Abstract
Objective: l-Methylfolate has been shown in retrospective and prospective studies to enhance antidepressant response. The aim of this study was to prospectively assess change in depression severity and medication satisfaction in patients prescribed l-methylfolate within a naturalistic setting.
Method: Between November 2010 and April 2012, patients who reported being treated for major depressive disorder rated their experiences before and after 3 months on the prescription medical food l-methylfolate (Deplin) 7.5 mg or 15 mg, through an automated telephone system. Survey questions included the 9-item Patient Health Questionnaire (PHQ-9), as well as quality of life and medication satisfaction questions. The primary outcome was change in depression severity from baseline to endpoint.
Results: Of 554 patients, 502 reported that l-methylfolate was added to their existing antidepressant and 52 were treated with l-methylfolate alone, without an antidepressant. Enrolled participants reported a mean reduction of 8.5 points (58.2% decrease) in their PHQ-9 score (mean baseline PHQ-9 score = 14.6, mean follow-up PHQ-9 score = 6.1; P = .000); 376 (67.9%) responded to treatment (50% reduction in baseline PHQ-9 score) and 253 (45.7%) achieved remission (follow-up PHQ-9 score < 5) after an average of 95 days of therapy. In addition, patients achieved significant reductions in self-reported impairment in their work/home/social life (P = .000). Medication satisfaction with l-methylfolate (mean satisfaction score = 7.0) was significantly higher than with prior medication (mean satisfaction score = 5.2; P = .000).
Conclusions: Results show that in a naturalistic setting, patients managed with l-methylfolate achieved statistically significant improvements in self-reported depression symptoms and functioning and greater satisfaction with their medication treatment.
Clinical Points
▪ In this real-world study, patients with depression taking l-methylfolate reported significant improvements in depressive symptoms and functioning, with 67.9% of patients responding and 45.7% achieving remission over 12 weeks.
▪ The results of this study also showed patient medication satisfaction and compliance was very high, with over 90% of patients reporting taking every dose or nearly every dose of l-methylfolate.
Studies have shown a link between folate deficiency and neuropsychiatric disorders. In particular, depressive symptoms are the most common neuropsychiatric manifestation of folate deficiency.1 Folate levels have been found to be inversely associated with depressive symptoms2 and with longer duration of depressive episodes.3 Depressed patients with folate deficiency showed a poorer response to standard treatment with antidepressants.4 Therefore, for patients with low plasma or red blood cell folate levels, folate augmentation during antidepressant treatment may improve patient outcomes.5
Folate is a B vitamin that occurs naturally in food as dihydrofolate and in vitamins and supplements as synthetic folic acid. Dihydrofolate and synthetic folic acid are metabolized in the body into l-5-methyltetrahydrofolate (l-5-MTHF), also known as l-methylfolate—the only form of folate that can cross the blood-brain barrier. l-Methylfolate is a cofactor in the production of monoamines serotonin, dopamine, and norepinephrine, which are involved in the regulation of mood and the mechanisms of actions of antidepressants.6 The bioavailability of l-methylfolate is higher compared to folic acid.7 Moreover, up to 70% of depressed patients have a genetic variant of the methylenetetrahydrofolate reductase enzyme that compromises their ability to convert dietary folate or synthetic folic acid to l-methylfolate.8 l-Methylfolate supplementation may thus improve response to antidepressants that affect monoamines among depressed patients who do not respond adequately.
l-Methylfolate (7.5 and 15 mg) has been shown in retrospective and prospective studies to enhance antidepressant response. A double-blind, placebo-controlled trial of l-methylfolate among folate-deficient patients with major depressive disorder showed that adding l-methylfolate 7.5 or 15 mg to standard therapy significantly improved clinical and social recovery.9 A double-blind multicenter study showed that adding l-methylfolate 7.5 or 15 mg to standard psychotropic medication significantly improved clinical recovery in depressed patients with folate deficiency.10 A retrospective analysis comparing patients treated with either selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) monotherapy versus those treated with a combination of an SSRI/SNRI antidepressant and l-methylfolate (7.5 mg or 15 mg) found that initiating therapy in major depressive disorder with l-methylfolate plus an SSRI/SNRI was more effective in improving depressive symptoms, led to more rapid improvement, and had fewer therapy discontinuations, while having the same rate of adverse effects as SSRI or SNRI monotherapy.11 In a recent randomized, double-blind, placebo-controlled trial,12 l-methylfolate 15 mg/d added to SSRI therapy was found to be superior to SSRI therapy alone, with twice as many patients responding to adjunctive l-methylfolate in 30 days compared to adjunctive placebo. Although data support the effectiveness of l-methylfolate added to an antidepressant medication, there are few data in real-world clinical settings.
The goal of this study is to assess the impact of l-methylfolate on depression severity, quality of life, and medication satisfaction among patients prescribed l-methylfolate within a naturalistic clinical setting.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
METHOD
Data for this study were obtained between November 2010 and April 2012 from surveys of patients who were enrolled in a patient experience program. To be eligible for the program, patients needed to be at least 18 years old and must have been prescribed l-methylfolate (Deplin 7.5 mg or 15 mg) by their physician for the treatment of major depressive disorder. Participants were offered the opportunity to participate in the study by their physician. During the enrollment process through a live phone agent, patients provided oral informed consent (consent was recorded). Once enrolled, patients were asked to complete a baseline survey prior to using l-methylfolate and a follow-up survey at 90 days after treatment initiation. Patients who completed the final survey were given the option to receive a savings offer or a $5 Starbucks card. Surveys were conducted through an interactive voice response system.
The baseline survey included questions on demographics (age and sex) and disease and treatment history. Patients were asked about how long they had symptoms of depression, medications taken for depression, satisfaction with these medications, and the impact of depression on quality of life. They also were administered the 9-item Patient Health Questionnaire (PHQ-9).13 The follow-up survey after approximately 90 days included questions on depression medications taken with l-methylfolate, compliance and satisfaction with l-methylfolate, impact on quality of life, and the PHQ-9.
Quality of life was measured on a 1 to 5 scale based on how difficult the depression symptoms made it for the patients to “do their work, take care of things at home, or get along with other people,” with 1 indicating that it was “extremely difficult” and 5 that it was “not at all difficult.” Medication satisfaction was measured on a 1 to 9 scale, with 1 indicating that the patient was “not at all satisfied” and 9 that the patient was “very satisfied.”
The analysis group was limited to patients with a baseline PHQ-9 score of at least 5. The primary outcome measure was change from baseline survey to endpoint survey in depression severity as measured by the PHQ-9 score. Treatment response was defined as a reduction of 50% or more in the baseline PHQ-9 score,13 and remission was considered to have been achieved if the PHQ-9 score was < 515 after 90 days of treatment. Change from baseline to endpoint survey in the individual items making up the PHQ-9 was also investigated. Secondary outcomes included change in quality of life and medication satisfaction.
Patient characteristics and outcomes of interest were summarized using frequency distributions or descriptive statistics (means, medians, and standard deviations) as appropriate. Statistical significance between baseline and follow-up responses was tested using Wilcoxon signed ranks test for continuous or ordinal variables and McNemar test or χ2 test for nominal variables. Analyses were performed on the overall audience and by each of these baseline depression severity groups: PHQ-9 score 5–9, 10–14, 15–19, and 20–27. Kroenke et al13 has shown that a PHQ-9 score of at least 10 is associated with a high likelihood of major depression. SPSS v20 (SPSS Inc, Chicago, Illinois) was used for all statistical analyses.
RESULTS
Patient Characteristics
A total of 594 patient experience program participants completed the baseline and follow-up surveys; of these, 554 patients had baseline PHQ-9 scores ≥ 5 and were included in the analysis. Table 1 shows a summary of the baseline patient characteristics. The majority of patients were women. The study was an approximately equal distribution across the 4 depression severity groups of interest.
Table 1.
Patient Characteristics and Medication Behavior (N = 554)
| Variable | Value |
| Patient Characteristic | |
| Gender, n (%) | |
| Female | 424 (76.5) |
| Male | 114 (20.6) |
| Unknown | 16 (2.9) |
| Age, total, mean ± SD, y | 49.9 ± 14.1 |
| Age group, n (%), y | |
| 18–34 | 79 (14.3) |
| 35–44 | 110 (19.9) |
| 45–54 | 140 (25.3) |
| 55–64 | 141 (25.5) |
| ≥ 65 | 84 (15.2) |
| Duration of depression symptoms at baseline, n (%) | |
| ≤ 6 mo | 27 (4.9) |
| 7 mo–12 mo | 30 (5.4) |
| 1–2 y | 53 (9.6) |
| > 2 y | 427 (77.1) |
| Not sure | 17 (3.1) |
| Depression severity measure (PHQ-9 score) at baseline, n (%) | |
| 5–9 | 131 (23.6) |
| 10–14 | 158 (28.5) |
| 15–19 | 143 (25.8) |
| 20–27 | 122 (22.0) |
| Medication Behavior | |
| Use of l-methylfolate during the study, n (%) | |
| Monotherapy | 52 (9.4) |
| Adjuvant therapy | 502 (90.6) |
| Depression medications taken with l-methylfolate, n (%)a | |
| SSRI (citalopram, escitalopram, paroxetine, fluoxetine, sertraline) | 234 (42.2) |
| SNRI (venlafaxine, desvenlafaxine, duloxetine) | 168 (30.3) |
| Bupropion | 124 (22.4) |
| Trazodone | 53 (9.6) |
| Other | 215 (38.8) |
| Compliance with l-methylfolate, n (%) | |
| Took every dose or nearly every dose of l-methylfolate as directed | 503 (90.8) |
| Took most doses of l-methylfolate | 38 (6.9) |
| Took half or less than half of the doses of l-methylfolate | 13 (2.3) |
Patients may have been taking more than 1 medication, so percentage totals are > 100%.
Abbreviations: PHQ-9 = 9-item Patient Health Questionnaire, SNRI = selective-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor.
Experience With l-Methylfolate
During the study, most patients took l-methylfolate as an augmentation medication, and compliance with l-methylfolate was very high, with 90.8% of patients reporting having taken every dose or nearly every dose (Table 1).
Treatment Response
To assess the effect of l-methylfolate on depression severity, we compared baseline PHQ-9 scores with PHQ-9 scores after an average of 95 days (range, 70–285) of l-methylfolate treatment. Participants reported a mean reduction of 8.5 (SD = 6.3) points in their PHQ-9 score (P = .000); the mean baseline PHQ-9 score was 14.6 (SD = 5.8) and mean endpoint score was 6.1 (SD = 5.0). Additionally, each of the 4 baseline depression severity groups had statistically significant mean reductions in their PHQ-9 scores (P = .000; Figure 1A).
Figure 1.

Change in Depression Severity Score
A. Mean Change in PHQ-9 Score by Baseline Severity
B. Mean Change in Individual PHQ-9 Items by Baseline Severity
Abbreviation: PHQ-9 = 9-item Patient Health Questionnaire.
An analysis of individual items that make up the PHQ-9 score was conducted; Figure 1B shows statistically significant reductions across all the 9 items for the overall audience. Note that the 2 items with the lowest mean reductions (“Too slow or too fidgety” and “Thoughts of dying/hurting self”) also had the lowest baseline scores. Each of the 4 baseline depression severity groups (Figure 1B) showed statistically significant reductions in each item (P value = .000 for each PHQ-9 item for each baseline severity group).
A total of 376 patients (67.9%) responded to treatment with l-methylfolate (Figure 2A) (ie, had a 50% reduction in PHQ-9 score). Figure 2A shows treatment response rate by baseline depression severity—for each of the severity groups, at least 60% of the patients responded to treatment, with response rate trending higher with greater baseline severity. The remission rate was 45.7% (Figure 2B). Table 2 shows treatment response rates and remission rates across different patient subgroups. Treatment response rates are similar for patients who take l-methylfolate as monotherapy and for patients who take it as adjuvant therapy. Among patients who had been experiencing depression symptoms for more than 2 years, the remission rate was 41.9%, and the remission rate among patients who had been experiencing depression symptoms for 2 years or less was 58.2%.
Figure 2.
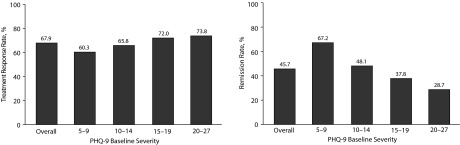
Treatment Response and Remission Rates by 9-item Patient Health Questionnaire (PHQ-9) Baseline Severity A. Treatment Response Rates B. Remission Rates
Table 2.
Treatment Response and Remission Rates by Patient Subgroup
| Patient Subgroup | Patients, n | Change in PHQ-9 Score, mean (SD) | Responded to Treatment, n (treatment response rate, %) |
Achieved Remission, n (remission rate, %) |
| Overall | 554 | −8.5 (6.3) | 376 (67.9) | 253 (45.7) |
| Gender | ||||
| Female | 424 | −8.7 (6.4) | 287 (67.7) | 189 (44.6) |
| Male | 114 | −8.0 (5.8) | 79 (69.3) | 59 (51.8) |
| Age group, y | ||||
| 18–34 | 79 | −8.6 (5.6) | 50 (63.3) | 39 (49.4) |
| 35–44 | 110 | −8.9 (6.6) | 80 (72.7) | 56 (50.9) |
| 45–54 | 140 | −8.6 (6.2) | 89 (63.6) | 60 (42.9) |
| 55–64 | 141 | −8.4 (6.7) | 100 (70.9) | 60 (42.6) |
| ≥ 65 | 84 | −7.7 (6.1) | 57 (67.9) | 38 (45.2) |
| Monotherapy vs adjuvant therapy | ||||
| Monotherapy | 52 | −7.7 (5.7) | 34 (65.4) | 27 (51.9) |
| Augmentation | 502 | −8.6 (6.3) | 342 (68.1) | 226 (45.0) |
| Duration of depression symptoms | ||||
| ≤ 2 y | 110 | −8.7 (6.4) | 82 (74.5) | 64 (58.2) |
| > 2 y | 427 | −8.7 (6.4) | 282 (66.0) | 179 (41.9) |
| Not sure | 17 | −8.7 (6.4) | 12 (70.6) | 10 (58.8) |
Abbreviation: PHQ-9 = 9-item Patient Health Questionnaire.
Quality of Life
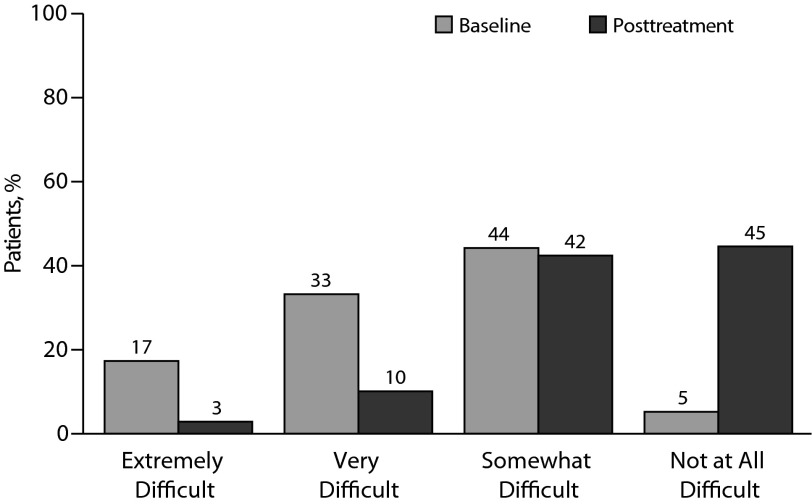
Patients achieved significant improvement in self-reported work, home, and social functioning (Figure 3). The proportion of patients who reported that their symptoms have made it very or extremely difficult to function in these areas decreased from 50% to 13% (P = .000).
Figure 3.
Impact of Depression on Quality of Lifea
aPatient response to “How difficult have these problems made it for you to do your work, take care of things at home, or get along with other people?” Note that “… these problems …” refers to the symptoms assessed on the 9-item Patient Health Questionnaire, which immediately preceded this question on the surveys.
Medication Satisfaction
Patients reported a mean satisfaction rating of 7.0 on a 1 to 9 scale, with 1 being “not at all satisfied” and 9 being “very satisfied.” Among patients who had been previously treated at baseline, a mean satisfaction with l-methylfolate of 7.0 was significantly higher than the mean satisfaction of 5.2 with their prior treatment (P = .000). Among patients with no prior treatment, mean medication satisfaction was 7.4.
DISCUSSION
The results of this naturalistic study showed that patients treated with l-methylfolate achieved statistically significant improvements in depression symptoms as measured by the PHQ-9 score and overall quality of life measure. There was also a high mean medication satisfaction. These improvements were observed across patient groups with varying depression severity levels and across other patient subgroups as well, including patients who reported having depression symptoms for more than 2 years. Note also that lower depression severity groups had higher remission rates (Figure 2B), possibly explained by the fact that patients with lower baseline PHQ-9 scores would have a smaller hurdle to overcome to achieve remission.
These real-world patient experience trial results provide a complement to controlled clinical trials data showing efficacy of l-methylfolate as part of major depressive disorder therapy. Information from patients about their experiences with and impressions of l-methylfolate helps in understanding the potential benefits of l-methylfolate in depression management. Patients’ perceptions of their response to treatment can play a key role in treatment adherence and, ultimately, in the outcomes of treatment. In a literature review of several studies across 12 diseases, Boswell et al15 found a positive correlation between medication adherence and clinical outcomes. In particular, the 2 articles on depression both showed a positive relationship between adherence and clinical outcomes.
There are several limitations with this survey program. The survey was designed to collect responses directly from patients about their experiences with l-methylfolate using automated media. Patients therefore had no opportunity to provide additional insight about their experiences. In addition, patients enrolled in the program and answered the surveys voluntarily; selection bias may be present in that these patients may be different from those who chose not to enroll or not to respond to the surveys. No data were available to determine the extent to which any differences may have existed among participants versus nonparticipants. The results from depressive patients choosing to participate and complete surveys may not represent the experiences of all depressive patients taking l-methylfolate as part of their depression disorder treatment. All data presented were based on self-reported impressions from patients responding to closed-ended questions; no clinical evaluations were conducted. Finally, this was not a controlled trial; it is unclear how a placebo or an alternative agent would have fared in this population.
Despite these limitations, information from real-world patients about their experiences with a medication is important. Clinical trials typically use stringent selection criteria, which may make participants unrepresentative of clinical patients. These trials are conducted under controlled conditions and often include small sample sizes. While clearly the “gold standard” for assessing clinical efficacy and safety, most controlled clinical trials are not designed to capture the experiences of everyday patients using a treatment in their own environment. Survey programs like the one presented here have the advantage of being able to include a large number of patients and may reflect the experience of patients in typical clinical settings. This information may be useful to clinicians and patients actively engaged in depression management.
Drug names: bupropion (Wellbutrin, Aplenzin, and others), citalopram (Celexa and others), desvenlafaxine (Pristiq), duloxetine (Cymbalta), escitalopram (Lexapro and others), fluoxetine (Prozac and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others), venlafaxine (Effexor and others).
Potential conflicts of interest: Ms Barrentine is an employee of Pamlab. Dr Shelton has received grant/research/clinical trial support from Bristol-Myers Squibb, Eli Lilly, Elan, Euthymics Bioscience, Forest, Janssen, Naurex, Novartis, Otsuka, Pamlab, Repligen, Ridge Diagnostics, St Jude Medical, and Takeda and has served as a consultant to Bristol-Myers Squibb, Cyberonics, Eli Lilly, Medtronic, Pamlab, Pfizer, Ridge Diagnostics, and Takeda. Dr Manning has served as a consultant to AstraZeneca, Eli Lilly, and Takeda-Lundbeck and has served on the speakers or advisory boards of AstraZeneca, Otsuka, and Takeda-Lundbeck. Dr Tipa is an employee of InfoMedics.
Funding/support: This study was conducted by InfoMedics, Inc, Reading, Massachusetts. Funding was provided by Pamlab, Covington, Louisiana. Pamlab manufactures Deplin.
Role of the sponsor: Pamlab had a role in the design of the study, the interpretation of the data, and the review and approval of the manuscript for submission. Pamlab had no role in the conduct of the study, data collection, or analysis. Pamlab provided funding for writing and editing of the manuscript.
References
- 1.Alpert JE, Fava M. Nutrition and depression: the role of folate. Medscape Psychiatry & Mental Health eJoural. 1997;2(1) doi: 10.1111/j.1753-4887.1997.tb06468.x. http://www.medscape.com/viewarticle/431514 . Accessed June 3, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Beydoun MA, Shroff MR, Beydoun HA, et al. Serum folate, vitamin B-12, and homocysteine and their association with depressive symptoms among U.S. adults. Psychosom Med. 2010;72(9):862–873. doi: 10.1097/PSY.0b013e3181f61863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levitt AJ, Joffe RT. Folate, B12, and life course of depressive illness. Biol Psychiatry. 1989;25(7):867–872. doi: 10.1016/0006-3223(89)90266-7. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds EH, Preece JM, Bailey J, et al. Folate deficiency in depressive illness. Br J Psychiatry. 1970;117(538):287–292. [PubMed] [Google Scholar]
- 5.Fava M. Augmenting antidepressants with folate: a clinical perspective. J Clin Psychiatry. 2007;68(suppl 10):4–7. [PubMed] [Google Scholar]
- 6.Stahl SM. Novel therapeutics for depression: l-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007;12(10):739–744. doi: 10.1017/s1092852900015418. [DOI] [PubMed] [Google Scholar]
- 7.Willems FF, Boers GH, Blom HJ, et al. Pharmacokinetic study on the utilisation of 5-methyltetrahydrofolate and folic acid in patients with coronary artery disease. Br J Pharmacol. 2004;141(5):825–830. doi: 10.1038/sj.bjp.0705446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly CB, McDonnell AP, Johnston TG, et al. The MTHFR C677T polymorphism is associated with depressive episodes in patients from Northern Ireland. J Psychopharmacol. 2004;18(4):567–571. doi: 10.1177/0269881104047285. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey PS, Toone BK, Carney MW, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392–395. doi: 10.1016/0140-6736(90)91942-4. [DOI] [PubMed] [Google Scholar]
- 10.Passeri M, Cucinotta D, Abate G, et al. Oral 5′-methyltetrahydrofolic acid in senile organic mental disorders with depression: results of a double-blind multicenter study. Aging (Milano) 1993;5(1):63–71. doi: 10.1007/BF03324128. [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg LD, Oubre AY, Daoud YA. l-methylfolate plus SSRI or SNRI from treatment initiation compared to SSRI or SNRI monotherapy in a major depressive episode. Innov Clin Neurosci. 2011;8(1):19–28. [PMC free article] [PubMed] [Google Scholar]
- 12.Papakostas G, et al. l-methylfolate adjunctive therapy for selective serotonin reuptake inhibitors (SSRIs) for major depressive disorder: results of 2 randomized, double-blind trials. Am J Psychiatry. 2012;169(12):1267–1274. doi: 10.1176/appi.ajp.2012.11071114. [DOI] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trangle M, Dieperink B, Gabert T, et al. Institute for Clinical Systems Improvement. Major Depression in Adults in Primary Care. https://www.icsi.org/guidelines__more/catalog_guidelines_and_more/catalog_guidelines/catalog_behavioral_health_guidelines/depression/. Updated May 2012. Accessed June 4, 2013. [Google Scholar]
- 15.Boswell KA, Cook CL, Burch SP, et al. Associating medication adherence with improved outcomes: a systematic literature review. Am J Man Care. 2012;4(4):e97–e108. [Google Scholar]