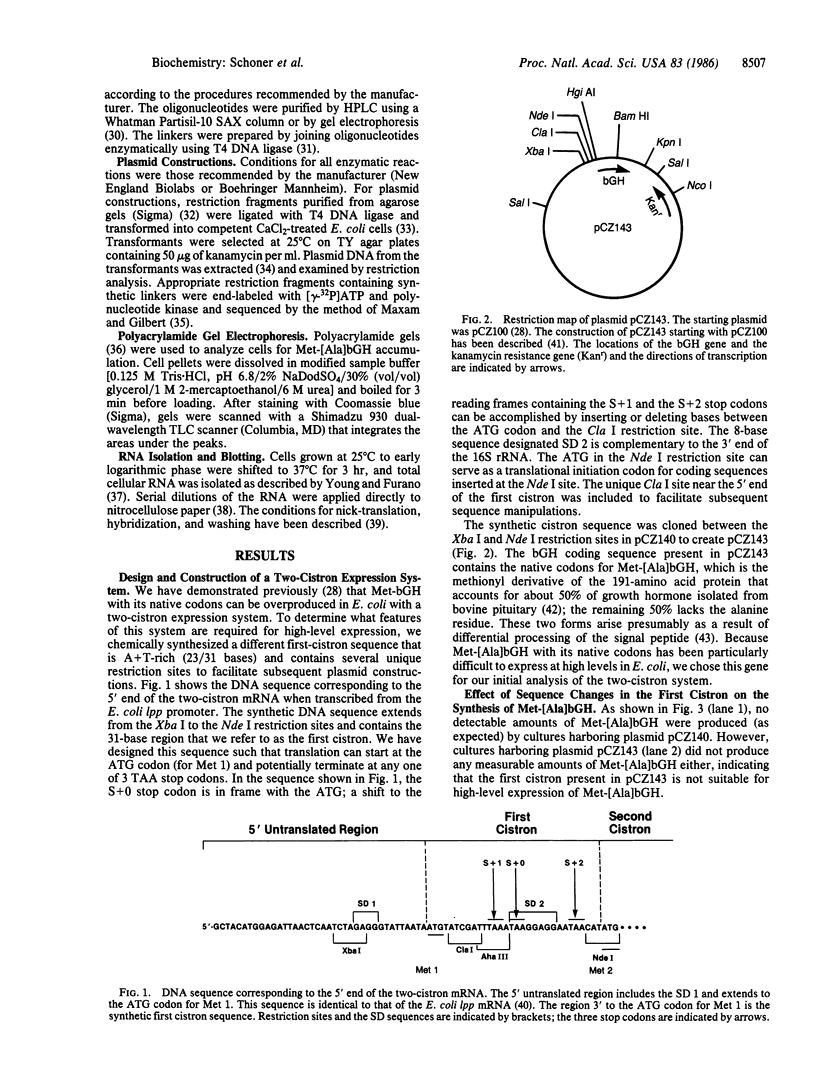
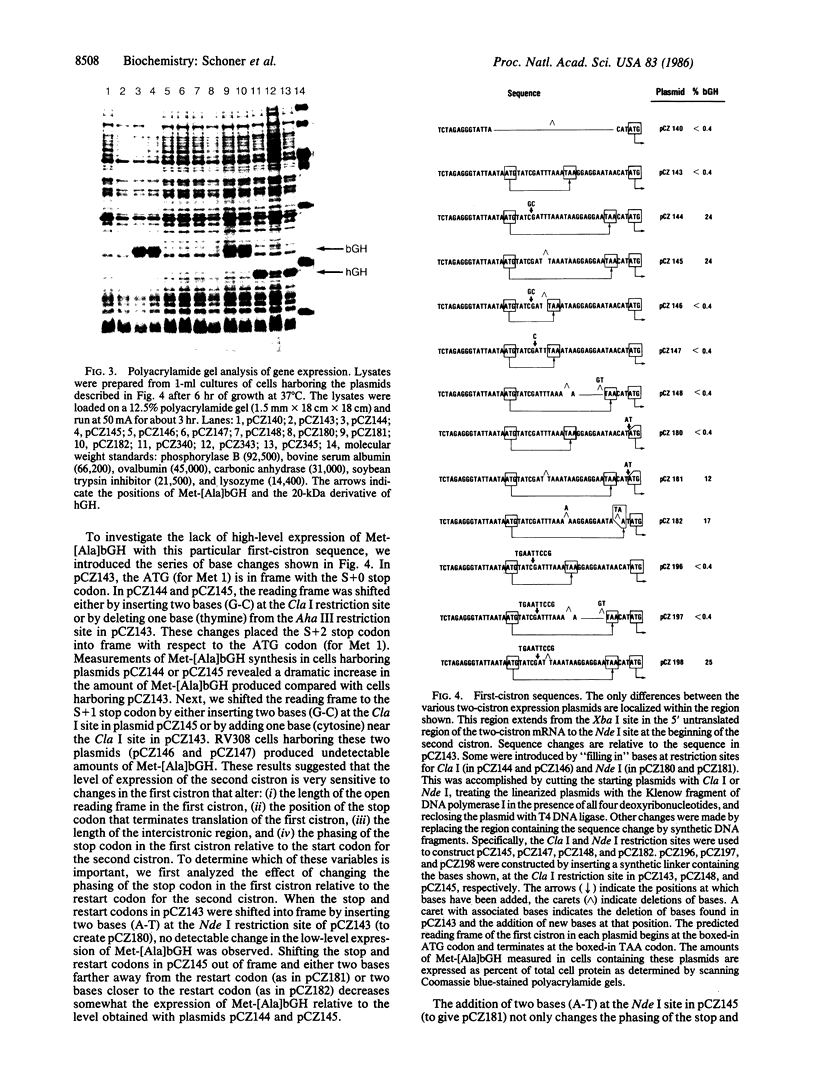
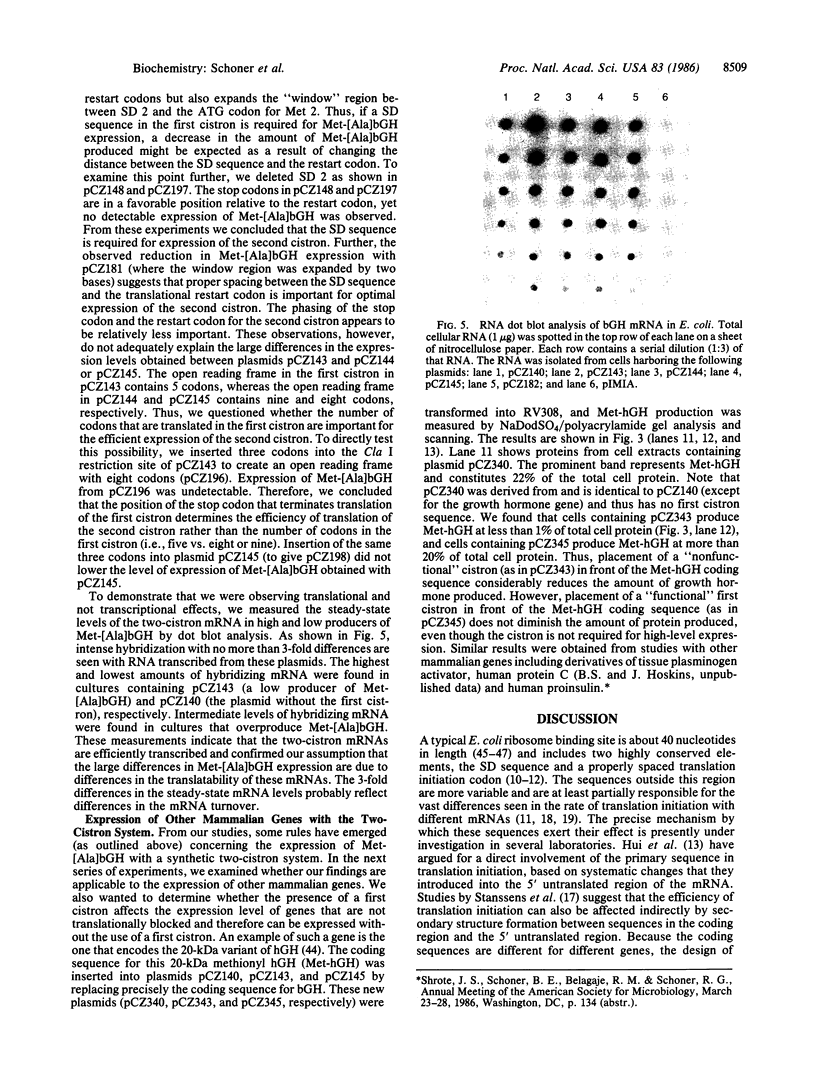
Abstract
A synthetic two-cistron expression system was constructed for the high-level expression of eukaryotic genes in Escherichia coli. This system was designed to overcome translational inhibition of mRNAs containing eukaryotic sequences. The first cistron in this system is a 31-base A + T-rich synthetic sequence that provides for efficient translation initiation. The second cistron contains the protein coding sequence for the eukaryotic gene. Insertion of the first cistron between the 5' untranslated region of the mRNA and the protein coding region separates the two and thereby potentially minimizes the formation of local secondary structures that might prevent ribosomes from binding and initiating translation. The 31-base cistron contains three nonsense codons (TAA), one in each of the three translational reading frames, and an 8-base Shine-Dalgarno sequence that is complementary to the 3' end of the 16S rRNA. The effects of translation of the first cistron in all three reading frames on the expression of the second cistron was examined. The most efficient expression of the second cistron seemed to occur when the stop codon that terminates translation of the first cistron is located 3' to the Shine-Dalgarno sequence and close to the AUG start codon for the second cistron. When the Shine-Dalgarno sequence was deleted from the first cistron, no detectable expression of the second cistron was observed. This two-cistron system has been used to express the gene encoding methionylalanyl bovine growth hormone with its native codons and the gene encoding methionyl human growth hormone at a level greater than 20% of total cell protein. In the case of human growth hormone, we show that the amount of gene product is not significantly diminished by placing a "functional" first cistron in front of a gene that can be expressed without a cistron.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughman G., Nomura M. Localization of the target site for translational regulation of the L11 operon and direct evidence for translational coupling in Escherichia coli. Cell. 1983 Oct;34(3):979–988. doi: 10.1016/0092-8674(83)90555-x. [DOI] [PubMed] [Google Scholar]
- Berkhout B., van Duin J. Mechanism of translational coupling between coat protein and replicase genes of RNA bacteriophage MS2. Nucleic Acids Res. 1985 Oct 11;13(19):6955–6967. doi: 10.1093/nar/13.19.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. L., Belagaje R., Ryan M. J., Khorana H. G. Chemical synthesis and cloning of a tyrosine tRNA gene. Methods Enzymol. 1979;68:109–151. doi: 10.1016/0076-6879(79)68010-2. [DOI] [PubMed] [Google Scholar]
- Crowl R., Seamans C., Lomedico P., McAndrew S. Versatile expression vectors for high-level synthesis of cloned gene products in Escherichia coli. Gene. 1985;38(1-3):31–38. doi: 10.1016/0378-1119(85)90200-8. [DOI] [PubMed] [Google Scholar]
- Danner D. B. Recovery of DNA fragments from gels by transfer to DEAE-paper in an electrophoresis chamber. Anal Biochem. 1982 Sep 1;125(1):139–142. doi: 10.1016/0003-2697(82)90394-3. [DOI] [PubMed] [Google Scholar]
- Das A., Urbanowski J., Weissbach H., Nestor J., Yanofsky C. In vitro synthesis of the tryptophan operon leader peptides of Escherichia coli, Serratia marcescens, and Salmonella typhimurium. Proc Natl Acad Sci U S A. 1983 May;80(10):2879–2883. doi: 10.1073/pnas.80.10.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Yanofsky C. A ribosome binding site sequence is necessary for efficient expression of the distal gene of a translationally-coupled gene pair. Nucleic Acids Res. 1984 Jun 11;12(11):4757–4768. doi: 10.1093/nar/12.11.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gait M. J., Singh M., Sheppard R. C., Edge M. D., Greene A. R., Heathcliffe G. R., Atkinson T. C., Newton C. R., Markham A. F. Rapid synthesis of oligodeoxyribonucleotides. IV. Improved solid phase synthesis of oligodeoxyribonucleotides through phosphotriester intermediates. Nucleic Acids Res. 1980 Mar 11;8(5):1081–1096. doi: 10.1093/nar/8.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. J., L'Italien J. J., Pilacinski W. P., Glassman D. L., Krzyzek R. A. High-level expression in Escherichia coli of biologically active bovine growth hormone. DNA. 1985 Aug;4(4):273–281. doi: 10.1089/dna.1985.4.273. [DOI] [PubMed] [Google Scholar]
- Gheysen D., Iserentant D., Derom C., Fiers W. Systematic alteration of the nucleotide sequence preceding the translation initiation codon and the effects on bacterial expression of the cloned SV40 small-t antigen gene. Gene. 1982 Jan;17(1):55–63. doi: 10.1016/0378-1119(82)90100-7. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gold L., Stormo G., Saunders R. Escherichia coli translational initiation factor IF3: a unique case of translational regulation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7061–7065. doi: 10.1073/pnas.81.22.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. L., Chen J., Schaefer L., Lengyel P., Weissman S. M. Nucleotide sequence of a ribosome attachment site of bacteriophage f2 RNA. Biochem Biophys Res Commun. 1970 Jun 5;39(5):883–888. doi: 10.1016/0006-291x(70)90406-7. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Débarbouillé M., Schwartz M. A role for mRNA secondary structure in the control of translation initiation. Nature. 1982 Feb 18;295(5850):616–618. doi: 10.1038/295616a0. [DOI] [PubMed] [Google Scholar]
- Hindley J., Staples D. H. Sequence of a ribosome binding site in bacteriophage Q-beta-RNA. Nature. 1969 Dec 6;224(5223):964–967. doi: 10.1038/224964a0. [DOI] [PubMed] [Google Scholar]
- Hui A., Hayflick J., Dinkelspiel K., de Boer H. A. Mutagenesis of the three bases preceding the start codon of the beta-galactosidase mRNA and its effect on translation in Escherichia coli. EMBO J. 1984 Mar;3(3):623–629. doi: 10.1002/j.1460-2075.1984.tb01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iserentant D., Fiers W. Secondary structure of mRNA and efficiency of translation initiation. Gene. 1980 Apr;9(1-2):1–12. doi: 10.1016/0378-1119(80)90163-8. [DOI] [PubMed] [Google Scholar]
- Kelley R. L., Yanofsky C. Trp aporepressor production is controlled by autogenous regulation and inefficient translation. Proc Natl Acad Sci U S A. 1982 May;79(10):3120–3124. doi: 10.1073/pnas.79.10.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. H., ASH L. The nitrogen terminal end-groups of hypophyseal growth hormone. J Biol Chem. 1953 Jul;203(1):419–424. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis U. J., Bonewald L. F., Lewis L. J. The 20,000-dalton variant of human growth hormone: location of the amino acid deletions. Biochem Biophys Res Commun. 1980 Jan 29;92(2):511–516. doi: 10.1016/0006-291x(80)90363-0. [DOI] [PubMed] [Google Scholar]
- Maurer R., Meyer B., Ptashne M. Gene regulation at the right operator (OR) bacteriophage lambda. I. OR3 and autogenous negative control by repressor. J Mol Biol. 1980 May 15;139(2):147–161. doi: 10.1016/0022-2836(80)90302-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. 1984 Jun 28-Jul 4Nature. 309(5971):810–812. doi: 10.1038/309810a0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Pirtle R. M., Pirtle I. L., Takeishi K., Inouye M. Messenger ribonucleic acid of the lipoprotein of the Escherichia coli outer membrane. II. The complete nucleotide sequence. J Biol Chem. 1980 Jan 10;255(1):210–216. [PubMed] [Google Scholar]
- Ray P. N., Pearson M. L. Evidence for post-transcriptional control of the morphogenetic genes of bacteriophage lambda. J Mol Biol. 1974 May 5;85(1):163–175. doi: 10.1016/0022-2836(74)90135-1. [DOI] [PubMed] [Google Scholar]
- Ray P. N., Pearson M. L. Functional inactivation of bacteriophage lambda morphogenetic gene in RNA. Nature. 1975 Feb 20;253(5493):647–650. doi: 10.1038/253647a0. [DOI] [PubMed] [Google Scholar]
- Roberts T. M., Kacich R., Ptashne M. A general method for maximizing the expression of a cloned gene. Proc Natl Acad Sci U S A. 1979 Feb;76(2):760–764. doi: 10.1073/pnas.76.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoner B. E., Hsiung H. M., Belagaje R. M., Mayne N. G., Schoner R. G. Role of mRNA translational efficiency in bovine growth hormone expression in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5403–5407. doi: 10.1073/pnas.81.17.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoner B., Schoner R. G. Distribution of IS5 in bacteria. Gene. 1981 Dec;16(1-3):347–352. doi: 10.1016/0378-1119(81)90093-7. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Roa M., Débarbouillé M. Mutations that affect lamB gene expression at a posttranscriptional level. Proc Natl Acad Sci U S A. 1981 May;78(5):2937–2941. doi: 10.1073/pnas.78.5.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Sias S., Adelman J., de Boer H. A., Hayflick J., Jhurani P., Goeddel D. V., Heyneker H. L. Efficient bacterial expression of bovine and porcine growth hormones. DNA. 1983;2(1):37–45. doi: 10.1089/dna.1.1983.2.37. [DOI] [PubMed] [Google Scholar]
- Shepard H. M., Yelverton E., Goeddel D. V. Increased synthesis in E. coli of fibroblast and leukocyte interferons through alterations in ribosome binding sites. DNA. 1982;1(2):125–131. doi: 10.1089/dna.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Stanssens P., Remaut E., Fiers W. Alterations upstream from the Shine-Dalgarno region and their effect on bacterial gene expression. Gene. 1985;36(3):211–223. doi: 10.1016/0378-1119(85)90176-3. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
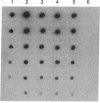
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Varadarajan R., Szabo A., Boxer S. G. Cloning, expression in Escherichia coli, and reconstitution of human myoglobin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5681–5684. doi: 10.1073/pnas.82.17.5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- Whitehorn E. A., Livak K. J., Petteway S. R., Jr The effects of hybrid ribosome-binding-site variants on the expression of human interferon-beta in Escherichia coli. Gene. 1985;36(3):375–379. doi: 10.1016/0378-1119(85)90194-5. [DOI] [PubMed] [Google Scholar]
- Young F. S., Furano A. V. Regulation of the synthesis of E. coli elongation factor Tu. Cell. 1981 Jun;24(3):695–706. doi: 10.1016/0092-8674(81)90096-9. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Hui A., Comstock L. J., Wong E., Vasser M. Portable Shine-Dalgarno regions: a system for a systematic study of defined alterations of nucleotide sequences within E. coli ribosome binding sites. DNA. 1983;2(3):231–235. doi: 10.1089/dna.1983.2.231. [DOI] [PubMed] [Google Scholar]