Abstract
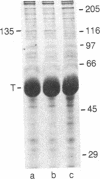
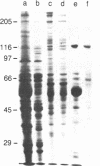
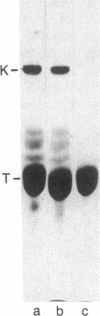
Recently, a protein called kinesin was described, which is capable of inducing movement of inert particles along microtubules. To purify this protein from bovine brain, we used the ability of kinesin to bind to taxol-stabilized microtubules in the presence of inorganic tripolyphosphate. The brain kinesin preparation contained one major polypeptide of 135 kDa and four minor polypeptides of 45-70 kDa. The minor polypeptides were eluted from a gel-permeation chromatography column at the same position as the major component. All the polypeptides of the preparation were capable of binding to the microtubules under identical conditions. The kinesin molecule is most probably a complex of these polypeptides. Brain kinesin had a very low ATPase activity (0.06-0.08 mumol X min-1 X mg-1 in 3 mM Mg2+ at pH 6.7). ATPase activity was strongly stimulated by microtubules (Vmax = 4.6 mumol per min per mg of kinesin). Microtubule-activated kinesin ATPase had a Km for ATP between 10 and 12 X 10(-6) M and a Kapp for microtubules (i.e., polymerized tubulin concentration required for a half-maximal activation) of 12-14 X 10(-6) M. Kinesin had a significant ATPase activity even without microtubules if 2 mM Ca2+ was substituted for Mg2+ (Vmax = 1.6 mumol X min-1 X mg-1; Km = 800 X 10(-6) M). Kinesin is therefore a mechanochemical ATPase that is activated by microtubules.
Full text
PDF
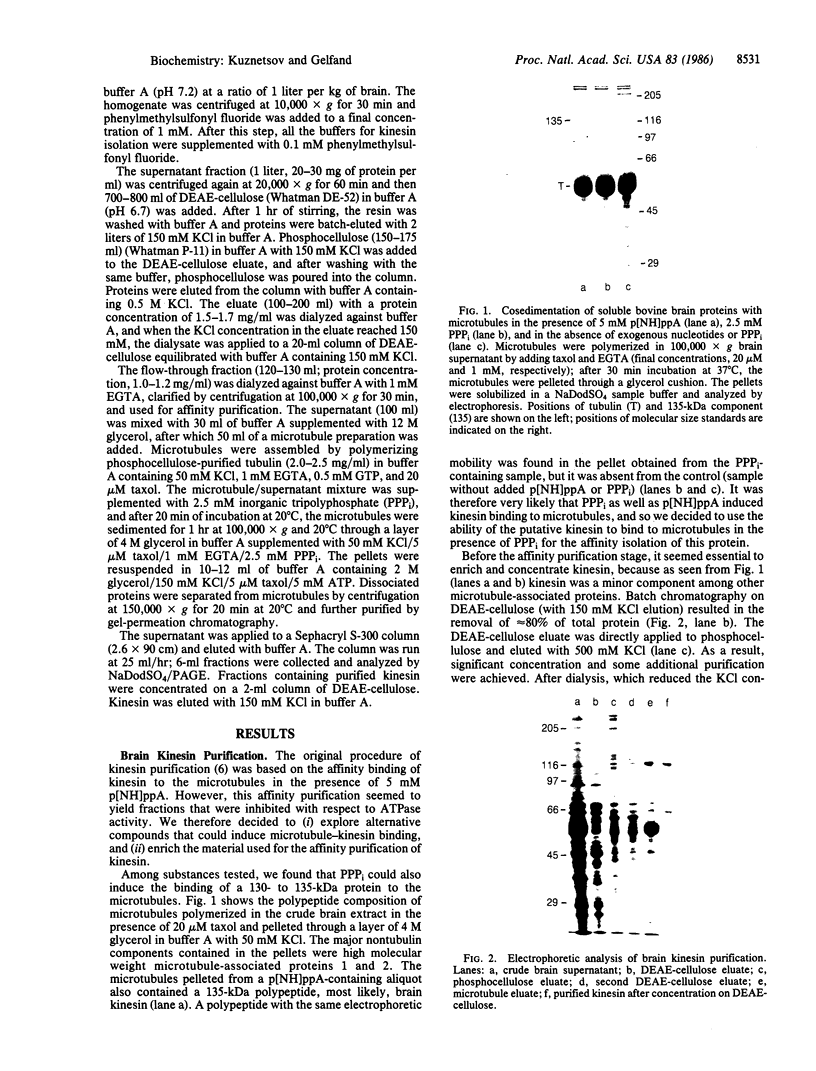
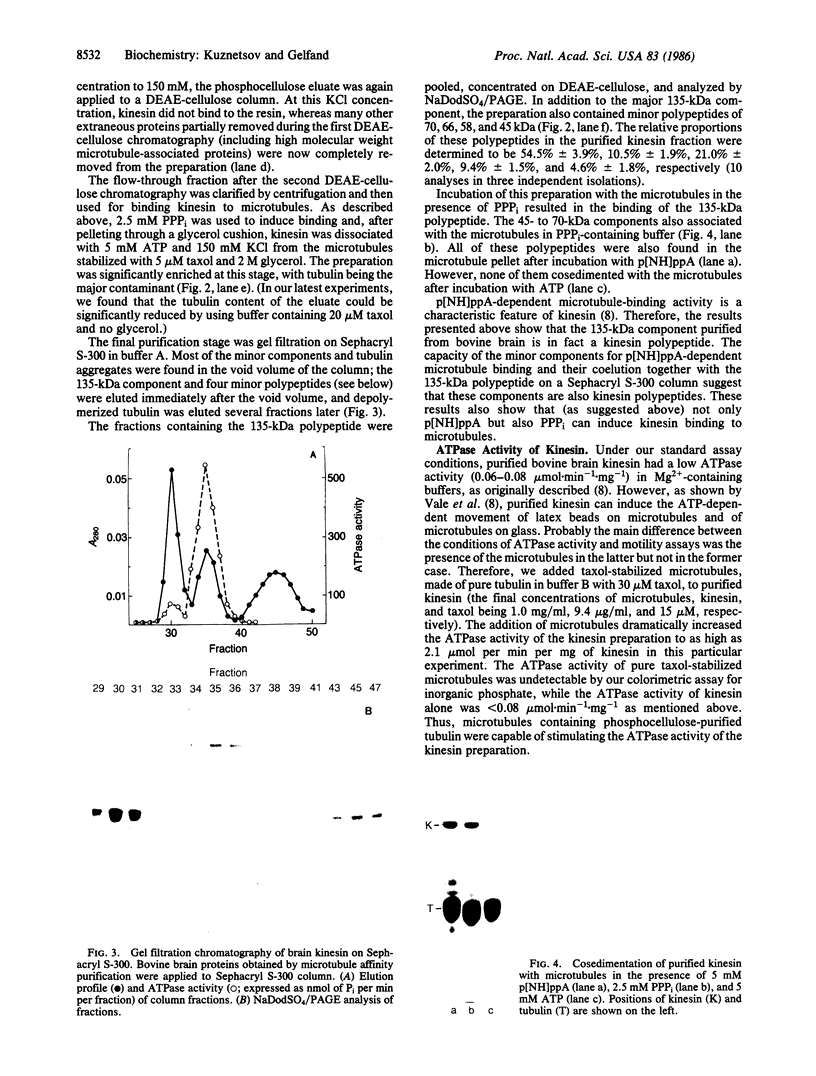
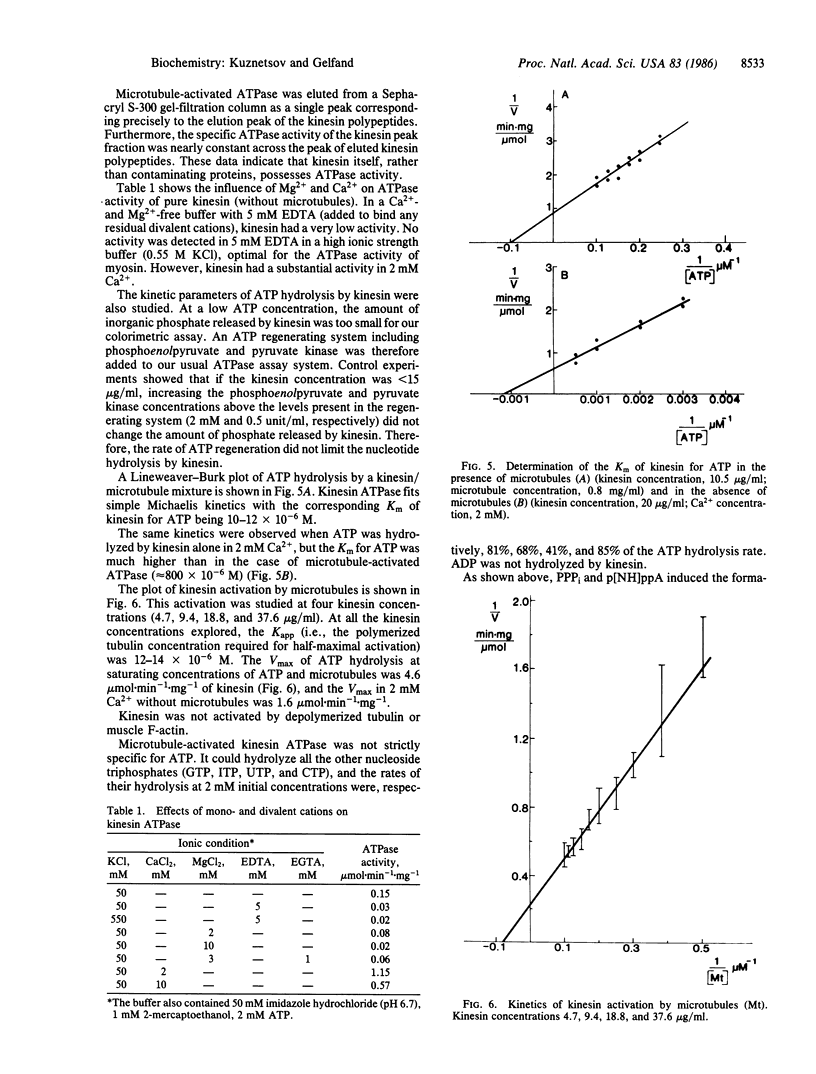

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Allen N. S. Video-enhanced microscopy with a computer frame memory. J Microsc. 1983 Jan;129(Pt 1):3–17. doi: 10.1111/j.1365-2818.1983.tb04157.x. [DOI] [PubMed] [Google Scholar]
- Allen R. D., Weiss D. G., Hayden J. H., Brown D. T., Fujiwake H., Simpson M. Gliding movement of and bidirectional transport along single native microtubules from squid axoplasm: evidence for an active role of microtubules in cytoplasmic transport. J Cell Biol. 1985 May;100(5):1736–1752. doi: 10.1083/jcb.100.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S. T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985 Sep 5;317(6032):73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J., Allen R. D. Fast axonal transport in extruded axoplasm from squid giant axon. Science. 1982 Dec 10;218(4577):1129–1131. doi: 10.1126/science.6183745. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasek R. J., Brady S. T. Attachment of transported vesicles to microtubules in axoplasm is facilitated by AMP-PNP. Nature. 1985 Aug 15;316(6029):645–647. doi: 10.1038/316645a0. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Omoto C. K., Johnson K. A. Activation of the dynein adenosinetriphosphatase by microtubules. Biochemistry. 1986 Jan 28;25(2):419–427. doi: 10.1021/bi00350a022. [DOI] [PubMed] [Google Scholar]
- Panusz H. T., Graczyk G., Wilmańska D., Skarzynski J. Analysis of orthophosphate-pyrophosphate mixtures resulting from weak pyrophosphatase activities. Anal Biochem. 1970 Jun;35(2):494–504. doi: 10.1016/0003-2697(70)90212-5. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin A. S. Energetics of the ribosome. Prog Nucleic Acid Res Mol Biol. 1978;21:39–62. doi: 10.1016/s0079-6603(08)60266-4. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Reese T. S., Sheetz M. P. Movement of organelles along filaments dissociated from the axoplasm of the squid giant axon. Cell. 1985 Feb;40(2):449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]