Abstract
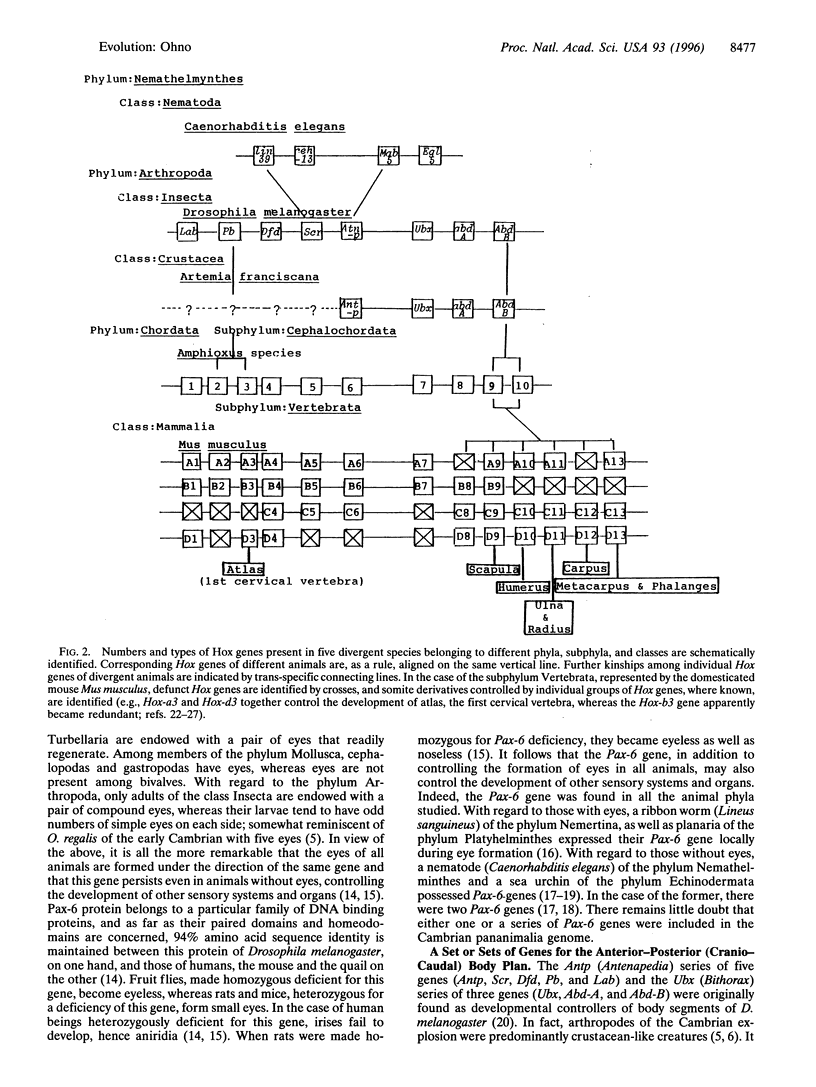
The toil by photosynthesizing cyanobacteria and blue-green algae of nearly three billion years appeared to have finally resulted in the sufficient accumulation of molecular oxygen. So, the stage was set for the emergence, at the ocean bottom, of diverse animals that were consumers of molecular oxygen. It now appears that this Cambrian explosion, during which nearly all the extant animal phyla have emerged, was of an astonishingly short duration, lasting only 6-10 million years. Inasmuch as only a 1% DNA base sequence change is expected in 10 million years under the standard spontaneous mutation rate, I propose that all those diverse animals of the early Cambrian period, some 550 million years ago, were endowed with nearly identical genomes, with differential usage of the same set of genes accounting for the extreme diversities of body forms. Some of the more pertinent genes that are thought to be included in the Cambrian pananimalia genome are as follows. (i) A gene for lysyloxidase that, in the presence of molecular oxygen, crosslinked collagen triple helices to produce ligaments and tendons, thus contributing to the stout bodies of the Cambrian animals. (ii) Genes for hemoglobin; these internal transporters of molecular oxygen are today seen sporadically in members of diverse animal phyla. (iii) The Pax-6 gene for eye formation; the eyes of a ribbon worm to a human are organized by this gene. In animals without eyes, the same gene organizes other sensory systems and organs. (iv) A series of Hox genes for the anterior-posterior (cranio-caudal) body plans: these genes are also present in all phyla of the kingdom Animalia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averof M., Akam M. Hox genes and the diversification of insect and crustacean body plans. Nature. 1995 Aug 3;376(6539):420–423. doi: 10.1038/376420a0. [DOI] [PubMed] [Google Scholar]
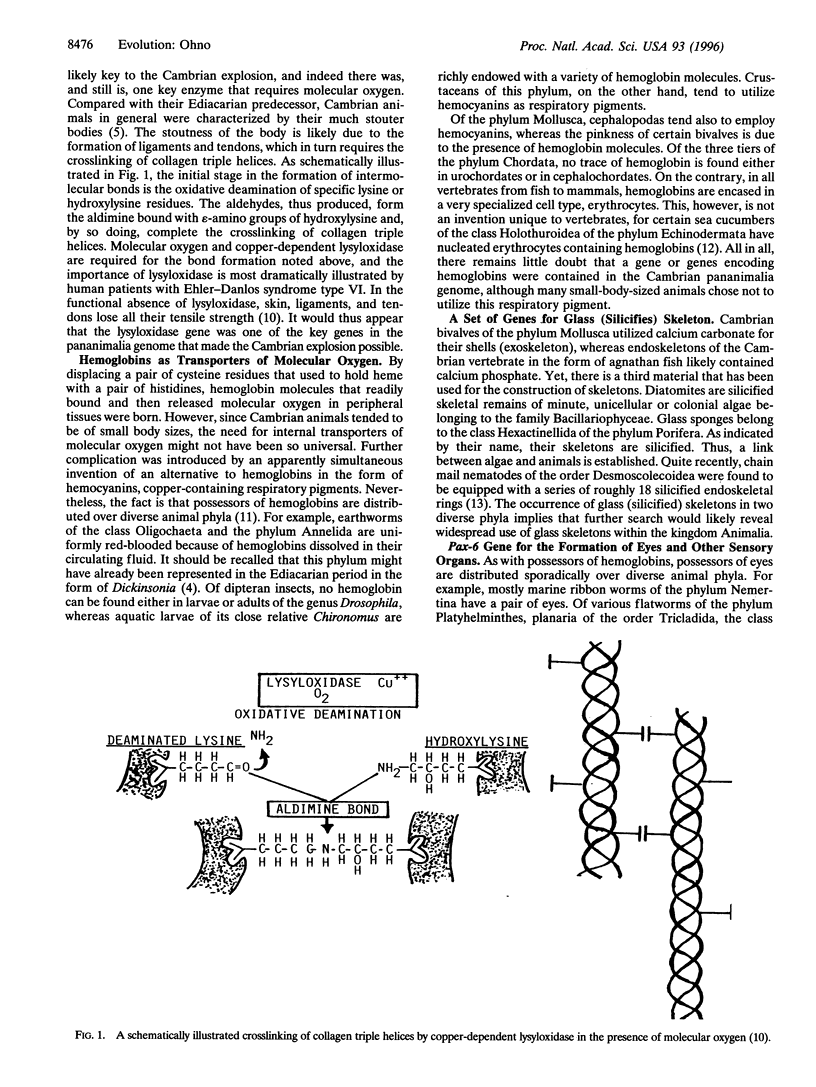
- Bailey A. J., Robins S. P., Balian G. Biological significance of the intermolecular crosslinks of collagen. Nature. 1974 Sep 13;251(5471):105–109. doi: 10.1038/251105a0. [DOI] [PubMed] [Google Scholar]
- Briggs D. E. Giant predators from the cambrian of china. Science. 1994 May 27;264(5163):1283–1284. doi: 10.1126/science.264.5163.1283. [DOI] [PubMed] [Google Scholar]
- Chisholm A. D., Horvitz H. R. Patterning of the Caenorhabditis elegans head region by the Pax-6 family member vab-3. Nature. 1995 Sep 7;377(6544):52–55. doi: 10.1038/377052a0. [DOI] [PubMed] [Google Scholar]
- Cloud P., Glaessner M. F. The ediacarian period and syste: metazoa inherit the Earth. Science. 1982 Aug 27;217(4562):783–792. doi: 10.1126/science.217.4562.783. [DOI] [PubMed] [Google Scholar]
- Condie B. G., Capecchi M. R. Mice with targeted disruptions in the paralogous genes hoxa-3 and hoxd-3 reveal synergistic interactions. Nature. 1994 Jul 28;370(6487):304–307. doi: 10.1038/370304a0. [DOI] [PubMed] [Google Scholar]
- Czerny T., Busslinger M. DNA-binding and transactivation properties of Pax-6: three amino acids in the paired domain are responsible for the different sequence recognition of Pax-6 and BSAP (Pax-5). Mol Cell Biol. 1995 May;15(5):2858–2871. doi: 10.1128/mcb.15.5.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. P., Witte D. P., Hsieh-Li H. M., Potter S. S., Capecchi M. R. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature. 1995 Jun 29;375(6534):791–795. doi: 10.1038/375791a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernández J., Holland P. W. Archetypal organization of the amphioxus Hox gene cluster. Nature. 1994 Aug 18;370(6490):563–566. doi: 10.1038/370563a0. [DOI] [PubMed] [Google Scholar]
- Hunter C. P., Kenyon C. Specification of anteroposterior cell fates in Caenorhabditis elegans by Drosophila Hox proteins. Nature. 1995 Sep 21;377(6546):229–232. doi: 10.1038/377229a0. [DOI] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Loosli F., Kmita-Cunisse M., Gehring W. J. Isolation of a Pax-6 homolog from the ribbonworm Lineus sanguineus. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):2658–2663. doi: 10.1073/pnas.93.7.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin L. G. Evolution of the vertebrate genome as reflected in paralogous chromosomal regions in man and the house mouse. Genomics. 1993 Apr;16(1):1–19. doi: 10.1006/geno.1993.1133. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Osumi-Yamashita N., Noji S., Ohuchi H., Koyama E., Myokai F., Matsuo N., Taniguchi S., Doi H., Iseki S. A mutation in the Pax-6 gene in rat small eye is associated with impaired migration of midbrain crest cells. Nat Genet. 1993 Apr;3(4):299–304. doi: 10.1038/ng0493-299. [DOI] [PubMed] [Google Scholar]
- McGinnis N., Kuziora M. A., McGinnis W. Human Hox-4.2 and Drosophila deformed encode similar regulatory specificities in Drosophila embryos and larvae. Cell. 1990 Nov 30;63(5):969–976. doi: 10.1016/0092-8674(90)90500-e. [DOI] [PubMed] [Google Scholar]
- Morris S. C. Burgess shale faunas and the cambrian explosion. Science. 1989 Oct 20;246(4928):339–346. doi: 10.1126/science.246.4928.339. [DOI] [PubMed] [Google Scholar]
- Quiring R., Walldorf U., Kloter U., Gehring W. J. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994 Aug 5;265(5173):785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Schopf J. W. Microfossils of the Early Archean Apex chert: new evidence of the antiquity of life. Science. 1993 Apr 30;260:640–646. doi: 10.1126/science.260.5108.640. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Emmons S. W. Specification of sense-organ identity by a Caenorhabditis elegans Pax-6 homologue. Nature. 1995 Sep 7;377(6544):55–59. doi: 10.1038/377055a0. [DOI] [PubMed] [Google Scholar]



