Abstract
Impaired ion regulation and dehydration is the primary pathophysiology in cystic fibrosis (CF) lung disease. A potential application of exhaled breath condensate (EBC) collection is to assess airway surface liquid ionic composition at baseline and in response to pharmacological therapy in CF. Our aims were to determine if EBC could detect differences in ion regulation between CF and healthy and measure the effect of the albuterol on EBC ions in these populations. Baseline EBC Cl−, DLCO and SpO2 were lower in CF (n = 16) compared to healthy participants (n = 16). EBC Cl− increased in CF subjects, while there was no change in DLCO or membrane conductance, but a decrease in pulmonary-capillary blood volume in both groups following albuterol. This resulted in an improvement in diffusion at the alveolar-capillary unit, and removal of the baseline difference in SpO2 by 90-minutes in CF subjects. These results demonstrate that EBC detects differences in ion regulation between healthy and CF individuals, and that albuterol mediates increases in Cl− in CF, suggesting that the benefits of albuterol extend beyond simple bronchodilation.
Keywords: diffusion capacity of the lungs for carbon monoxide and nitric oxide (DLCO/DLNO), peripheral oxygen saturation (SpO2), exhaled sodium, exhaled chloride
Introduction
Cystic fibrosis (CF) is the most common autosomal recessive genetic disease affecting 70,000 individuals worldwide. Mutation of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) chloride (Cl−) channel, which also regulates other ion channels, leads to impairments in Cl−, sodium (Na+) and fluid regulation across the epithelium of secretory tissues, which include the sweat glands, intestine, pancreas, and lungs. Although the pathological effects on all these tissues can be debilitating and reduce a CF patient’s quality of life, 90% of mortality is due to pulmonary complications.1 Impairments in ion regulation due to the lack of functional CFTR channel activity in the lungs causes pathological dehydration and impairs mucociliary clearance leading to stagnant mucus, airway obstruction and recurrent inflammation and infection, leading to structural damage that progresses to end stage lung disease for CF patients.2
Maintenance of airway surface liquid (ASL) depth is crucial for pulmonary defense and function by facilitating effective airway clearance. However, tight regulation of secretion or absorption of ions and the ensuing fluid exchange is also imperative in gas diffusion.3–5 Removal of excess fluid from the airways and alveoli is mediated primarily by the absorption of Na+ through epithelial sodium channels (ENaC).6,7 Increased Cl− secretion is the countervailing mechanism helping to maintain ASL fluid depth, occurring primarily through CFTR, but also to a lesser extent by calcium-activated chloride channels (CaCC).5,8,9 Proper ASL depth of approximately four microns is regulated through soluble mediators (nucleotides, purinergic and adrenergic agonists) and mechanical stress.5,9–11
β2-adrenergic receptor (ADBR2) agonists, such as endogenously-released epinephrine or exogenously administered albuterol, can play an important role in modulating ASL depth.5,12,13 The downstream activation of protein kinase A (PKA) that follows ADBR2 stimulation leads to an increase in the number of ENaCs on the apical portion of alveolar cells, and increased open probability of ENaC.14 Phosphorylation of the regulatory domain of CFTR by PKA causes channel activation, resulting in Cl− efflux to the apical side of airway epithelial cells.15,16 When both ENaC and CFTR are expressed in a cell, the effects of cAMP mimetics on ENaC invert, decreasing ENaC activity, due to the inhibitory action of CFTR on ENaC.17,18 β-agonists are amongst the most frequently used therapy in CF patients, primarily for their bronchodilatory properties, but their potential ability to modulate CFTR and ENaC activity and thereby alter ion regulation and fluid homeostasis in the lung would be beneficial in CF and has not been demonstrated in vivo.
The goal in the treatment of CF is to attenuate the yearly 2–3% decline in pulmonary function and ameliorate impairments in ion regulation.19 Regular clinic visits to perform spirometry measures allow the CF care team to monitor a patient’s lung function, identifying if the current therapy regimen is sufficient or if there has been is a decline in pulmonary health that requires changes in prescribed treatment or admission to the hospital. In contrast, the ability to assess if the prescribed pharmacological therapy is improving Cl− efflux and reducing Na+ absorption to better hydrate the lungs, through assessment of the ion composition of ASL or the transepithelial ion flux in vivo, remains relatively elusive. In the development of new compounds for CF targeting the channels (CFTR, ENaC and CaCC) or the pathways of channel activation and ASL homeostasis (adrenergic, purinergic, and nucleotides), the only currently available methods to assess ion flux are nasal potential difference and sweat chloride measurement techniques which although helpful are not ideal. As assessment of ion regulation of the nasal epithelia and in the sweat glands can only help one to speculate what is happening in the airways and alveoli; exhaled breath condensate (EBC) collection may be a more direct way to measure ion regulation in the airways.
EBC collection is a technique being investigated as a non-invasive means to assess ASL composition. A potential new application for EBC is to indirectly assess ionic composition of ASL, which could thereby provide a means of assessing the degree of impairments in ion flux in the CF lung and whether a therapy is effective at improving ion dysregulation for these patients. EBC is believed to contain small droplets of ASL, formed by shear force of turbulent air flow over the ASL as it passes through the airways.20 The composition of EBC is considered to be a dilute, surrogate marker of ASL composition.21–24
The goals of this study were to determine if EBC collection could detect differences ion regulation between CF patients and healthy controls and measure the effects of albuterol on EBC ion composition in these populations. We hypothesized that EBC Cl− and Na+ would be lower in CF patients compared to healthy subjects due to reduction in Cl− secretion and hyperabsorption of Na+ that characterizes the disease pathology. Further, we hypothesized that albuterol administration would alter ion regulation of the ASL and result in a decrease in EBC Na+ and an increase in EBC Cl− in healthy subjects, but the extent and direction of these changes for CF patients was less clear. We thought the lack of CFTR in the CF patients may limit the increase in Cl− secretion and have a diminished effect on EBC Na+ due to the already overactive state of ENaC. Second, since the mechanisms driving the proposed benefits of β-agonist therapy in CF are still unclear though thought to be primarily bronchodilation, we sought to determine the influence of albuterol on lung diffusing capacity parameters and peripheral oxygen saturation (SpO2) in CF subjects. We expected albuterol would mediate an improvement in lung diffusing capacity in patients with CF.
Methods
Study design
All subjects completed two visits. The first visit was a screening visit where baseline spirometry and a maximal exercise test were performed. The findings regarding the CF patients’ responses to exercise in comparison to healthy subjects has been described previously.25 On the second visit, subjects completed baseline measurements of pulmonary function, diffusion of the lungs for carbon monoxide and nitric oxide (DLCO/DLNO), and a 25-minute EBC collection. Then subjects received nebulized albuterol and measurements of pulmonary function, DLCO/DLNO and EBC were completed at 30-, 60- and 90-minutes post-albuterol. Additionally, nine of the healthy individuals completed a third visit where they received the placebo saline following the identical procedure as the albuterol visit to determine if albuterol was indeed stimulating changes in EBC Na+ and Cl−.
Subjects
Sixteen CF patients with mild to moderate CF (FEV1 > 40% predicted), who were carriers of at least one ΔF508 allele, and clinically diagnosed with a positive sweat test (sweat Cl− value > 60 mmol/L) were recruited through the University of Arizona Cystic Fibrosis Center. The ΔF508 mutation is the most common CFTR mutation expressed in at least one allele in 90% of CF patients. This mutation causes the CFTR channel to fold improperly and generally remain trapped and degraded in the endoplasmic reticulum rather than being inserted into the plasma membrane. The functionality of ΔF508 CFTR that is expressed in the membrane is diminished. Sixteen healthy subjects recruited through advertizing posted around the university and by word of mouth served as frequency-matched controls (Table 1). The protocol was reviewed and approved by the University of Arizona Institutional Review Board, all participants provided written informed consent prior to the study, and all aspects of the study were performed according to the Declaration of Helsinki.
Table 1.
Subject demographics.
| HEALTHY | CF | |||
|---|---|---|---|---|
| ALL | HETEROZYGOUS ΔF508 | HOMOZYGOUS ΔF508 | ||
| N | 16 | 16 | 3 | 13 |
| Homozygous ΔF508 n (%) | 0 | 13(81) | – | – |
| Gender (% female) | 50 | 25 | 0 | 69 |
| Age (years) | 25 ± 6 | 22 ± 8 | 25 ± 12 | 22 ± 7 |
| Height (cm) | 169 ± 8 | 166 ± 8 | 166 ± 11 | 166 ± 8 |
| Weight (kg) | 64 ± 9 | 60 ± 9 | 66 ± 12 | 58 ± 8 |
| BMI (kg/m2) | 23 ± 3 | 22 ± 3 | 24 ± 1 | 21 ± 3 |
| BSA (m2) | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.1 |
| Hemoglobin (g/dL) | 14.3 ± 1.3 | 14.6 ± 1.5 | 15.8 ± 0.6 | 14.2 ± 1.5 |
| VO2PEAK (% predicted) | 108 ± 35 | 54 ± 24* | 54 ± 11 | 53 ± 26 |
Note: Values are mean ± SD *P < 0.01 vs. healthy. Statistics not performed on ΔF508 groups.
Abbreviations: CF, cystic fibrosis; BMI, body mass index; BSA, body surface area; VO2peak, maximal oxygen consumption.
Protocol
On the day of the study visit, all subjects abstained from taking their β-agonist the morning of the visit and arrived in a 2-hour fasted state. Subjects were then outfitted with a 12-lead electrocardiogram (ECG; Marquette electronics, Milwaukee, WI) to allow for continuous monitoring of heart rhythms and heart rate (HR). Blood pressure was also monitored by cuff auscultation throughout the visit. Baseline assessment of DLCO/DLNO, pulmonary function (maximal expiratory flow volume, MEFV) and EBC ionic composition were completed. The subjects then inhaled albuterol (2.5 mg diluted in 3 mL normal saline) using a Power Neb2 nebulizer (Drive Medical, Port Washington, NY). Subjects were seated and breathing quietly with a nosepiece until all the liquid had been nebulized, usually taking between 10–12 minutes. Subjects were cued to take a larger inhalation every two minutes to assist with the dispersion of the nebulized particles into the lower airways. Once nebulization was completed, subjects rested for 10 minutes to allow for treatment onset before the first post-EBC collection was initiated. In total, three separate 25-minute EBC collections were completed at the approximate time points of 30-, 60- and 90-minutes post nebulization. After each EBC sample collection, subjects suspended breathing on the collection device to perform DLCO/DLNO and MEFV measurements. Peripheral oxygen saturation was assessed by pulse oximetry with a finger sensor (Nellcor N-600 Pulse Oximeter, Bolder, CO) at the midpoint of each EBC collection (12 minutes). Blood samples were collected at the midpoint of each EBC collection allowing for measurement of serum hemoglobin (Hb) by a cyanide-free hemoglobin method on an ADVIA 2120 Hematology system and serum ions (Na+, K− and Cl−) by ion-selective electrodes both at the University of Arizona Center Pathology Laboratory. The additional placebo visit completed by the healthy subjects was identical to what has been described, except saline (3 mL) was nebulized instead of albuterol. A schematic of the sequence and timing of measurements during the visit is provided (Fig. 1).
Figure 1.
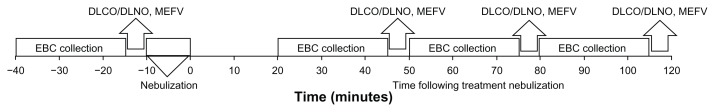
Timeline of measurements during visit.
Experimental procedures
Collection of Exhaled Breath Condensate
Exhaled breath condensate was collected for 25 minutes using a Jager EcoSceen cooling unit (Cardinal Health, Yorba Linda, CA) a system we have previously described.27 Briefly, an individual breathes on a mouthpiece and his/her exhaled breath flows through the condensing unit where it is precipitated and the droplets are collected in the sample collection cup. A new condenser and collection cup were used for at each time point (baseline, 30, 60 and 90) for each subject. Previous research confirmed that salivary amylase was not present in EBC samples collected from the EcoScreen.24 Additionally, gross contamination is avoided in the EcoScreen design by the elbow joint connecting the mouthpiece to the condensing unit, which is at an angle where exhaled air can be redirected to the condenser, but any saliva travels back down to the mouth and can be swallowed by the subject rather than into the condenser.
Quantification of EBC ion concentrations
Sodium and K+ concentrations from the EBC samples were measured using an atomic absorption spectrophotometer (Analyst 100; Perkin-Elmer, Norwalk, CT) as previously described.27 Potassium was measured with an emission wavelength of 766.5 nm. Anion analysis was conducted using a Dionex AS11-HC column. For Cl− analysis, samples were run using a Dionex AS11-HC column with a 100uL injection loop. For each sample run, the eluent gradient was created from sodium hydroxide (2 M) and nanopure water (Barnstead Nanopure, Waltham, MA) with the eluent concentration increasing linearly from 30 to 200 mM over 18 minutes at a flow rate of 1.5 mL per minute. The calibration curve was prepared from Cl− ion chromatography standard (SPEX Certiprep) to provide a detection range from 5 to 250 μM Cl−.
The calculation of net chloride efflux was used to account for the paracellular reabsorption of Cl− that will follow the reabsorption of Na+ to maintain electroneutral ion flux. Thus, the net chloride efflux calculation used was the gross chloride concentration plus the absolute value of the percent change in sodium from baseline multiplied by the gross chloride concentration for each time point:
The dilution factor for each sample was calculated using the total cation method28:
Measurement of diffusion of the lungs for carbon monoxide, alveolar-capillary membrane conductance and pulmonary-capillary blood volume
We followed the baseline and 30-, 60-, 90-minutes post-nebulization EBC collections with measures of the diffusion capacity of the lungs for carbon monoxide and nitric oxide (DLCO/DLNO) using a rebreathing technique described previously.29–32 Briefly, a 5-liter anesthesia bag was filled with 1575 mL of gas containing 0.7% acetylene, 9% helium 9% He, 0.3% carbon monoxide (C18O), 35% O2 and 40 PPM NO (diluted immediately before the test in the bag from an 800 PPM gas mixture). At the end of a normal expiration (end-expiratory lung volume, EELV), the subjects were switched into the rebreathe bag and instructed to nearly empty the bag with each breath for 8–10 consecutive breaths while his/her respiratory rate was controlled at 32 breaths/minute using a metronome. Gas concentration was measured using a Perkin Elmer MGA-1100 mass spectrometer (Wesley, MA) and NO analyzer (Sievers Instruments, Boulder, CO), which was integrated with custom analysis software for the assessment of DLCO/DLNO.
The diffusion capacity of the lungs for carbon monoxide is based on the contribution of both the membrane conductance and the hemoglobin binding.33 The rate of disappearance of the gases with each breath was calculated from the slope of the exponential disappearance for each gas with respect to helium using custom software.29 Unlike DLCO, DLNO is theoretically based solely on membrane conductance as nitric oxide is scavenged 280 times faster by hemoglobin than O2, so its uptake into the blood is nearly instantaneous. For this reason, DLNO is considered a relatively direct measure of membrane conductance (DMNO), as the diffusion resistance of the blood is trivial.33–36 The DMNO value is then used to calculate the DM for carbon monoxide (DMCO) by adjusting for differences in diffusion constants based on molecular weight and solubility between the two gases, as described previously.25,33 Recent work by Ceridon et al.37 has demonstrated that a correction factor of 2.11 is most appropriate and, as such, was used in this study. Pulmonary-capillary blood volume (VC) was then calculated from the DLCO measured by subtracting the resistance to diffusion associated with alveolar-capillary barrier (DMCO) and correcting for differences in the rate of uptake and binding to hemoglobin (1/θ) due to differences in hemoglobin (Hb) concentrations (ie, correcting the measured Hb measured at each time point for the normal Hb concentration 14.6 g/dL) and the alveolar pressure of oxygen as described previously.25 Since DLCO was assessed using the rebreathe technique, predicted values could not be calculated as no standard equations have been established. Alveolar volume (VA) was also concurrently assessed using helium during the rebreathing technique.
Pulmonary function testing and assessment of airway function
Spirometry was performed according to American Thoracic Society guidelines using the Medical Graphics CPXD (Minneapolis, MN) to determine: forced vital capacity (FVC), forced expiratory volume in one second of the FVC (FEV1) and forced expiratory flow at 25–75% of the FVC (FEF25–75).38 For each subject, the FVC maneuver was performed a minimum of three times such that three repeatable values were obtained (measured values of FVC and FEV1 within 150 mL of each other) on the first visit. On the albuterol and saline visits spirometry was performed following each diffusing capacity maneuver at each time point. Predicted values for all pulmonary function measures were based on predicted equations from NHANES III.39
Statistical analysis
All statistical comparisons were performed using the SPSS statistical software package under the consultation of a statistician (v. 16.0, Chicago, IL). Prior to analysis, data was examined to ensure no missing data or outliers. There were four single missing data points that were replaced with mean for that parameter and time point based on group, healthy or CF as described by Cohen et al.40 Separate one-way repeated measures Friedman tests for healthy and CF groups were performed to assess differences between parameters across the four time points (baseline, 30, 60 and 90-minutes post). Planned comparisons for the effect of albuterol within a group were assessed by paired-sample Wilcoxon signed-rank tests to determine if there was a significant change from baseline to 60- and 90-minutes post with a Bonferroni correction of 0.02 used to determine significance. Independent-samples Mann-Whitney U tests were performed to examine the differences at rest and at all time points between healthy and CF subjects with a Bonferroni correction of 0.01 used for the multiple comparisons conducted. In addition to significance test statistics, standardized effect size as an estimate of the strength of the relationship was calculated according to Cohen’s d as the difference in means between the two groups divided by the pooled standard deviation. Effect sizes were classified based on Cohen’s classification: 0.20 = small effect, 0.50 = medium effect and 0.80 = large effect.41 Regression analysis was conducted to determine the influence of lung diffusion vs. bronchodilation on the observed improvements in SpO2. All data are presented as mean ± SD unless indicated.
Results
The purpose of this study was two-fold; first, we wanted to determine if the collection of EBC was sensitive enough to detect differences in ionic composition between healthy and CF individuals and if EBC composition could be modified in response to a stimulus. Our second aim in this study was to investigate if the potential benefits of the short acting beta-agonist albuterol in CF patients include altering the ionic composition of the ASL, utilizing EBC as a surrogate measure of the ASL.
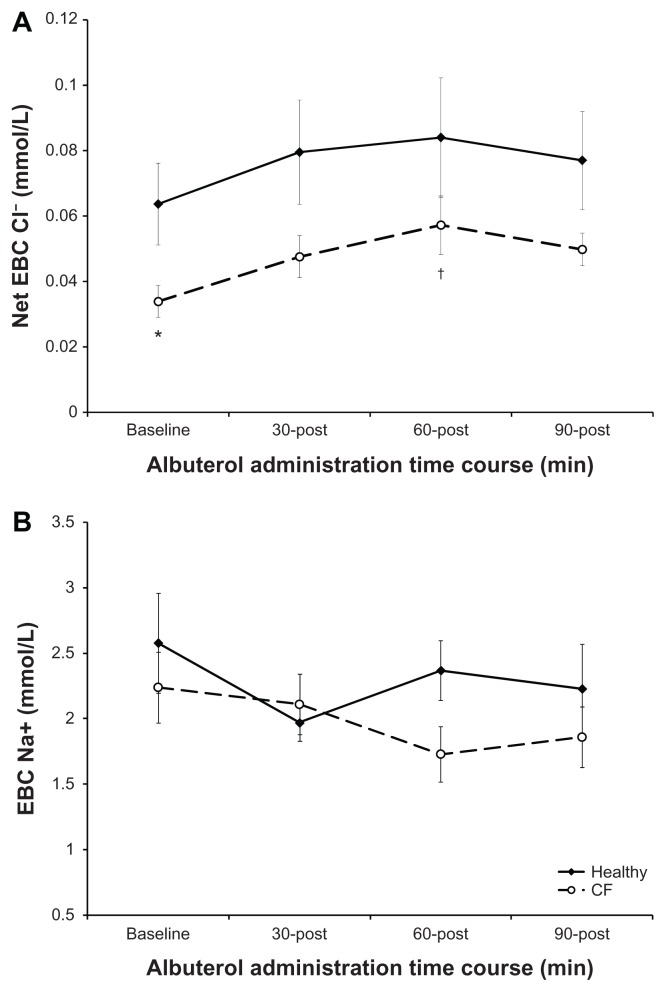
There were no differences in age, height, weight or body mass index (BMI) between the two matched groups (Table 1). As expected and previously reported, there was a significant difference in aerobic capacity (VO2PEAK) and pulmonary function assessed on the screening visit of the study (Tables 1 and 2).25 Within the CF population, 81% were homozygous for the ΔF508 mutation, and all three compound heterozygotes were male. EBC Cl− concentrations at baseline were significantly lower in CF compared to healthy subjects, which follows the pathological premise of CF (Fig. 2A). There was no difference in EBC Na+ between the two conditions at baseline (Fig. 2B). In addition to presenting raw EBC Cl− values, to represent total Cl− flux, we calculated net EBC Cl− which accounts for the Cl− influx that would follow Na absorption and detract from the measured EBC Cl− (see methods section for formula). Raw EBC Cl− tended to increase following albuterol, peaking at 60 minutes post-CF in patients. Albuterol increased chloride efflux as described by net EBC Cl−, which was significant by 60-minutes post albuterol administration in CF patients. In contrast, although healthy patients also tended to increase EBC Cl−, there was no significant change with albuterol. Further, the increase in EBC Cl− in CF patients following albuterol resulted in abolition of the difference in EBC Cl− between healthy and CF groups that was present at baseline. Albuterol did not result in any significant change in EBC Na+ in either healthy or CF subjects.
Table 2.
Baseline pulmonary function parameters.
| HEALTHY | CF | |||
|---|---|---|---|---|
| ALL | HETEROZYGOUS ΔF508 | HOMOZYGOUS ΔF508 | ||
| FVC (L) | 4.6 ± 1.3 | 3.5 ± 0.9* | 3.8 ± 1.1 | 3.4 ± 0.9 |
| FVC (% predicted) | 101 ± 19 | 82 ± 20* | 85 ± 13 | 82 ± 21 |
| (FEV)1 (L) | 3.7 ± 0.8 | 2.5 ± 0.8* | 2.7 ± 0.9 | 2.4 ± 0.9 |
| (FEV)1 (% predicted) | 97 ± 16 | 70 ± 25* | 74 ± 21 | 69 ± 26 |
| (FEV)1 (/FVC (%) | 83 ± 1 | 70 ± 1* | 71 ± 9 | 70 ± 1 |
| (FEF)25–75 (mL) | 3.8 ± 1.0 | 1.9 ± 1.1* | 2.0 ± 1.0 | 1.9 ± 1.2 |
| (FEF)25–75 (% predicted) | 93 ± 24 | 51 ± 31* | 51 ± 29 | 51 ± 33 |
Note: Values are mean ± SD *P < 0.01 vs. healthy. Statistics not performed on ΔF508 groups.
Abbreviations: CF, cystic fibrosis; FVC, forced vital capacity; FEV1, forced expiratory volume after one second of FVC; FEF25–75, forced expiratory flow at 25–75% of FVC.
Figure 2.
Effect of albuterol on EBC Ions in healthy and CF subjects. Panel A: Net EBC Cl− (see methods for equation). Panel B: EBC Na+. The open circles represent subjects with CF with the response overtime shown as the broken line. The closed diamonds represent healthy subjects with the response overtime shown as the solid line. The error bars represent the standard error of the mean.
Notes: *P < 0.01 healthy vs. CF and †P < 0.02 baseline vs. time post in CF subjects.
In our evaluation of albuterol-mediated vs. saline-mediated changes in EBC ions in the healthy subjects, we found that the raw EBC ion concentrations were different between days, even at baseline suggesting a difference in dilution. After applying a dilution factor based on the ratio of serum to EBC cations, we found that there was no difference between treatments at baseline and that there was minimal response following both albuterol and saline nebulization (data available in supplemental table). After noting the strong influence of dilution in our EBC samples we compared dilution corrected EBC Na+, Cl− and Net Cl−, between healthy and CF subjects. Even with the dilution correction, CF subjects still presented lower EBC Cl− at baseline and noted a significant increase in EBC Cl− with albuterol at the 60 minute time point (Table 3). The variability in dilution factor between subjects was fairly broad. The average dilution factor across all time periods was 34 (range 9–73) in healthy and 36 (range 15–80) in CF subjects.
Table 3.
Raw EBC Cl− and Na+ and dilution correction.
| HEALTHY | CF | P-VALUE | EFFECT SIZE (D) | |
|---|---|---|---|---|
| EBC Na(+) (mmol/L) | ||||
| Baseline | 2.58 ± 1.51 | 2.24±1.09 | – | – |
| 30 minutes | 1.97 ± 0.58 | 2.11±0.93 | – | – |
| 60 minutes | 2.37 ± 0.94 | 1.73±0.85 | – | – |
| 90 minutes | 2.23 ± 1.34 | 1.86±0.93 | – | – |
| Corrected for Dilution EBC Na+(mmol/L) | ||||
| Baseline | 58.7 ± 11.5 | 53.2±6.3 | – | – |
| 30 minutes | 60.2 ± 1.7 | 53.1±12.0 | – | – |
| 60 minutes | 60.8 ± 1.1 | 54.6±8.4 | – | – |
| 90 minutes | 60.4 ± 1.1 | 55.0±11.3 | – | – |
| EBC Cl−(mmol/L) | ||||
| Baseline | 0.064 ± 0.05 | 0.036 ± 0.02 | 0.05 | – |
| 30 minutes | 0.059 ± 0.045 | 0.035 ± 0.017 | 0.77 | – |
| 60 minutes | 0.057 ± 0.045 | 0.041 ± 0.022 | 0.65 | – |
| 90 minutes | 0.054 ± 0.039 | 0.034 ± 0.01 | 0.68 | – |
| Corrected for Dilution EBC Cl−(mmol/L) | ||||
| Baseline | 1.37 ± 0.89 | 0.75 ± 0.36 | 0.16 | 0.58 |
| 30 minutes | 1.94 ± 1.51 | 0.85 ± 0.26 | 0.32 | 0.65 |
| 60 minutes | 1.33 ± 0.90 | 1.35 ± 0.88 | 0.96 | - |
| 90 minutes | 1.79 ± 1.16 | 1.10 ± 0.51 | 0.13 | 0.65 |
| Net EBC Cl−(mmol/L) | ||||
| baseline | 0.064 ± 0.05 | 0.037 ± 0.02 | 0.05 | 0.57 |
| 30 minutes | 0.078 ± 0.143 | 0.048 ± 0.031 | 0.45 | 0.54 |
| 60 minutes | 0.084 ± 0.073 | 0.057 ± 0.038† | 0.62 | 0.43 |
| 90 minutes | 0.077 ± 0.06 | 0.050 ± 0.016† | 0.38 | 0.51 |
| Corrected for Dilution Net EBC Cl−(mmol/L) | ||||
| Baseline | 1.37 ± 0.89 | 0.75 ± 0.36 | 0.06 | |
| 30 minutes | 3.09 ± 3.46 | 1.38 ± 0.66 | 0.31 | 0.28 |
| 60 minutes | 2.58 ± 2.11 | 2.76 ± 3.11† | 0.35 | 0.26 |
| 90 minutes | 3.16 ± 2.39 | 1.93 ± 1.30 | 0.48 | 0.45 |
Notes:
P < 0.02 vs. baseline; P-value column Healthy vs. CF. Values are mean ± SD.
Abbreviation: CF, cystic fibrosis.
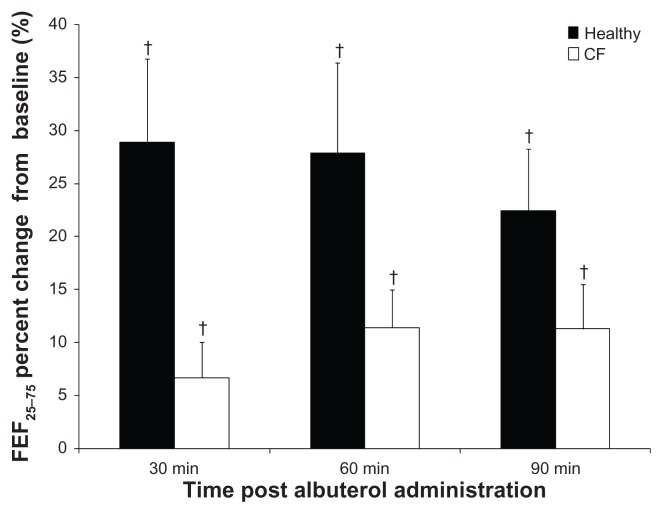
To evaluate the beneficial effects of albuterol in CF, we measured pulmonary function, diffusion capacity of the lungs, and SpO2. As expected, albuterol administration resulted in bronchodilation that was apparent based on the percent change in FEF25–75 in both groups, but the response was reduced in the CF patients (Fig. 3). Small improvements were noted in the FVC of CF patients who demonstrated an average 4% increase following albuterol which was opposite of healthy subjects who noted an average 1% decline. For both healthy and CF subjects there was an increase in FEV1 with albuterol, averaging 7% change in healthy and 5% in CF subjects, but this was only significant for the healthy subjects at 60-minutes post.
Figure 3.
Bronchodilation in response to albuterol in healthy and CF subjects. The percent change in force expiratory flow at 25–75% of FVC (FEF25–75) for each time point (30, 60 and 90-minutes post). CF subjects are represented by white bars and healthy subjects are represented by black bars. The error bars represent the standard error of the mean.
Note:†P < 0.02 baseline.
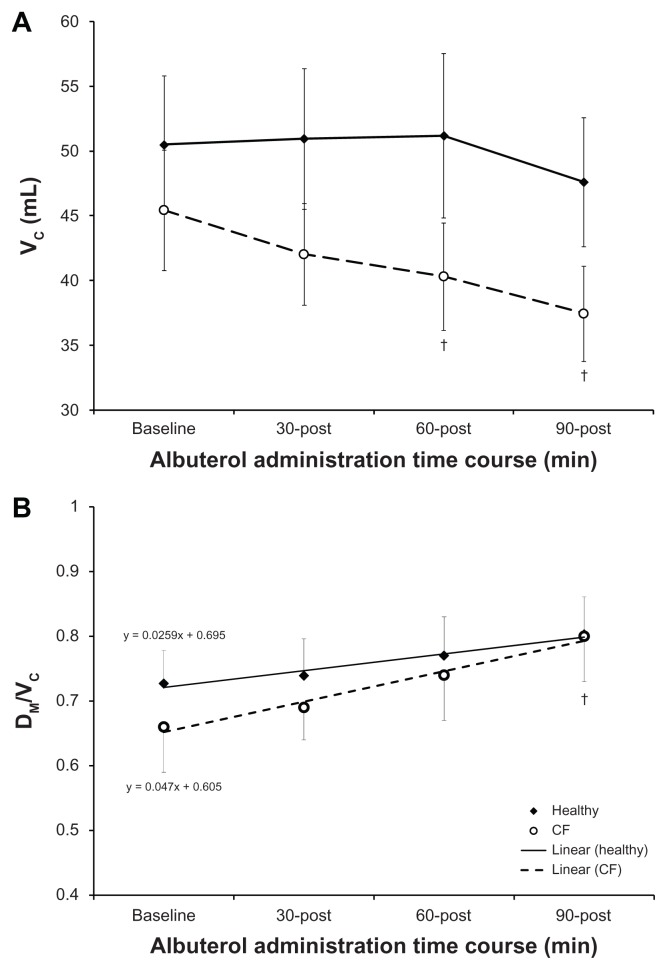
At baseline, the diffusion capacity of the lungs was impaired in CF. Specifically, DLCO was reduced and evaluation of the components of DLCO demonstrated that this reduction was due to a reduction in membrane conductance (DLNO and DM) and ventilation (alveolar ventilation, VA) (Table 4). There was no difference between conditions when DLCO was correct for VA or when DM was correct for pulmonary-capillary blood volume (VC). Administration of albuterol resulted in no change in DLCO, DLNO or DM in either group; as such, the differences between healthy and CF subjects remained. Both healthy and CF subjects demonstrated a decline in VC following albuterol, which was significant at 60- and 90-minutes post for CF patients (Fig. 4A). This decline in VC improved the ratio of membrane conductance to pulmonary perfusion (DM/VC) for CF patients, significantly increasing DM/VC from baseline by 90-minutes post albuterol. Evaluation of the response to albuterol for DM/VC demonstrated that the improvement was greater for CF patients (slope: 0.0259 vs. 0.047, healthy vs. CF respectively; Figure 4B). Additionally, SpO2 was significantly though not clinically different in these mild to moderate CF patients when compared to healthy subjects (Table 4). Albuterol increased SpO2 in CF patients such that there was no longer a difference between the healthy and CF subjects by 90-minutes post administration. Further, albuterol resulted in small increases (2 mmHg) alveolar oxygen tension (PAO2) for CF patients, which is trivial when oxyhemoglobin saturation is >90% on the flat portion of the dissociation curve. However, this effect of albuterol would be clinically beneficial for more severe CF patients, whose saturation may drop below 80% or in conditions when the work of breathing is increased such as with exercise where we have demonstrated a diffusion limitation.
Table 4.
Effect of albuterol on diffusion capacity of the lungs and peripheral oxygen saturation.
| HEALTHY | CF | P-VALUE | EFFECT SIZE (D) | |
|---|---|---|---|---|
| DLCO (ml/min/mmHg) | ||||
| Baseline | 21.5 ± 5.8 | 17.3 ± 4.4 | 0.04 | 0.83 |
| 30 minutes | 21.6 ± 5.3 | 17.4 ± 4.3 | 0.03 | 0.87 |
| 60 minutes | 21.6 ± 6.2 | 17.0 ± 4.4 | 0.03 | 0.87 |
| 90 minutes | 21.2 ± 5.5 | 17.1 ± 4.9 | 0.06 | 0.79 |
| DLNO (mL/min/mmHg) | ||||
| Baseline | 70.3 ± 18.9 | 55.0 ± 15.0 | 0.03 | 0.9 |
| 30 minutes | 70.8 ± 16.7 | 56.4 ± 15.0 | 0.04 | 0.91 |
| 60 minutes | 72.1 ± 19.2 | 56.2 ± 16.6 | 0.04 | 0.88 |
| 90 minutes | 73.0 ± 20.6 | 58.5 ± 17.8 | 0.13 | 0.75 |
| VA(mL) | ||||
| Baseline | 2794 ± 677 | 2022 ± 402 | 0.01 | 1.44 |
| 30 minutes | 2737 ± 752 | 1915 ± 440 | 0.01 | 1.38 |
| 60 minutes | 2777 ± 693 | 1933 ± 399 | 0.01 | 1.55 |
| 90 minutes | 2685 ± 711 | 1898 ± 404 | 0.01 | 1.41 |
| DLCO/VA(min/mmHg/L) | ||||
| Baseline | 7.8 ± 1.2 | 8.6 ± 1.4 | – | – |
| 30 minutes | 8.0 ± 1.3 | 9.2 ± 1.8 | – | – |
| 60 minutes | 7.8 ± 1.2 | 8.8 ± 1.3 | – | – |
| 90 minutes | 8.0 ± 1.2 | 9.1 ± 1.8 | – | – |
| DM(mL/min/mmHg) | ||||
| baseline | 33.5 ± 9.1 | 26.1 ± 7.1 | 0.03 | 0.92 |
| 30 minutes | 33.9 ± 8.3 | 26.7 ± 7.1 | 0.04 | 0.93 |
| 60 minutes | 34.3 ± 9.3 | 26.7 ± 7.9 | 0.04 | 0.89 |
| 90 minutes | 34.6 ± 9.8 | 27.9 ± 9.8 | 0.14 | 0.73 |
| PAO2(mL) | ||||
| Baseline | 155.3 ± 11.4 | 160.2 ± 7.4 | - | - |
| 30 minutes | 156.7 ± 10.1 | 162.4 ± 8.7 | – | – |
| 60 minutes | 156.4 ± 9.9 | 162.5 ± 8.6 | – | – |
| 90 minutes | 156.5 ± 11.2 | 162.7 ± 9.6 | – | – |
| SpO2(%) | ||||
| Baseline | 99 ± 1 | 98 ± 1 | 0.01 | 1.02 |
| 30 minutes | 100 ± 0 | 98 ± 1 | 0.01 | 1.52 |
| 60 minutes | 99 ± 1 | 98 ± 1 | 0.02 | 0.91 |
| 90 minutes | 99 ± 1 | 99 ± 1† | 0.11 | 0.59 |
Notes: Values are mean ± SD.
P < 0.02 vs. baseline P-value column Healthy vs. CF.
Abbreviations: CF, cystic fibrosis; DLCO, diffusion of the lungs for carbon monoxide; DLNO, diffusion capacity of the lungs for nitric oxide; VA, alveolar volume; DLCO/VA, DLCO corrected for VA; DM, alveolar-capillary membrane conductance; PAO2, alveolar oxygen tension; SpO2, peripheral oxygen saturation.
Figure 4.
Effect of albuterol on pulmonary capillary blood volume and gas diffusion at the individual alveolar-capillary unit (DM/VC) in healthy and CF subjects. Panel A: Pulmonary capillary blood volume (VC). Panel B: Gas Diffusion at the Individual Alveolar-capillary Unit (DM/VC). The open circles represent subjects with CF with the response overtime shown as the broken line. The closed diamonds represent healthy subjects with the response overtime shown as the solid line. The error bars represent the standard error of the mean.
Notes:†baseline vs. time post in CF subjects.
Since CF subjects also experienced significant bronchodilation with albuterol, we performed regression analyses to assess the influence of bronchodilation vs. improvements in diffusion in facilitating the observed improvement in SpO2. DLCO/VA explained 13% whereas FEF25–75 could accounted for 1% of the variability of SpO2 in CF subjects (F = 2.04, P = 0.18, R = 0.36, R2 = 0.13; F = 0.12, P = 0.73, R = 0.09, R2 = 0.01; DLCO/VA and FEF25–75 respectively). When both DLCO/VA and FEF25–75 were included in the model there was no improvement in the predictability (R2 = 0.13), but DLCO/VA accounted for the largest portion of the explained variation (β = 0.37, P = 0.21; β = −0.04, P = 0.89, DLCO/VA and FEF25–75 respectively).
Discussion
We collected EBC to assess the effects of albuterol on ion regulation in the lungs. We found that CF subjects had lower EBC Cl−, which is in agreement with the reduced Cl− conductance that has been previously observed in CF subjects using nasal potential difference.42–44 We observed an increase in EBC Cl− following albuterol in both CF and healthy subjects, which was significant in the CF patients by 60 minutes, eliminating the difference in EBC Cl− between the healthy and CF subjects. These results suggest that albuterol may indeed improve ion dysregulation in CF, as there was no longer a detectable difference in EBC Cl− between groups after albuterol administration. This response to albuterol is compatible with a mechanistic understanding of CFTR activity in response to adrenergic stimulation. Previous in vivo research has demonstrated that ADBR2 activation stimulates CFTR, as this cAMP-dependent channel requires phosphorylation by PKA in addition to ATP to open the channel and facilitate conductance of Cl− in normal airway epithelium.45,46 However, 80% of our CF patient population was homozygous for the ΔF508 mutation. Until recently, the present state of knowledge based on heterologous expression studies was that these patients had no ΔF508 CFTR present in the apical surface of airway epithelia, and that PKA failed to open CFTR in CF airway epithelium.45,47 This notion would conflict with our findings; how could CFTR activity and Cl− efflux be increasing if there are no channels present to activate?
More recent work has disagreed with this idea, demonstrating ΔF508 CFTR protein expression in ciliated respiratory epithelia from CF patients is no different from non-CF tissue,48 that ΔF508 homozygous patients have CFTR expression but its expression is in a reduced number of cells (22% vs. 56%; CF vs. healthy),49 and that the ΔF508 mutation does reduce expression, but there are functional CFTR channels that do make it to the membrane and are active although channel kinetics are altered (reduced response to PKA and open-time probability).26,50 Additionally, the use of the long acting β2-agonist salmeterol has salmeterol has been shown to restore CFTR function (83% increase in ΔF508 CFTR mediated Cl− efflux) in airway submucosal gland serous cells,51 and that PKA can mediate dimerization of CFTR, which can increase membrane expression by inhibiting endocytic retrieval.52,46 This provides a potential mechanism for ΔF508 homozygous CF patients to still be capable of Cl− efflux given stimulation. The fact that ΔF508 CFTR expression and function remains controversial highlights that in order to validate the collection of EBC as a technique for studying ion regulation in the lung, future work comparing the changes in EBC Na+ and Cl− to that of changes in ion flux assessed utilizing the standardized nasal potential difference technique to see if the two responses are in agreement is warranted. One cannot identify where the collected EBC droplets are being produced (larger airways, smaller airways, alveoli); as such, comparison of the ionic composition of an EBC sample to a bronchoalveolar lavage sample taken with deionized water would improve our understanding of the source of our EBC sample.
The use of a β-agonist as a therapeutic modality in CF is commonplace, being prescribed in over 80% of individuals with CF, although the conclusive support demonstrating the benefits of this therapy in this patient population is limited.53 The main reason it is prescribed is to treat airway obstruction and hyperresponsiveness, by dilating the airways to alleviate these asthmatic symptoms.54 Previous research has demonstrated an improvement in pulmonary function both with short-term and long-term β-agonist use, and this has been the primary hypothesis for the noted improvements in SpO2 in CF.55,56 It has also been well established that β-agonists increase ciliary beat frequency, and that administration of the β-agonist terbutaline increases mucociliary clearance (MCC) in CF subjects when compared to healthy subjects.57,58 Our work adds to this previous work suggesting that bronchodilation is not the only beneficial effect of β-agonists such as albuterol.
In this study we found that, at baseline, subjects with CF exhibit lower DLCO than their healthy counterparts, agreeing with previous work.59,60 We were also able to assess the components of DLCO: DM as well as VC by measuring DLNO in conjunction with DLCO, to identify if this impairment is due to a limitation in DM, VC or both. Utilizing concurrent assessment of DLCO and DLNO, we found that DM, but not VC were lower in our CF population. Since there was no difference between conditions when DLCO was corrected for VA or when DM was corrected for VC, these differences seem to be explained by ventilation-perfusion mismatching due to gross lung damage and limited functional surface area. Albuterol could not mediate any attenuation of the diffusion impairments for CF patients as there was no change in DLCO, DLNO or DM following albuterol in either group. However, albuterol did result in a significant improvement in gas diffusion at the alveolar-capillary functional unit expressed by the parameter DM/VC, which improved at greater rate in CF patients such that by 90-minutes post, DM/VC was similar to what was observed in healthy subjects. Since there was no change in membrane conductance (DLNO or DM) with albuterol, the improvement in gas transfer at the functional unit can be attributed to the significant decline in VC. We hypothesize that the decrease in VC is the result of β-adrenergic mediated pulmonary vasodilation decreasing vascular tone. Causing a derecruitment of vessels in the areas surrounding these areas of dilation, and bronchodilation-mediated increases in alveolar oxygen tension (PAO2); oxygen-mediated relaxation. In essence, this process is the opposite of hypoxia-induced recruitment via vasoconstriction.61
Albuterol resulted in small increases (2 mmHg) in PAO2 for CF patients, which is in support of our hypothesis that the pulmonary capillaries are responding to changes in PAO2 to allow better ventilation-perfusion (V/Q) matching. Additionally, it has been demonstrated that β-agonists blunt the acute hypoxic vasopressor response.62 We believe that the increase in SpO2 that we observed in CF patients is the result of this better matching of alveolar ventilation and diffusing capacity to the pulmonary blood flow. Although the increases in EBC Cl− lead us to hypothesize that albuterol can partially and temporarily improve ion dysregulation and allow for better hydration of the lungs and mucus, along with its enhancement of mucociliary beat frequency, which can help to clear stagnant mucus and improve the rate of diffusion by decreasing the distance of diffusion, on this single dose scale it is most likely that albuterol is improving V/Q matching to provide the clinically modest increases in SpO2. Previous research in patients with idiopathic fibrosis noted that DLCO would have to be reduced by half in order to see significant desaturation63 and our regression analysis, in which we found that the improvements in DLCO/VA explained the greatest portion of the improvement in SpO2 rather than bronchodilation, both support our explanation of albuterol facilitating better matching of ventilation and diffusion with perfusion as the cause of the observed increase in SpO2.
In this study, we utilized healthy subjects to compare what a normal response in EBC ions would be to albuterol, but we did not include the placebo control of saline within the CF patients. As the common protocol for albuterol administration in CF patients is nebulization, we did not compare the effects of albuterol dissolved in saline to that of saline alone, as our goal was not to partition whether the benefits of albuterol in CF were due to saline helping to wet the airways, exogenous stimulation of ADBR2 in the lung, or both, but to determine if albuterol could positively alter sodium and chloride in the ASL, and to see if these changes could be detect in EBC. However, we did compare the effects of nebulized albuterol and saline in a subset of our healthy subjects, where we found the response to either treatment to be minimal. The changes in EBC Na+ and Cl− in response to albuterol were not the same between CF subjects and healthy subjects, and previous work found that isotonic saline does not mediate the improvements in MCC that hypertonic saline demonstrates in CF patients, suggesting that the changes in EBC Cl− we observed were not due to saline alone, but also adrenergic stimulation. When we compared EBC Na+ concentrations based on a single nucleotide polymorphism gene encoding ENaC in healthy individuals, we found that individuals with the channel characterized by greater basal activity had lower baseline EBC Na+, which we feel adds support to the validity of this technique.64 Additionally, the difference in the baseline values within the healthy subjects between the saline and albuterol visits highlights the need for correcting for dilution of the ASL droplet by water vapor during exhalation.
Limitations
Although these findings provide a clearer picture of the components of gas diffusion, a drawback of the assessment of DLCO using the DLCO/DLNO rebreathe technique is that it does not allow for the calculation of the percentage predicted, because no equations have been established; equations are only available for the single breath assessment. Percent-predicted values allow for easier comparison between studies, especially since there are different methods (single breath, wash-in, and now more recently DLCO with DLNO rebreathe), but work by Dressel et al.65 demonstrated that the destruction of lung epithelia in CF, visualized with computed tomography, was better correlated with membrane conductance quantified using the assessment of DLNO rather than measurement of DLCO, further validating the use of this method for assessing diffusing capacity of the lungs in this patient population.
The sample size for this study was relatively small; as such, the trends with large effect sizes noted in this study would likely have been significant with the recruitment of a larger sample size, but this was not possible for this study due to the available patient population. Also, pulse oximetry is not as sensitive as direct arterial blood draws. Given that SpO2 was a secondary parameter in this study, the added invasiveness of arterial sampling, and the results of a meta-analysis that demonstrated a strong relationship between SpO2 measures,66 it is not likely that the accuracy of our SpO2 were affected. This is especially the case at the higher levels of saturation seen in this study, as pulse oximeters tend to lose sensitivity at low saturations. However, future studies with arterial O2 measurement and recruitment of a larger subject population are needed to confirm our findings.
Conclusions
Our findings that CF patients have lower EBC Cl− at baseline when compared to healthy control subjects, and that albuterol increased EBC Cl−, improved DM/VC and normalized SpO2, support our hypothesis: EBC was able to detect differences in ionic composition of the ASL between healthy and CF individuals. Also, adrenergic stimulation, via albuterol, of CFTR mediated Cl− efflux was detectable in EBC, and the benefits of albuterol administration in this patient population extend beyond simple bronchodilation. We, like others, have found that EBC is not a perfected measure of ASL composition yet,67 but our work suggests that the collection of EBC may be a technique that has the potential to help researchers and clinicians to understand at a more cellular level if current and developing drugs for CF patients are indeed enhancing Cl− secretion and/or inhibiting Na+ absorption, as well as whether this is positively affecting lung fluid homeostasis, as in vitro mechanistic work has suggested.
Supplementary Material
Table 1.
Raw EBC Cl− and Na+ and dilution correction in healthy subjects in response to albuterol and saline.
| HEALTHY SUBJECTS | ALBUTEROL | SALINE |
|---|---|---|
| EBC Na+(mmol/L) | ||
| Baseline | 2.6 ± 1.7 | 2.0 ± 0.4 |
| 30 minutes | 2.1 ± 0.6 | 1.7 ± 0.6 |
| 60 minutes | 2.5 ± 1.0 | 1.7 ± 0.6 |
| 90 minutes | 2.3 ± 1.6 | 2.0 ± 0.7 |
| EBC Cl−(mmol/L) | ||
| Baseline | 0.068 ± 0.044 | 0.035 ± 0.03 |
| 30 minutes | 0.067 ± 0.041 | 0.027 ± 0.01 |
| 60 minutes | 0.068 ± 0.048 | 0.025 ± 0.007 |
| 90 minutes | 0.058 ± 0.039 | 0.033 ± 0.018 |
| Corrected for Dilution EBC Na+(mmol/L) | ||
| Baseline | 61.0 ± 0.8 | 60.7 ± 0.8 |
| 30 minutes | 60.8 ± 1.1 | 60.8 ± 0.8 |
| 60 minutes | 60.6 ± 1.3 | 61.0 ± 0.8 |
| 90 minutes | 60.6 ± 0.7 | 60.6 ± 1.4 |
| Corrected for Dilution EBC Cl−(mmol/L) | ||
| Baseline | 1.80 ± 1.71 | 1.50 ± 1.30 |
| 30 minutes | 1.63 ± 0.92 | 1.23 ± 0.54 |
| 60 minutes | 1.36 ± 0.85 | 0.96 ± 0.40 |
| 90 minutes | 2.19 ± 2.25 | 1.55 ± 1.61 |
| Dilution Factor | ||
| Baseline | 27.2 ± 10.4 | 30.9 ± 5.5 |
| 30 minutes | 29.8 ± 8.1 | 41.8 ± 16.3 |
| 60 minutes | 30.1 ± 15.0 | 40.3 ± 13.2 |
| 90 minutes | 32.8 ± 14.7 | 35.6 ± 16.7 |
Acknowledgements
We would like to thank the CF and healthy subjects who willingly donated their time and effort to be involved in this study.
Footnotes
COMPETING INTERESTS: WJM has received consultancy fees from Genentech and the Cystic Fibrosis Foundation, speaker’s fees from AAAAI and ATS, royalties from Elsevier, and grants or grants pending to his institution from the University of Washington, Seattle, Mt Sinai Hospital/Research Institute, NHLBI, Cystic Fibrosis Foundation and NIAID. Other authors disclose no potential conflicts of interest.
Author Contributions
CMW, WTL, NAC, MAM, EMS were responsible for study design, data collection and analysis and manuscript writing. WJM, CLD, HP were responsible for recruitment and manuscript writing. All authors reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
FUNDING: This study was funded by the University of Arizona Clinical Scholars program and University of Arizona Foundation, Caldwell Health Sciences Research Fellowship and National Institute of Health (1R01HL108962–01).
References
- 1.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2006 Annual data report to the center directors. Bethesda: Cystic Fibrosis Foundation; 2008. [Google Scholar]
- 2.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 3.Potter JL, Matthews LW, Lemm J, Spector S. Human pulmonary secretions in health and disease. Ann N Y Acad Sci. 1963;106:692–7. doi: 10.1111/j.1749-6632.1963.tb16677.x. [DOI] [PubMed] [Google Scholar]
- 4.Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–8. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 5.Chambers LA, Rollins BM, Tarran R. Liquid movement across the surface epithelium of large airways. Respir Physiol Neurobiol. 2007;159(3):256–70. doi: 10.1016/j.resp.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitkänen OM, Smith D, O’Brodovich H, Otulakowski G. Expression of alpha-, beta-, and gamma-hENaC mRNA in the human nasal, bronchial, and distal lung epithelium. Am J Respir Crit Care Med. 2001;163(1):273–6. doi: 10.1164/ajrccm.163.1.9909114. [DOI] [PubMed] [Google Scholar]
- 7.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 8.Matsui H, Davis CW, Tarran R, Boucher RC. Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J Clin Invest. 2000;105(10):1419–27. doi: 10.1172/JCI4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Button B, Boucher RC University of North Carolina Virtual Lung Group. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol. 2008;163(1–3):189–201. doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–61. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 11.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127(5):591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutlu GM, Koch WJ, Factor P. Alveolar epithelial beta 2-adrenergic receptors: their role in regulation of alveolar active sodium transport. Am J Respir Crit Care Med. 2004;170(12):1270–5. doi: 10.1164/rccm.200404-470CP. [DOI] [PubMed] [Google Scholar]
- 13.Bossard F, Silantieff E, Lavazais-Blancou E, et al. β1, β2, and β3 adrenoceptors and Na+/H+ exchanger regulatory factor 1 expression in human bronchi and their modifications in cystic fibrosis. Am J Respir Cell Mol Biol. 2011;44(1):91–8. doi: 10.1165/rcmb.2009-0372OC. [DOI] [PubMed] [Google Scholar]
- 14.Chen XJ, Eaton DC, Jain L. Beta-adrenergic regulation of amiloride-sensitive lung sodium channels. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L609–20. doi: 10.1152/ajplung.00356.2001. [DOI] [PubMed] [Google Scholar]
- 15.Haws C, Krouse ME, Xia Y, Gruenert DC, Wine JJ. CFTR channels in immortalized human airway cells. Am J Physiol. 1992;263(6 Pt 1):L692–707. doi: 10.1152/ajplung.1992.263.6.L692. [DOI] [PubMed] [Google Scholar]
- 16.Hwang TC, Nagel G, Nairn AC, Gadsby DC. Regulation of the gating of cystic fibrosis transmembrane conductance regulator C1 channels by phosphorylation and ATP hydrolysis. Proc Natl Acad Sci U S A. 1994;91(11):4698–702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT. Na transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986;78:1245–52. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem. 1997;272(22):14037–40. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- 19.Davis PB. The decline and fall of pulmonary function in cystic fibrosis: new models, new lessons. J Pediatr. 1997;131(6):789–90. doi: 10.1016/s0022-3476(97)70019-2. [DOI] [PubMed] [Google Scholar]
- 20.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10(2):105–16. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 21.Knowles MR, Robinson JM, Wood RE, et al. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J Clin Invest. 1997;100(10):2588–95. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002;110(1):28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 23.Griese M, Noss J, Schramel P. Elemental and ion composition of exhaled air condensate in cystic fibrosis. J Cyst Fibros. 2003;2(3):136–42. doi: 10.1016/S1569-1993(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 24.Zacharasiewicz A, Wilson N, Lex C, et al. Repeatability of sodium and chloride in exhaled breath condensates. Pediatr Pulmonol. 2004;37(3):273–5. doi: 10.1002/ppul.10431. [DOI] [PubMed] [Google Scholar]
- 25.Wheatley CM, Foxx-Lupo WT, Cassuto NA, et al. Impaired lung diffusing capacity for nitric oxide and alveolar-capillary membrane conductance results in oxygen desaturation during exercise in patients with cystic fibrosis. J Cyst Fibros. 2011;10(1):45–53. doi: 10.1016/j.jcf.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Dalemans W, Barbry P, Champigny G, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991;354(6354):526–8. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 27.Wheatley CM, Cassuto NA, Foxx-Lupo WT, Snyder EM. Variability in measures of exhaled breath na, influence of pulmonary blood flow and salivary na. Clin Med Insights Circ Respir Pulm Med. 2010;4:25–34. doi: 10.4137/ccrpm.s4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Effros RM, Biller J, Foss B, et al. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med. 2003;168(12):1500–5. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]
- 29.Snyder EM, Johnson BD, Beck KC. An open-circuit method for determining lung diffusing capacity during exercise: comparison to rebreathe. J Appl Physiol (1985) 2005;99(5):1985–91. doi: 10.1152/japplphysiol.00348.2005. [DOI] [PubMed] [Google Scholar]
- 30.Snyder EM, Beck KC, Hulsebus ML, Breen JF, Hoffman EA, Johnson BD. Short-term hypoxic exposure at rest and during exercise reduces lung water in healthy humans. J Appl Physiol (1985) 2006;101(6):1623–32. doi: 10.1152/japplphysiol.00481.2006. [DOI] [PubMed] [Google Scholar]
- 31.Wheatley CM, Baldi JC, Cassuto NA, Foxx-Lupo WT, Snyder EM. Glycemic control influences lung membrane diffusion and oxygen saturation in exercise-trained subjects with type 1 diabetes: alveolar-capillary membrane conductance in type 1 diabetes. Eur J Appl Physiol. 2011;111(3):567–78. doi: 10.1007/s00421-010-1663-8. [DOI] [PubMed] [Google Scholar]
- 32.Wheatley CM, Foxx-Lupo WT, Cassuto NA, et al. Impaired lung diffusing capacity for nitric oxide and alveolar-capillary membrane conductance results in oxygen desaturation during exercise in patients with cystic fibrosis. J Cyst Fibros. 2011;10(1):45–53. doi: 10.1016/j.jcf.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Tamhane RM, Johnson RL, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120(6):1850–6. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- 34.ROUGHTON FJ, FORSTER RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11(2):290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 35.Hsia CC. Recruitment of lung diffusing capacity: update of concept and application. Chest. 2002;122(5):1774–83. doi: 10.1378/chest.122.5.1774. [DOI] [PubMed] [Google Scholar]
- 36.Hsia CC, Raskin P. The diabetic lung: relevance of alveolar microangiopathy for the use of inhaled insulin. Am J Med. 2005;118(3):205–11. doi: 10.1016/j.amjmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Ceridon ML, Beck KC, Olson TP, Bilezikian JA, Johnson BD. Calculating alveolar capillary conductance and pulmonary capillary blood volume: comparing the multiple- and single-inspired oxygen tension methods. J Appl Physiol (1985) 2010;109(3):643–53. doi: 10.1152/japplphysiol.01411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 39.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 40.Cohen J, Cohen P, West G, Aiken L. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Hillsdale: Lawrence Erlbaum Associates, Inc; 2003. [Google Scholar]
- 41.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale: Lawrence Erlbaum Associates, Inc; 1969. [Google Scholar]
- 42.Schmitt L, Wiebel M, Frese F, Dehnert C, Zugck C, Bartsch P, Mairbaurl H. Exercise reduces airway Na-reabsorption in cystic fibrosis but not in exercise asthma. Eur Respir J. 2011;37(2):342–8. doi: 10.1183/09031936.00197309. [DOI] [PubMed] [Google Scholar]
- 43.Alsuwaidan S, Li Wan, Po A, Morrison G, et al. Effect of exercise on the nasal transmucosal potential difference in patients with cystic fibrosis and normal subjects. Thorax. 1994;49(12):1249–50. doi: 10.1136/thx.49.12.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hebestreit A, Kersting U, Basler B, Jeschke R, Hebestreit H. Exercise inhibits epithelial sodium channels in patients with cystic fibrosis. Am J Respir Crit Care Med. 2001;164(3):443–6. doi: 10.1164/ajrccm.164.3.2007168. [DOI] [PubMed] [Google Scholar]
- 45.Li M, McCann JD, Liedtke CM, Nairn AC, Greengard P, Welsh MJ. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988;331(6154):358–60. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- 46.Riordan JR. Assembly of functional CFTR chloride channels. Annu Rev Physiol. 2005;67:701–18. doi: 10.1146/annurev.physiol.67.032003.154107. [DOI] [PubMed] [Google Scholar]
- 47.Kreda SM, Mall M, Mengos A, et al. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell. 2005;16(5):2154–67. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kälin N, Claass A, Sommer M, Puchelle E, Tümmler B. DeltaF508 CFTR protein expression in tissues from patients with cystic fibrosis. J Clin Invest. 1999;103(10):1379–89. doi: 10.1172/JCI5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penque D, Mendes F, Beck S, et al. Cystic fibrosis F508del patients have apically localized CFTR in a reduced number of airway cells. Lab Invest. 2000;80(6):857–68. doi: 10.1038/labinvest.3780090. [DOI] [PubMed] [Google Scholar]
- 50.Drumm ML, Wilkinson DJ, Smit LS, et al. Chloride conductance expressed by delta F508 and other mutant CFTRs in Xenopus oocytes. Science. 1991;254(5039):1797–9. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- 51.Delavoie F, Molinari M, Milliot M, et al. Salmeterol restores secretory functions in cystic fibrosis airway submucosal gland serous cells. Am J Respir Cell Mol Biol. 2009;40(4):388–97. doi: 10.1165/rcmb.2008-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lukacs GL, Segal G, Kartner N, Grinstein S, Zhang F. Constitutive internalization of cystic fibrosis transmembrane conductance regulator occurs via clathrin-dependent endocytosis and is regulated by protein phosphorylation. Biochem J. 1997;328(Pt 2):353–61. doi: 10.1042/bj3280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176(10):957–69. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 54.Brand PL. Bronchodilators in cystic fibrosis. J R Soc Med. 2000;2000;93(Suppl 38):37–9. [PMC free article] [PubMed] [Google Scholar]
- 55.Hordvik NL, Sammut PH, Judy CG, Strizek SJ, Colombo JL. The effects of albuterol on the lung function of hospitalized patients with cystic fibrosis. Am J Respir Crit Care Med. 1996;154(1):156–60. doi: 10.1164/ajrccm.154.1.8680672. [DOI] [PubMed] [Google Scholar]
- 56.König P, Poehler J, Barbero GJ. A placebo-controlled, double-blind trial of the long-term effects of albuterol administration in patients with cystic fibrosis. Pediatr Pulmonol. 1998;25(1):32–6. doi: 10.1002/(sici)1099-0496(199801)25:1<32::aid-ppul3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 57.Wood RE, Wanner A, Hirsch J, Farrell PM. Tracheal mucociliary transport in patients with cystic fibrosis and its stimulation by terbutaline. Am Rev Respir Dis. 1975;111(6):733–8. doi: 10.1164/arrd.1975.111.6.733. [DOI] [PubMed] [Google Scholar]
- 58.Salathe M. Effects of beta-agonists on airway epithelial cells. J Allergy Clin Immunol. 2002;2002;110(Suppl 6):S275–81. doi: 10.1067/mai.2002.129412. [DOI] [PubMed] [Google Scholar]
- 59.Espiritu JD, Ruppel G, Shrestha Y, Kleinhenz ME. The diffusing capacity in adult cystic fibrosis. Respir Med. 2003;97(6):606–11. doi: 10.1053/rmed.2003.1487. [DOI] [PubMed] [Google Scholar]
- 60.Dressel H, Filser L, Fischer R, et al. Lung diffusing capacity for nitric oxide and carbon monoxide: dependence on breath-hold time. Chest. 2008;133(5):1149–54. doi: 10.1378/chest.07-2388. [DOI] [PubMed] [Google Scholar]
- 61.Cutaia M, Rounds S. Hypoxic pulmonary vasoconstriction. Physiologic significance, mechanism, and clinical relevance. Chest. 1990;97:706–18. doi: 10.1378/chest.97.3.706. [DOI] [PubMed] [Google Scholar]
- 62.Porcelli RJ, Viau AT, Naftchi NE, Bergofsky EH. beta-Receptor influence on lung vasoconstrictor responses to hypoxia and humoral agents. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(4):612–6. doi: 10.1152/jappl.1977.43.4.612. [DOI] [PubMed] [Google Scholar]
- 63.Agustí AG, Roca J, Gea J, Wagner PD, Xaubet A, Rodriguez-Roisin R. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1991;143(2):219–25. doi: 10.1164/ajrccm/143.2.219. [DOI] [PubMed] [Google Scholar]
- 64.Foxx-Lupo WT, Wheatley CM, Baker SE, Cassuto NA, Delamere NA, Snyder EM. Genetic variation of the alpha subunit of the epithelial Na+ channel influences exhaled Na+ in healthy humans. Respir Physiol Neurobiol. 2011;179(2–3):205–11. doi: 10.1016/j.resp.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dressel H, Filser L, Fischer R, et al. Lung diffusing capacity for nitric oxide and carbon monoxide in relation to morphological changes as assessed by computed tomography in patients with cystic fibrosis. BMC Pulm Med. 2009;9:30. doi: 10.1186/1471-2466-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jensen LA, Onyskiw JE, Prasad NG. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart Lung. 1998;27(6):387–408. doi: 10.1016/s0147-9563(98)90086-3. [DOI] [PubMed] [Google Scholar]
- 67.Davis MD, Montpetit A, Hunt J. Exhaled breath condensate: an overview. Immunol Allergy Clin North Am. 2012;32(3):363–75. doi: 10.1016/j.iac.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1.
Raw EBC Cl− and Na+ and dilution correction in healthy subjects in response to albuterol and saline.
| HEALTHY SUBJECTS | ALBUTEROL | SALINE |
|---|---|---|
| EBC Na+(mmol/L) | ||
| Baseline | 2.6 ± 1.7 | 2.0 ± 0.4 |
| 30 minutes | 2.1 ± 0.6 | 1.7 ± 0.6 |
| 60 minutes | 2.5 ± 1.0 | 1.7 ± 0.6 |
| 90 minutes | 2.3 ± 1.6 | 2.0 ± 0.7 |
| EBC Cl−(mmol/L) | ||
| Baseline | 0.068 ± 0.044 | 0.035 ± 0.03 |
| 30 minutes | 0.067 ± 0.041 | 0.027 ± 0.01 |
| 60 minutes | 0.068 ± 0.048 | 0.025 ± 0.007 |
| 90 minutes | 0.058 ± 0.039 | 0.033 ± 0.018 |
| Corrected for Dilution EBC Na+(mmol/L) | ||
| Baseline | 61.0 ± 0.8 | 60.7 ± 0.8 |
| 30 minutes | 60.8 ± 1.1 | 60.8 ± 0.8 |
| 60 minutes | 60.6 ± 1.3 | 61.0 ± 0.8 |
| 90 minutes | 60.6 ± 0.7 | 60.6 ± 1.4 |
| Corrected for Dilution EBC Cl−(mmol/L) | ||
| Baseline | 1.80 ± 1.71 | 1.50 ± 1.30 |
| 30 minutes | 1.63 ± 0.92 | 1.23 ± 0.54 |
| 60 minutes | 1.36 ± 0.85 | 0.96 ± 0.40 |
| 90 minutes | 2.19 ± 2.25 | 1.55 ± 1.61 |
| Dilution Factor | ||
| Baseline | 27.2 ± 10.4 | 30.9 ± 5.5 |
| 30 minutes | 29.8 ± 8.1 | 41.8 ± 16.3 |
| 60 minutes | 30.1 ± 15.0 | 40.3 ± 13.2 |
| 90 minutes | 32.8 ± 14.7 | 35.6 ± 16.7 |