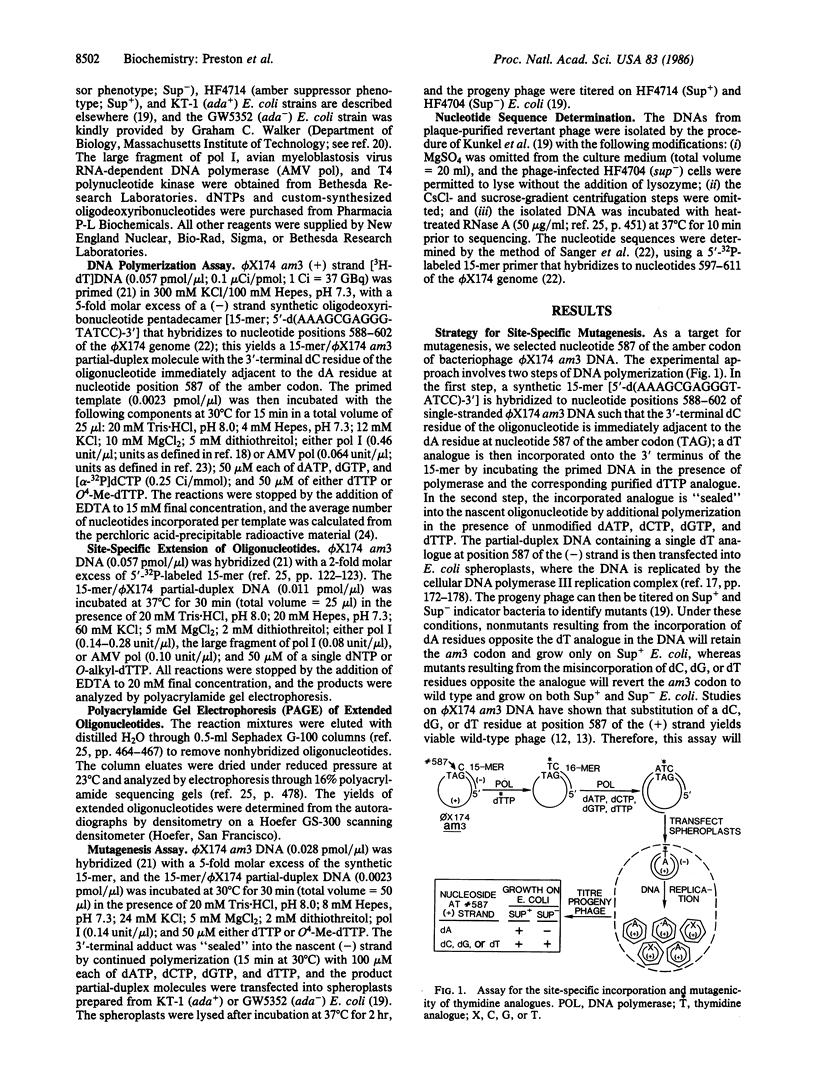
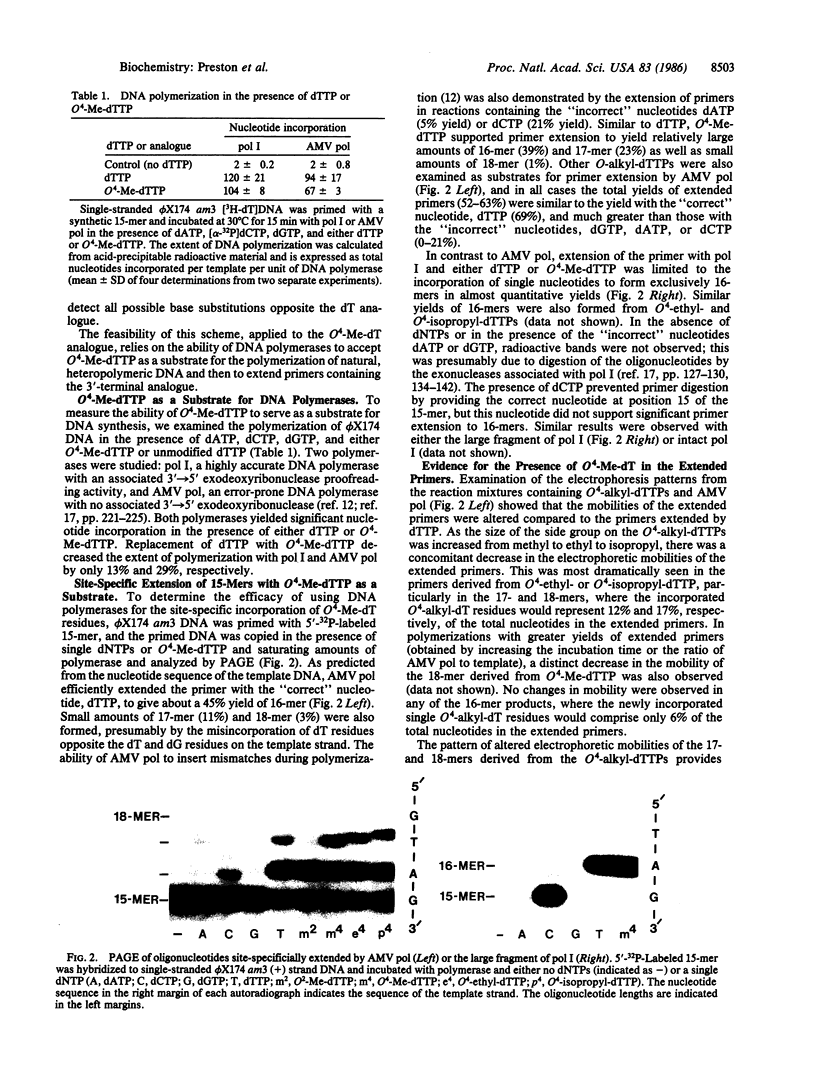
Abstract
O4-Alkylthymine-DNA adducts have been implicated as causative lesions in chemical mutagenesis and carcinogenesis. To directly assess the mutagenic potential of these adducts in vivo, we have designed an enzymatic technique for introducing nucleotide analogues at predetermined sites of biologically active DNA. Escherichia coli DNA polymerase I was used in vitro to incorporate a single O4-methylthymine residue at the 3' terminus of an oligonucleotide primer opposite the adenine residue of the amber codon in bacteriophage phi X174 am3 DNA. After further extension of the primer with unmodified nucleotides, the partial-duplex product was transfected into E. coli spheroplasts. Replication of the site-specifically methylated DNA in E. coli deficient in O4-methylthymine-DNA methyltransferase (ada-) yielded 10-fold more mutant progeny phage than replication of nonmethylated DNA; no increase in mutation frequency was observed after replication in repair-proficient (ada+) E. coli. The DNA from 20 independently isolated mutant plaques all contained A.T----G.C transitions at the original site of O4-methylthymine incorporation. These data demonstrate that O4-methylthymine induces base-substitution mutations in E. coli and suggest that this adduct may be involved in mutagenesis by N-nitroso methylating agents. This enzymatic technique for site-specific mutagenesis provides an alternative to the chemical synthesis of oligonucleotides containing altered bases.
Full text
PDF
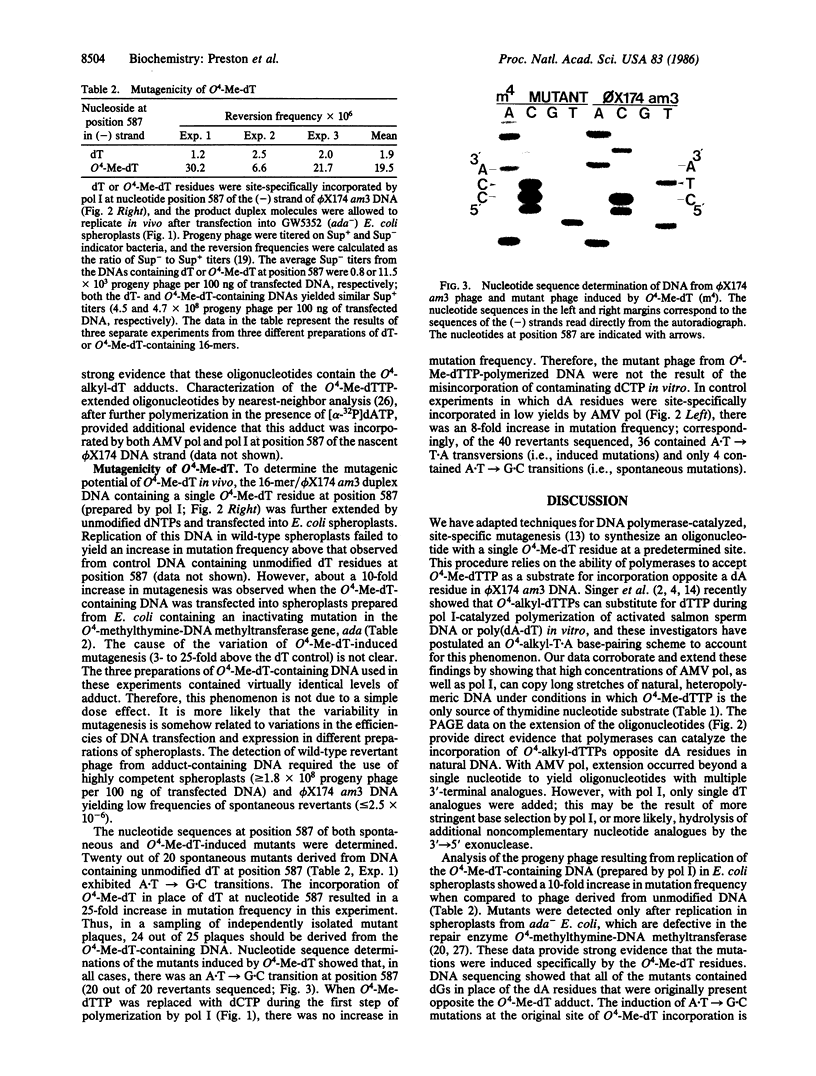

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbotts J., Loeb L. A. On the fidelity of DNA replication: use of synthetic oligonucleotide-initiated reactions. Biochim Biophys Acta. 1985 Jan 29;824(1):58–65. doi: 10.1016/0167-4781(85)90029-6. [DOI] [PubMed] [Google Scholar]
- Battula N., Loeb L. A. The infidelity of avian myeloblastosis virus deoxyribonucleic acid polymerase in polynucleotide replication. J Biol Chem. 1974 Jul 10;249(13):4086–4093. [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Demple B., Sedgwick B., Robins P., Totty N., Waterfield M. D., Lindahl T. Active site and complete sequence of the suicidal methyltransferase that counters alkylation mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(9):2688–2692. doi: 10.1073/pnas.82.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. A., Saffhill R. The incorporation of O6-methyldeoxyguanosine and O4-methyldeoxythymidine monophosphates into DNA by DNA polymerases I and alpha. Nucleic Acids Res. 1983 Jun 25;11(12):4185–4193. doi: 10.1093/nar/11.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houts G. E., Miyagi M., Ellis C., Beard D., Beard J. W. Reverse transcriptase from avian myeloblastosis virus. J Virol. 1979 Feb;29(2):517–522. doi: 10.1128/jvi.29.2.517-522.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. C., Guttenplan J. B. Evidence for a major premutagenic ethyldeoxythymidine-DNA adduct in an in vivo system: N-nitroso-N-ethylurea-treated Salmonella typhimurium. Carcinogenesis. 1985 Oct;6(10):1513–1516. doi: 10.1093/carcin/6.10.1513. [DOI] [PubMed] [Google Scholar]
- JOSSE J., KAISER A. D., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VIII. Frequencies of nearest neighbor base sequences in deoxyribonucleic acid. J Biol Chem. 1961 Mar;236:864–875. [PubMed] [Google Scholar]
- Lemotte P. K., Walker G. C. Induction and autoregulation of ada, a positively acting element regulating the response of Escherichia coli K-12 to methylating agents. J Bacteriol. 1985 Mar;161(3):888–895. doi: 10.1128/jb.161.3.888-895.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffhill R. In vitro miscoding of alkylthymines with DNA and RNA polymerases. Chem Biol Interact. 1985 Feb-Apr;53(1-2):121–130. doi: 10.1016/s0009-2797(85)80090-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Silber J. R., Fry M., Martin G. M., Loeb L. A. Fidelity of DNA polymerases isolated from regenerating liver chromatin of aging Mus musculus. J Biol Chem. 1985 Jan 25;260(2):1304–1310. [PubMed] [Google Scholar]
- Singer B., Abbott L. G., Spengler S. J. Assessment of mutagenic efficiency of two carcinogen-modified nucleosides, 1,N6-ethenodeoxyadenosine and O4-methyldeoxythymidine, using polymerases of varying fidelity. Carcinogenesis. 1984 Sep;5(9):1165–1171. doi: 10.1093/carcin/5.9.1165. [DOI] [PubMed] [Google Scholar]
- Singer B., Chavez F., Spengler S. J. O4-Methyl-, O4-ethyl-, and O4-isopropylthymidine 5'-triphosphates as analogues of thymidine 5'-triphosphate: kinetics of incorporation by Escherichia coli DNA polymerase I. Biochemistry. 1986 Mar 25;25(6):1201–1205. doi: 10.1021/bi00354a001. [DOI] [PubMed] [Google Scholar]
- Singer B., Kröger M., Carrano M. O2- and O4-alkyl pyrimidine nucleosides: stability of the glycosyl bond and of the alkyl group as a function of pH. Biochemistry. 1978 Apr 4;17(7):1246–1250. doi: 10.1021/bi00600a018. [DOI] [PubMed] [Google Scholar]
- Singer B., Spengler S. J., Fraenkel-Conrat H., Kuśmierek J. T. O4-Methyl, -ethyl, or -isopropyl substituents on thymidine in poly(dA-dT) all lead to transitions upon replication. Proc Natl Acad Sci U S A. 1986 Jan;83(1):28–32. doi: 10.1073/pnas.83.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Spengler S., Bodell W. J. Tissue-dependent enzyme-mediated repair or removal of O-ethyl pyrimidines and ethyl purines in carcinogen-treated rats. Carcinogenesis. 1981;2(10):1069–1073. doi: 10.1093/carcin/2.10.1069. [DOI] [PubMed] [Google Scholar]
- Singer B., Sági J., Kuśmierek J. T. Escherichia coli polymerase I can use O2-methyldeoxythymidine or O4-methyldeoxythymidine in place of deoxythymidine in primed poly(dA-dT).poly(dA-dT) synthesis. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4884–4888. doi: 10.1073/pnas.80.16.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater J. P., Tamir I., Loeb L. A., Mildvan A. S. The mechanism of Escherichia coli deoxyribonucleic acid polymerase I. Magnetic resonance and kinetic studies of the role of metals. J Biol Chem. 1972 Nov 10;247(21):6784–6794. [PubMed] [Google Scholar]
- Snow E. T., Foote R. S., Mitra S. Kinetics of incorporation of O6-methyldeoxyguanosine monophosphate during in vitro DNA synthesis. Biochemistry. 1984 Sep 11;23(19):4289–4294. doi: 10.1021/bi00314a006. [DOI] [PubMed] [Google Scholar]
- Stöhrer G., Osband J. A., Alvarado-Urbina G. Site-specific modification of the lactose operator with acetylaminofluorene. Nucleic Acids Res. 1983 Aug 11;11(15):5093–5102. doi: 10.1093/nar/11.15.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenberg J. A., Dyroff M. C., Bedell M. A., Popp J. A., Huh N., Kirstein U., Rajewsky M. F. O4-ethyldeoxythymidine, but not O6-ethyldeoxyguanosine, accumulates in hepatocyte DNA of rats exposed continuously to diethylnitrosamine. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1692–1695. doi: 10.1073/pnas.81.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakour R. A., James E. A., Loeb L. A. Site specific mutagenesis: insertion of single noncomplementary nucleotides at specified sites by error-directed DNA polymerization. Nucleic Acids Res. 1984 Aug 24;12(16):6615–6628. doi: 10.1093/nar/12.16.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]