months after treatment (Hammar, Lund, & Hugdahl, 2003b), but normalized after a 10-year follow-up period (Hammar & Ardal, 2012). Because these previous studies used a single display size, RT slope and intercept data were not available to determine whether the participant group differences were associated with either the comparison process (slope), encoding/response processes (intercept), or both. These visual search studies also did not include older adults, which is relevant for LLD, because even in the context of normal aging, the speed of elementary perceptual processing declines with increasing age (Madden, 2001; Salthouse, 1996; Salthouse & Madden, 2007), and age-related decline in the speed of search and comparison processes also occurs, particularly in conjunction search conditions (Hommel, Li, & Li, 2004; Kramer & Madden, 2008; Madden & Whiting, 2004).
The purpose of the current study was to compare visual search performance in older adults with acute depression to a comparison sample with no history of depression, in order to better differentiate cognitive components of processing speed in LLD. Based on prior studies (Hammar & Ardal, 2012; Hammar et al., 2003a, 2003b), it is not clear whether the depression-related difference in visual search is associated specifically with the comparison or encoding/response components of search, or instead reflects more general, nonspecific slowing. We compared feature-search and conjunction-search conditions and predicted a greater relative deficit in conjunction search for LLD participants compared to nondepressed controls, as in Hammar et al. (2003a), and particularly in RT slope data. That is, we predicted that the depression-related effect would be relatively greater in the comparison stage of the conjunction search condition (i.e., a higher RT slope), which involves more attentional control and is presumably more sensitive to inefficiency of visual search.
Method
Participants
Sixty-four participants, 60-85 years of age, consented to complete visual search as an adjunct to a larger National Institute of Mental Health-sponsored treatment study (Steffens et al., 2004). All depressed participants (n = 32) met DSM-IV criteria for major depressive disorder (MDD) as diagnosed using the Diagnostic Interview Schedule (Robins, Helzer, Croughan, & Ratcliff, 1981) and confirmed with a clinical evaluation by a geriatric psychiatrist. Exclusion criteria included: 1) another major psychiatric illness, including bipolar disorder, schizophrenia, or dementia; 2) history of alcohol or drug abuse or dependence; 3) primary neurologic illness, including dementia; and 4) medical illness, medication use, or disability that would prevent the participant from completing neurocognitive testing. Depressed participants were recruited from clinics, referrals, and advertisements, and were enrolled in a treatment study that is described elsewhere (Steffens et al., 2004). Among the depressed group, 15 participants reported an age of first MDD onset before age 50, 9 participants reported onset after age 50, and 8 participants could not identify a specific age of onset. Nondepressed comparison participants (n = 32) were community-dwelling individuals recruited through advertisements.
Eligible participants had a non-focal neurological examination, no self-report of neurologic or psychiatric illness, and no evidence of a current or past psychiatric disorder based on the Diagnostic Interview Schedule. Individuals with comorbid anxiety disorders were not excluded, as long as MDD was the primary diagnosis. Individuals were screened for dementia at time of enrollment based on an established protocol that included review of a comprehensive clinical evaluation, consultation with referring physicians, and cognitive screening with the Mini-Mental State Examination (MMSE; Folstein, Folstein, & Fanjiang, 2001; Folstein, Folstein, & McHugh, 1975). This study was approved by the Duke University Institutional Review Board.
Visual Search Task
The visual search task consisted of a feature search condition and a conjunction search condition (Figure 1). On each trial, participants made a yes/no response manually, regarding the presence of a target item (a black vertical bar) in a visual display. One hand was designated for each response key, which was alternated in a counterbalanced fashion across participants. In the feature search condition, the distractor items were black horizontal bars, and thus the target, when present, differed from all of the distractors in the orientation feature value (vertical target versus horizontal distractor). In the conjunction condition, the distractors were both black horizontal bars and white vertical bars, and thus the target was a conjunction of an individual feature value from each distractor. There were 4 blocks of trials composed of 2 feature-search blocks and 2 conjunction-search blocks. The blocks were presented in a run order that alternated condition, counterbalanced for each participant group so that each block was presented an equal number of times at each position in the run order. Display size was either 3, 7, or 11 items. Each block was composed of 96 trials, with 16 trials for each combination of target present/absent and display size, presented randomly, yielding 192 trials per task condition and 384 total trials. Stimuli appeared on a white background presented on a 17″ computer monitor. The display was created on an imaginary 2 × 2 grid with an overall visual angle of approximately 8° at a viewing distance of 60 cm (viewing was unconstrained). The placement of target items on the grid was counterbalanced so that the target appeared in each of the 4 quadrants of the grid an equal number of times. The task was programmed using E-prime 2 software (Psychological Software Tools, Pittsburgh, PA).
Figure 1.
Example of visual search stimuli by search type.
Neuropsychological and Mood Assessment
Neuropsychological and mood assessment was conducted by trained psychometric technicians under the supervision of a licensed clinical neuropsychologist (GGP), during the same visit as visual search assessment. For the purposes of the current study, we selected Parts A and B of the Trail Making Test (Reitan, 1992), and the Symbol-Digit Modalities Test (SDMT; Smith, 1982) as comparison measures of information processing speed/attentional control. We additionally calculated a difference score of the time to complete Trail Making Part B minus the time to complete Trail Making Part A; the subtraction of Part A from Part B is regarded by some to reflect a purer measure of the executive function demands of Part B (Drane, Yuspeh, Huthwaite, & Klingler, 2002).The complete neuropsychological assessment is described elsewhere (Steffens et al., 2004). Depression severity was assessed using the Beck Depression Inventory-2 (BDI-2; Beck, Steer, & Brown, 1996).
Statistical Analysis
We used independent samples t tests to evaluate the significance of differences in age and education level between depressed and nondepressed participants, and chi-square tests to evaluate group differences in sex and race. Independent samples t tests were also used to evaluate group differences on the traditional neuropsychological measures, with Satterthwaite’s t tests used in cases of unequal variance.
Visual search performance was based on correct response trials, with separate analysis of error rates. For each participant, we calculated median RT for correct responses within each condition; summary data are presented as means (across participants) of the median RTs. To examine visual search performance, we first used an analysis of variance (ANOVA) of the RT data, with participant group (control, depressed) as a between-subjects variable and search condition (feature, conjunction), target (present, absent), and display size (3, 7, 11) as within-subjects variables. This initial ANOVA provided information regarding the additive and interactive effects of the independent variables in the outcome RT measure. We followed this ANOVA with analyses of the linear slope and intercept values of the RT × Display Size function, obtained for each participant and task condition. This latter type of analysis separates different components of search RT relevant for our hypotheses. Specifically, the slope represents the comparison time per display item, and the intercept represents the remaining portion of RT attributable to visual encoding and response processes (Duncan & Humphreys, 1989; Eckstein, 2011; Schneider & Shiffrin, 1977; Wolfe, 1998a).
As noted previously, steeper slope values indicate a longer comparison of each display item, which reflects a more inefficient search. Higher intercept values indicate more time associated with encoding and response processes. We examined RT slopes and intercepts as separate dependent variables in ANOVA models with group (depressed vs. nondepressed) as a between-subjects variable, and within-subjects variables of search (feature vs. conjunction) and target (present vs. absent). For all analyses, we specified rejection of the null hypothesis at a p-value of .05.
Results
Descriptive statistics and results of t tests appear in Table 1. There were no significant differences between depressed and nondepressed individuals with respect to age, education level, sex, or race. As expected, depressed individuals did have significantly higher depression symptom severity as assessed by the BDI-2. On traditional neuropsychological measures, there were no significant differences in performance between depressed and nondepressed groups.
Table 1. Demographic, Clinical, and Neuropsychological Characteristics of the Study Groups.
| Depressed |
Control |
||||
|---|---|---|---|---|---|
| Characteristic | M | (SD) | M | (SD) | statistical test |
| Age (years) | 67.66 | (7.17) | 69.53 | (6.41) | t(62) = 1.10 |
| Education (years) | 16.22 | (2.52) | 16.13 | (2.27) | t(62) = −0.16 |
| % Women | 53.13 | 56.25 | χ2 = 0.06 | ||
| % Caucasian | 87.50 | 87.50 | χ2 = 0.01 | ||
| BDI-2 | 19.93 | (8.16) | 1.1× | (1.55) | t(33.24) = −12.84a* |
| MMSE | 28.44 | (1.46) | 28.44 | (1.44) | t(62) = 0.01 |
| SDMT | 40.47 | (9.10) | 43.28 | (9.98) | t(62) = 1.18 |
| TMT A (sec) | 37.65 | (12.63) | 38.84 | (12.58) | t(62) = 0.38 |
| TMT B (sec) | 92.97 | (42.32) | 79.39 | (39.26) | t(61) = −1.32 |
| TMT Diff (sec) | 55.31 | (36.78) | 41.32 | (34.49) | t(61) = −1.56 |
Note. n = 32 per group, with one missing participant on TMT B. BDI-2 = Beck Depression Inventory-2 (Beck, et al., 1996). MMSE = Mini-Mental State Examination (Folstein, et al., 2001). SDMT = Symbol-Digit Modalities Test (Smith, 1982). TMT = Trail Making Test, subtests A and B (Reitan, 1992). TMT Diff = Trail Making Difference Score (TMT B – TMT A).
Value based on Satterthwaite t-test for unequal variance.
p < .01
Error Rate
Error rates were less than 2% across the feature search condition and less than 3% across the conjunction search condition. There were no differences in error rate between depressed and nondepressed groups.
Visual Search
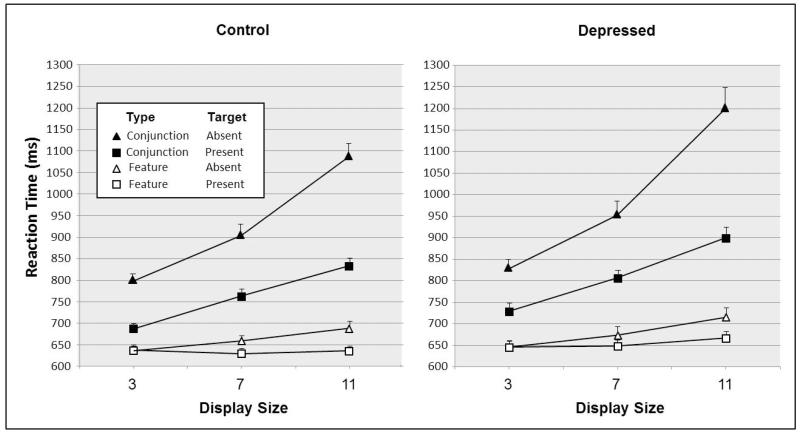
We obtained the median RT for correct responses for each participant in each task condition. The means of these median values are presented in Figure 2. Analysis of variance (ANOVA) of these median RTs, with a between-subjects variable of participant group and within-subjects variables of search condition, target presence, and display size, yielded significant main effects for search condition, F(1, 62) = 478.50, p < .001, target presence, F(1, 62) = 123.36, p < .001, and display size, F(2, 124) = 340.53, p < .001. These effects represent, respectively, a 217 ms increase in RT for conjunction search relative to feature search, a 101 ms increase in RT for target-absent responses relative to target-present responses, and a relatively monotonic increase in RT as a function of increasing display size. The depressed and nondepressed groups did not differ significantly in overall RT. Significant interaction terms were obtained for Group × Display Size, F(2, 124) = 6.06, p = .003, Group × Search Condition, F(1, 62) = 3.98, p = .050, Target Presence × Display Size, F(2, 124) = 81.20, p < .001, Search Condition × Display Size, F(2, 124) = 216.97, p < .001, Search Condition × Target Presence, F(2, 124) = 132.54, p < .001, and Search Condition × Target Presence × Display Size, F(2, 124) = 30.51, p < .001. The Group × Search Condition effect represents a greater increase in RT for conjunction search relative to feature search for the depressed group (237 ms) relative to the control group (198 ms). The Search Condition × Target Presence interaction occurred because target-absent RT was 176 ms higher than target-present RT for conjunction search, whereas the corresponding difference was only 26 ms for feature search.
Figure 2.
Visual search reaction time (RT) for depressed (Panel A) and control (Panel B) participants as a function of conjunction versus feature search type, target presence, and display size.
Values are means, across participants, of each participant’s median RT for correct responses. Error bars are standard errors of the means.
The efficiency of visual search was examined in an analysis of the slope and intercept of the linear function relating RT to display size, obtained for each participant. Mean slope and intercept values for the RT × Display Size functions are presented in Table 2. Analysis of the RT slopes yielded significant main effects for group, F(1, 62) = 6.62, p = .013, search condition, F(1, 62) = 281.98, p < .001, and target presence, F(1, 62) = 108.05, p < .001, as well as an interaction for Search Condition × Target Presence, F(1, 62) = 33.25, p < .001. The main effects represent: 1) an overall slower comparison process for depressed individuals (20 ms per item) than for control participants (15 ms per item), 2) slower comparison during conjunction search (31 ms per item) relative to feature search (5 ms per item), and 3) slower comparison for target-absent displays (24 ms per item) relative to target-present displays (11 ms per item).
Table 2. Slope and Intercept Values of Reaction Time × Display Size Functions, by Search Type, Target Presence, and Group.
| Depressed |
Control |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | Slope | Intercept | ||||||
|
| |||||||||
| Search | Target | M | (SD) | M | (SD) | M | (SD) | M | (SD) |
| feature | present | 2.72 | (4.19) | 633.43 | (81.20) | −0.30 | (3.83) | 634.13 | (74.24) |
| feature | absent | 8.69 | (7.83) | 616.42 | (86.24) | 6.61 | (5.69) | 614.23 | (64.22) |
| conjunction | present | 21.14 | (9.82) | 662.59 | (112.51) | 18.42 | (7.10) | 631.08 | (73.14) |
| conjunction | absent | 46.57 | (24.65) | 667.30 | (110.60) | 36.06 | (16.56) | 676.82 | (109.49) |
Note. n = 32 per group; data are presented as means (across participants) for each task condition. Values are milliseconds. Slope = slope of the linear RT × Display Size function (comparison time per item); intercept = intercept of the linear RT × Display Size function (encoding and response time).
Analysis of the RT intercepts yielded significant main effects for search condition, F(1, 62) = 18.28, p < .001, but not for group or target presence. There was an interaction for Search Condition × Target Presence, F(1, 62) = 11.51, p = .001. The main effect of search condition represents an overall slower perceptual encoding and response in the conjunction search condition (659 ms) compared to the feature search condition (625 ms). The interaction term indicates a significant slowing in encoding and response processes on target-absent trials relative to target-present trials in the conjunction search condition (26 ms), whereas the corresponding difference in the feature search condition (18 ms) was not significant.
Correlations between Neuropsychological and RT Measures
We also examined the correlation between search slopes and traditional neuropsychological tests representing the broad constructs of information processing speed, attentional control, and visual tracking (Table 3). Significant correlations were present in 8 out of 32 tests, though only 2 of the tests remained significant after Bonferroni correction.
Table 3. Pearson correlations of visual search slope with neuropsychological measures.
| Neuropsychological test |
||||
|---|---|---|---|---|
| Search condition/target presence | SDMT | TMT A | TMT B | TMT Diff |
| Depressed participants | ||||
| feature/present | −.40* | .54**† | .40* | 0.27 |
| feature/absent | −56**† | .34 | .34 | 0.28 |
| conjunction/present | −.20 | .11 | .33 | 0.34 |
| conjunction/absent | −.39* | .29 | .24 | 0.17 |
|
| ||||
| Control participants | ||||
| feature/present | −.17 | .04 | −.09 | −0.10 |
| feature/absent | −.13 | −.16 | −.12 | −0.09 |
| conjunction/present | −.45** | .16 | .52** | 0.54** |
| conjunction/absent | .06 | −.06 | .10 | 0.16 |
Note. n = 32 per group, with one missing participant on TMT B. SDMT = Symbol-Digit Modalities Test (Smith, 1982). TMT-A = Trail Making Test Part A. TMT-B = Trail Making Test Part B (Reitan, 1992). TMT Diff = Trail Making Difference Score (TMT B – TMT A).
p < .05.
p < .01.
Remained significant after Bonferroni correction
Discussion
The visual search results (Figure 2) replicated previous findings with regard to differences between feature and conjunction search (Wolfe, 1998a, 1998b). Overall, the RT slopes were < 10 ms per item for feature search, consistent with a highly efficient comparison process for both LLD and control participants. In contrast, the conjunction search slopes were higher in magnitude and exhibited an approximately 2:1 ratio for target-absent trials relative to target-present trials, indicating a less efficient (i.e., more attention-demanding) search that terminated when a target was identified.
Comparison of the participant groups provided evidence for response slowing in LLD, associated to some degree with the attentional demands of conjunction search. Analyses of the mean RT data including display size yielded significant effects for the two-way interactions between group and display size, and between group and search condition. The Group × Display Size interaction represents a more pronounced increase in mean RT for LLD participants compared to controls in relation to increasing display size. This interaction is both supported and extended by the main effect of group in the slope analysis, which reflects higher slope values for display size (i.e., RT per item) in LLD participants compared to controls. Because there was no main effect of group in the analysis of the intercept, the slope finding specifically suggests that speed of comparing successive display items was slower in LLD than controls (slope), rather than speed of other encoding/response processes (intercept).The Group × Search Condition interaction indicates a more profound slowing of mean RT in conjunction search relative to feature search; however, the interaction between group and search condition was not significant for either RT slopes or intercepts. Thus, while LLD patients were differentially sensitive to the attentional demands of conjunction search in the mean RT data, consistent with Hammar and colleagues (Hammar et al., 2003a), this effect was not sufficiently large to be evident within either the RT slope or intercept data. Because mean RT captures all of the different cognitive processes related to visual search, it is possible that the LLD effect is associated with relatively small decrements in several component processes, which are not sufficiently isolated by the slope/intercept analysis. It will be important to study visual search performance in acutely depressed young adults to determine if the effects of age and depression can be more completely separated.
Performance on four neuropsychological measures of information processing speed and attentional control were not significantly different between LLD and control groups (Table 1), which suggests that the group difference in the RT slope of visual search detected LLD-related slowing that was not detectable by these measures. The lack of LLD-related differences on traditional neuropsychological measures in the current study may reflect the fact that it was designed and powered to detect differences in visual search rather than neuropsychological differences, as previous studies have found differences between depressed and nondepressed older adults on Trail Making Test and SDMT with larger samples sizes (Butters et al., 2004; Sheline et al., 2006). In addition, no group differences in visual search were detected either in the encoding and response processes represented by the RT intercepts, or in overall mean RT of visual search. It is possible that group differences in these aspects of visual search may be found with task changes that make conjunction search more difficult, or which otherwise make search more inefficient. To our knowledge, visual search has not been studied previously in LLD, and the analysis of search slope and intercept functions provides new information about slowing at a specific comparison stage of information processing, rather than a more general slowing of information processing speed.
The current results should be evaluated with respect to the cognitive neuroscience of both LLD and visual search. Studies of LLD suggest that both processing speed and attentional control may be adversely affected by dysfunction of fronto-striatal networks (Alexopoulos, Kiosses, Klimstra, Kalayam, & Bruce, 2002; Sheline et al., 2006; Sheline et al., 2010), which may be due to aberrant brain activation or connectivity. Studies of attentional control in LLD have not used visual search specifically, but functional magnetic resonance imaging (fMRI) studies have found a pattern of greater task-related deactivation in the dorsolateral prefrontal cortex (DLPFC) in LLD patients (Aizenstein et al., 2009; Wang et al., 2008), which is a key region in the dorsal attention network and in the intrinsic connectivity of the “task positive” network (Fox, Snyder, Barch, Gusnard, & Raichle, 2005; Kelly, Uddin, Biswal, Castellanos, & Milham, 2008). Studies of visual search in nondepressed and nonelderly samples using fMRI have identified a transiently activated ventral attention system that appears to respond to the occurrence of attentional targets, and a dorsal attention network that demonstrates more sustained activation during search for targets, particularly under conditions of inefficient search (Anderson et al., 2007; Mantini, Corbetta, Perrucci, Romani, & Del Gratta, 2009; Parks & Madden, 2013; Shulman et al., 2003). Healthy older adults have exhibited relatively greater engagement of the frontoparietal network during attentionally demanding visual search (Madden et al., 2007). Thus, insufficient neural activation to cognitive demand in the DLPFC and dorsal attention network of depressed older adults may contribute to a plausible explanation for the slowing of visual search performance observed in the current study; however, this should be tested in fMRI paradigms specific to visual search.
A potential clinical implication of these results is that assessing processing speed over multiple, precisely timed trials can reveal subtle processing speed deficits in LLD compared to older adults with no history of depression. Response measurement over multiple trials with precision timing can produce a smaller standard error, which may favor the detection of significant effects at smaller sample sizes. Measures that can detect performance differences between depressed and nondepressed older adults at smaller sample sizes could be useful in clinical trials to treat cognitive impairments in depression, where smaller sample sizes can lower the cost and time to conduct trials. In addition, tasks designed with careful attention to minimizing confounds across conditions may be helpful in dissociating group differences in cognitive subprocesses, such as the current distinction between comparison speed and encoding/response speed in LLD.
Aside from clinical utility in depression, visual search tests may also have clinical utility in other populations, such as early Alzheimer’s disease (Tales et al., 2011; Tales, Haworth, Nelson, Snowden, & Wilcock, 2005), Parkinson’s disease (Mannan, Hodgson, Husain, & Kennard, 2008; Uc et al., 2006), and stroke (Hildebrandt, Schutze, Ebke, Brunner-Beeg, & Eling, 2005), as well as in functionally important issues such as the assessment and remediation of driving ability (Jehkonen, Saunamaki, Alzamora, Laihosalo, & Kuikka, 2012; Lavalliere, Simoneau, Tremblay, Laurendeau, & Teasdale, 2012; Uc et al., 2006). With respect to clinical practice, few tests from experimental paradigms provide the normative data necessary for clinical interpretation, and this shortcoming will need to be addressed before experimental paradigms like visual search can become useful tools for clinical neuropsychological assessment.
There are limitations to note with this study. For instance, we did not have sufficient data to analyze whether first depression onset in early or late life was related to search performance, which may be important if cognitive profiles differ by age of onset. Another potential limitation of the current study is that participants were enrolled in a naturalistic treatment study designed to optimize individual treatment response (Steffens, McQuoid, & Krishnan, 2002), as opposed to studies in which all participants receive the same pharmacologic treatment. While individuals with depression were typically treated with a selective serotonin reuptake inhibitor (SSRI) as the first option, they were individually treated with different types of antidepressants and combinations thereof. Although this constrains our ability to analyze potential effects of single anti-depressant agents, we believe it unlikely that medication differences in our study would have a systematic effect on visual search performance, because most antidepressant medications have small-to-nonexistent effects on cognitive performance (Podewils & Lyketsos, 2002; Siepmann, Grossmann, Muck-Weymann, & Kirch, 2003), and antidepressant treatment would presumably lessen rather than increase symptom severity. We note that the relatively high level of education in our sample may not generalize to samples with lower levels of education, though a prior study did not find education to moderate cognitive deficits between LLD and a nondepressed control group (Bhalla et al., 2005). Finally, while we did identify a novel finding regarding comparison speed during visual search in LLD, the generalizability to information processing speed in other cognitive domains such as working memory or episodic memory remains to be established. In summary, these results suggest a specific slowing in the comparison stage of visual search in LLD, rather than a general cognitive slowing. Although this research furthers the argument that slowing of some cognitive processes is a specific and core feature of LLD, the finding may not be unique to LLD and would benefit from replication in younger samples with MDD. This underscores the need to work toward identifying the relationship of specific components of cognitive slowing to depression across a range of ages, and how specific profiles of cognitive slowing are related to clinical phenotypes and neurocircuitry of LLD.
Acknowledgements
This research was supported by the following grants from the National Institutes of Health: R01 MH054846, P50 MH060451, K23 MH087741, K24 MH070027, R01 AG039684. We also thank Kyle Heidtman and Scott Davis for their assistance with manuscript preparation.
References
- Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. American Journal of Geriatric Psychiatry. 2009;17(1):30–42. doi: 10.1097/JGP.0b013e31817b60af. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. American Journal of Geriatric Psychiatry. 2002;10(1):98–106. [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M. Executive dysfunction and long-term outcomes of geriatric depression. Archives of General Psychiatry. 2000;57(3):285–290. doi: 10.1001/archpsyc.57.3.285. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Young RC, Meyers BS. Geriatric depression: age of onset and dementia. Biological Psychiatry. 1993;34(3):141–145. doi: 10.1016/0006-3223(93)90383-o. doi: 10.1016/0006-3223(93)90383-O. [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Mannan SK, Husain M, Rees G, Sumner P, Mort DJ. Involvement of prefrontal cortex in visual search. Experimental Brain Research. 2007;180(2):289–302. doi: 10.1007/s00221-007-0860-0. doi: 10.1007/s00221-007-0860-0. [DOI] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychological Medicine. 1996;26(3):591–603. doi: 10.1017/s0033291700035662. doi: 10.1017/S0033291700035662. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GA. Manual for the Beck Depression Inventory-II. San Antonio, TX; Psychological Corporation: 1996. [Google Scholar]
- Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B. Persistence of neuropsychologic deficits in the remitted state of late-life depression. American Journal of Geriatric Psychiatry. 2006;14(5):419–427. doi: 10.1097/01.JGP.0000203130.45421.69. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Zmuda MD, Seligman K, Mulsant BH, Pollock BG. Does education moderate neuropsychological impairment in late-life depression? International Journal of Geriatric Psychiatry. 2005;20(5):413–417. doi: 10.1002/gps.1296. doi: 10.1002/gps.1296. [DOI] [PubMed] [Google Scholar]
- Boone K, Lesser B, Miller B, Wohl M, Berman N, Lee A. Cognitive functioning in a geriatric depressed population: relationship of presence and severity of depression to neuropsychological scores. Neuropsychology. 1995;9:390–398. doi: 10.1037/0894-4105.9.3.390. [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61(6):587–595. doi: 10.1001/archpsyc.61.6.587. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Deluca J, Kalmar JH. Information processing speed in clinical populations. Taylor & Francis; New York: 2007. [Google Scholar]
- Demakis GJ, Sawyer TP, Fritz D, Sweet JJ. Incidental recall on WAIS-R digit symbol discriminates Alzheimer’s and Parkinson’s diseases. Journal of Clinical Psychology. 2001;57(3):387–394. doi: 10.1002/jclp.1020. doi: 10.1002/jclp.1020. [DOI] [PubMed] [Google Scholar]
- Drane DL, Yuspeh RL, Huthwaite JS, Klingler LK. Demographic characteristics and normative observations for derived-trail making test indices. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2002;15(1):39–43. [PubMed] [Google Scholar]
- Duncan J, Humphreys GW. Visual search and stimulus similarity. Psychological Review. 1989;96(3):433–458. doi: 10.1037/0033-295x.96.3.433. doi: 10.1037/0033-295X.96.3.433. [DOI] [PubMed] [Google Scholar]
- Eckstein MP. Visual search: a retrospective. J Vis. 2011;11(5) doi: 10.1167/11.5.14. doi: 10.1167/11.5.14. [DOI] [PubMed] [Google Scholar]
- Elliott R. The neuropsychological profile in unipolar depression. Trends in Cognitive Sciences. 1998;2(11):447–454. doi: 10.1016/s1364-6613(98)01235-2. doi: 10.1016/S1364-6613(98)01235-2. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, Fanjiang G. Mini-Mental State Examination: Clinical Guide and User’s Guide. Psychological Assessment Resources; Lutz, FL: 2001. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Barch DM, Gusnard DA, Raichle ME. Transient BOLD responses at block transitions. Neuroimage. 2005;28(4):956–966. doi: 10.1016/j.neuroimage.2005.06.025. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Hammar A, Ardal G. Effortful information processing in patients with major depression - A 10-year follow-up study. Psychiatry Research. 2012;198(3):420–423. doi: 10.1016/j.psychres.2011.11.020. doi: 10.1016/j.psychres.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Hammar A, Lund A, Hugdahl K. Selective impairment in effortful information processing in major depression. Journal of the International Neuropsychological Society. 2003a;9(6):954–959. doi: 10.1017/S1355617703960152. doi: 10.1017/S1355617703960152. [DOI] [PubMed] [Google Scholar]
- Hammar A, Lund A, Hugdahl K. Long-lasting cognitive impairment in unipolar major depression: a 6-month follow-up study. Psychiatry Research. 2003b;118(2):189–196. doi: 10.1016/s0165-1781(03)00075-1. doi: 10.1016/S0165-1781(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Schutze C, Ebke M, Brunner-Beeg F, Eling P. Visual search for item- and array-centered locations in patients with left middle cerebral artery stroke. Neurocase. 2005;11(6):416–426. doi: 10.1080/13554790500263511. doi: 10.1080/13554790500263511. [DOI] [PubMed] [Google Scholar]
- Hommel B, Li KZ, Li SC. Visual search across the life span. Developmental Psychology. 2004;40(4):545–558. doi: 10.1037/0012-1649.40.4.545. doi: 10.1037/0012-1649.40.4.545. [DOI] [PubMed] [Google Scholar]
- Jehkonen M, Saunamaki T, Alzamora AK, Laihosalo M, Kuikka P. Driving ability in stroke patients with residual visual inattention: a case study. Neurocase. 2012;18(2):160–166. doi: 10.1080/13554794.2011.568504. doi: 10.1080/13554794.2011.568504. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kiosses DN, Alexopoulos GS, Murphy C. Symptoms of striatofrontal dysfunction contribute to disability in geriatric depression. International Journal of Geriatric Psychiatry. 2000;15(11):992–999. doi: 10.1002/1099-1166(200011)15:11<992::aid-gps248>3.0.co;2-6. doi: 10.1002/1099-1166(200011)15:11<992::AID-GPS248>3.0.CO. [DOI] [PubMed] [Google Scholar]
- Kohler S, Thomas AJ, Barnett NA, O’Brien JT. The pattern and course of cognitive impairment in late-life depression. Psychological Medicine. 2010;40(4):591–602. doi: 10.1017/S0033291709990833. doi: 10.1017/S0033291709990833. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Psychology Press; New York: 2008. pp. 189–249. [Google Scholar]
- Lavalliere M, Simoneau M, Tremblay M, Laurendeau D, Teasdale N. Active training and driving-specific feedback improve older drivers’ visual search prior to lane changes. BMC Geriatrics. 2012;12:5. doi: 10.1186/1471-2318-12-5. doi: 10.1186/1471-2318-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. International Psychogeriatrics. 2007;19(1):125–135. doi: 10.1017/S1041610206003607. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. Journal of Affective Disorders. 2012;140(2):113–124. doi: 10.1016/j.jad.2011.10.023. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Speed and timing of behavioral processes. In: Birren JE, Schaie KW, editors. Handbook of the Psychology of Aging. Academic Press; San Diego, CA: 2001. pp. 288–312. [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiology of Aging. 2007;28(3):459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL. Age-related changes in visual attention. In: Costa PT, Siegler IC, editors. Recent advances in psychology and aging. Elsevier; Amsterdam: 2004. pp. 41–88. [Google Scholar]
- Mannan SK, Hodgson TL, Husain M, Kennard C. Eye movements in visual search indicate impaired saliency processing in Parkinson’s disease. Progress in Brain Research. 2008;171:559–562. doi: 10.1016/S0079-6123(08)00679-1. doi: 10.1016/S0079-6123(08)00679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Perrucci MG, Romani GL, Del Gratta C. Large-scale brain networks account for sustained and transient activity during target detection. Neuroimage. 2009;44(1):265–274. doi: 10.1016/j.neuroimage.2008.08.019. doi: 10.1016/j.neuroimage.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KM, Yaffe K, Langa KM, Sands L, Whooley MA, Covinsky KE. Additive effects of cognitive function and depressive symptoms on mortality in elderly community-living adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2003;58(5):M461–467. doi: 10.1093/gerona/58.5.m461. doi: 10.1093/gerona/58.5.M461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychological Medicine. 2000;30(3):679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- Parks EL, Madden DJ. Brain connectivity and visual attention. Brain Connectivity. 2013 doi: 10.1089/brain.2012.0139. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podewils LJ, Lyketsos CG. Tricyclic antidepressants and cognitive decline. Psychosomatics. 2002;43(1):31–35. doi: 10.1176/appi.psy.43.1.31. doi: 10.1176/appi.psy.43.1.31. [DOI] [PubMed] [Google Scholar]
- Potter GG, Kittinger JD, Wagner HR, Steffens DC, Krishnan KR. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29(12):2266–2271. doi: 10.1038/sj.npp.1300551. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- Potter GG, Wagner HR, Burke JR, Plassman BL, Welsh-Bohmer KA, Steffens DC. Neuropsychological Predictors of Dementia in Late-Life Major Depressive Disorder. American Journal of Geriatric Psychiatry. 2012 doi: 10.1016/j.jagp.2012.12.009. doi: 10.1016/j.jagp.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test: Manual for Administration and Scoring. Reitan Neuropsychological Laboratory; Tuscon, AZ: 1992. [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38(4):381–389. doi: 10.1001/archpsyc.1981.01780290015001. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. doi: 10.1037/0033-295X.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Madden DJ. Information processing speed and aging. In: Deluca J, Kalmar J, editors. Information processing speed in clinical populations. Psychology Press; New York: 2007. pp. 221–241. [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review. 1977;84:1–66. doi: 10.1037/0033-295X.84.1.1. [Google Scholar]
- Schweitzer I, Tuckwell V, O’Brien J, Ames D. Is late onset depression a prodrome to dementia? International Journal of Geriatric Psychiatry. 2002;17(11):997–1005. doi: 10.1002/gps.525. doi: 10.1002/gps.525. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biological Psychiatry. 2006;60(1):58–65. doi: 10.1016/j.biopsych.2005.09.019. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Disabato BM, Hranilovich J, Morris C, D’Angelo G, Pieper C. Treatment course with antidepressant therapy in late-life depression. American Journal of Psychiatry. 2012;169:1185–1193. doi: 10.1176/appi.ajp.2012.12010122. doi: 10.1176/appi.ajp.2012.12010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Archives of General Psychiatry. 2010;67(3):277–285. doi: 10.1001/archgenpsychiatry.2009.204. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d’Avossa G. Quantitative analysis of attention and detection signals during visual search. Journal of Neurophysiology. 2003;90(5):3384–3397. doi: 10.1152/jn.00343.2003. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Siepmann M, Grossmann J, Muck-Weymann M, Kirch W. Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology. 2003;168(3):293–298. doi: 10.1007/s00213-003-1448-4. doi: 10.1007/s00213-003-1448-4. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test-Manual. Western Psychological Services; Los Angeles: 1982. [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological Bulletin. 2013;139(1):81–132. doi: 10.1037/a0028727. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, McQuoid DR, Krishnan KR. The Duke Somatic Treatment Algorithm for Geriatric Depression (STAGED) approach. Psychopharmacology Bulletin. 2002;36(2):58–68. [PubMed] [Google Scholar]
- Steffens DC, Welsh-Bohmer KA, Burke JR, Plassman BL, Beyer JL, Gersing KR. Methodology and preliminary results from the neurocognitive outcomes of depression in the elderly study. Journal of Geriatric Psychiatry and Neurology. 2004;17(4):202–211. doi: 10.1177/0891988704269819. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- Tales A, Bayer AJ, Haworth J, Snowden RJ, Philips M, Wilcock G. Visual search in mild cognitive impairment: a longitudinal study. Journal of Alzeimers Disease. 2011;24(1):151–160. doi: 10.3233/JAD-2010-101818. doi: 10.3233/JAD-2010-101818. [DOI] [PubMed] [Google Scholar]
- Tales A, Haworth J, Nelson S, Snowden RJ, Wilcock G. Abnormal visual search in mild cognitive impairment and Alzheimer’s disease. Neurocase. 2005;11(1):80–84. doi: 10.1080/13554790490896974. doi: 10.1080/13554790490896974. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, O’Brien JT. Depression and cognition in older adults. Current Opinion in Psychiatry. 2008;21(1):8–13. doi: 10.1097/YCO.0b013e3282f2139b. doi: 10.1097/YCO.0b013e3282f2139b. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Sparks J, Rodnitzky RL, Dawson JD. Impaired visual search in drivers with Parkinson’s disease. Annals of Neurology. 2006;60(4):407–413. doi: 10.1002/ana.20958. doi: 10.1002/ana.20958. [DOI] [PubMed] [Google Scholar]
- Wang L, Krishnan KR, Steffens DC, Potter GG, Dolcos F, McCarthy G. Depressive state- and disease-related alterations in neural responses to affective and executive challenges in geriatric depression. American Journal of Psychiatry. 2008;165(7):863–871. doi: 10.1176/appi.ajp.2008.07101590. doi: 10.1176/appi.ajp.2008.07101590. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Visual search. In: Pashler H, editor. Attention. Psychology Press; East Sussex, UK: 1998a. pp. 13–73. [Google Scholar]
- Wolfe JM. What can 1 million trials tell us about visual search? Psychological Science. 1998b;9:33–39. [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1998;11(3):111–119. [PubMed] [Google Scholar]