Abstract
Acute lung injury (ALI) is a major cause of mortality and morbidity worldwide. The activation of peroxisome proliferator-activated receptor-α (PPARα) by its ligands, which include Wy-14643, has been implicated as a potential anti-inflammatory therapy. To address the beneficial efficacy of Wy-14643 for ALI along with systemic inflammation, the in vivo role of PPARα activation was investigated in a mouse model of lipopolysaccharide (LPS)-induced ALI. Using age-matched Ppara-null and wild-type mice, we demonstrate that the activation of PPARα by Wy-14643 attenuated LPS-mediated ALI. This was evidenced histologically by the significant alleviation of inflammatory manifestations and apoptosis observed in the lung tissues of wild-type mice, but not in the corresponding Ppara-null mice. This protective effect probably resulted from the inhibition of LPS-induced increases in pro-inflammatory cytokines and nitroxidative stress levels. These results suggest that the pharmacological activation of PPARα might have a therapeutic effect on LPS-induced ALI.
Keywords: Peroxisome proliferator-activated receptor-α, Lipopolysaccharide, Acute Lung Injury, Cytokines, Nitroxidative stress
1. Introduction
Acute lung injury (ALI), a severe complication often observed in intensive care units, is a syndrome characterized by serial pulmonary inflammatory responses including alveolar, epithelial and vascular endothelial injury, leukocyte infiltration, interstitial edema, and proteinous leakage into the alveolar space. Lipopolysaccharide (LPS), which is discharged during systemic Gram-negative bacterial infections, is thought to play a crucial role in the development of ALI. Recent data showed that the crude incidence of ALI was 78.9 per 100 000 person-years, which is a severe burden in health-care management [1]. Despite its high mortality rate, there is currently no effective ALI therapy; therefore, there remains an urgent need for the discovery of definitive and targeted drug therapies for ALI. Studies designed to address this issue often rely on animal models of LPS-induced ALI, a valuable tool in ALI research. These models are generated via either local (intranasal or intratracheal) or systemic (intravenous and intraperitoneal) administration of LPS for a short period, which is usually no more than 48 h [2]. The development of ALI by systemic LPS administration is clinically suited for mimicking sepsis.
PPARα belongs to the nuclear receptor superfamily of ligand-activated transcription factors that regulate lipid metabolism, inflammation, and cell differentiation [3]. Growing evidence links PPARα with a potent anti-inflammatory activity through the control of NF-κB and JNK activation [4,5], signal transducer and activator of transcription 1 (STAT1)-related inflammatory signaling in cultured neuronal cells [6], and LPS-induced liver toxicity in PPARα-null mice [7]. Although the natural PPARα ligands are fatty acids, pharmacological activators such as Wy-14643 and its structural derivatives have been widely used for exerting potent stimulatory effects on PPARα in research settings [8]. Recently, the effect of PPARα activation by Wy-14643 in the ALI model using Ppara-null mice was reported [8]. In that study, pretreatment of the mice with Wy-14643 decreased the levels of macrophage inflammatory protein-2, tumor necrosis factor-α and eicosanoids, and improved lung compliance and vascular leakage in LPS-exposed mice. However, the study did not assess histopathological changes, apoptosis, the potential role of various cytokines, or nitrosative stress in systemic LPS-induced ALI, and did not fully investigate the potential protective effects of PPARα activation. Therefore, the aims of the present work are to address these unresolved issues. In this study, LPS was administered intraperitoneally to induce systemic inflammation and the effects of PPARα activation on ALI and serum cytokines and development of nitroxidative stress were observed.
2. Materials and methods
2.1 Animals
Homozygous female age-matched Ppara-null mice (129/Svj background) and wild-type (WT) mice [stock number 2448 from the Jackson Laboratory (Bar Harbor, ME, USA)] were used in this study. All mice were maintained in a controlled environment and fed standard rodent chow and water ad libitum. At the age of 8–10 weeks, the animals were randomly divided into six groups (n = 3–4/group). Mice in the first and third groups (WT mice) and fourth and sixth groups (null mice) were pre-treated with Wy-14643 (dissolved in corn oil) at a dose of 50 mg/kg body weight intraperitoneally (i.p.). Two hours later, the second and third groups (WT mice) and fifth and sixth groups (null mice) were treated with LPS (Escherichia coli, serotype 0111:B4; Sigma-Aldrich, St. Louis, MO, USA) at a dose of 10 mg/kg body weight i.p. Lung tissues were excised from the mice, which were killed by asphyxiation at 20 h after LPS injection or at 22 h for the Wy-14643-treated groups (the first and fourth groups). All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
2.2 Histopathological analysis
The lung specimens from each mouse were fixed in 10% neutral formalin and embedded in paraffin, and then 4-μm thick sections were stained with hematoxylin and eosin (H&E). To assess the levels of lung injury, 10 areas in the lung parenchyma were graded by an experienced pathologist who was blind to group assignment, as previously reported [7].
2.3 Homogenized tissue sample preparation
Lung tissues were homogenized in 5 volumes of ice-cold TE buffer (50 mM Tris-HCl, pH 7.5, and 1 mM EDTA) containing protease and phosphatase inhibitor cocktails (Calbiochem, La Jolla, CA, USA).
2.4 Determination of serum cytokines
Serum levels of the cytokines interleukin-6 (IL-6) and interferon-gamma (IFN-γ) were determined using cytometry beads arrays (BD Bioscience, Palo Alto, CA, USA) following the manufacturer’s protocol.
2.5 Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Apoptotic cell death in lung tissues was determined using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon International Inc., Temecula, CA, USA). The numbers of TUNEL-positive apoptotic cells in the lung were counted in 10 high-power (×200) microscope fields, and the mean was calculated.
2.6 Immunohistochemistry and immunoblotting
Anti-3-nitrotyrosine (3-NT) antibody (Biocare Medical, Concord, CA, USA) was used for immuohistostaining of 3-nitrotyrosine according to the manufacturer’s protocol.For immunoblot analysis, whole lung lysates (40 μg of protein) were separated by 12% SDS-PAGE and subjected to immunoblot analyses using a specific anti-3-NT antibody (1:1000 dilution; Abcam, Cambridge, MA, USA), as previously described [7]. After removal of the primary antibodies followed by washing steps, the nitrocellulose membranes were incubated with an HRP-conjugated secondary goat anti-mouse antibody (1:5000 dilution in TE-buffered saline containing 5% milk and 0.05% Tween 20). Protein bands were detected by enhanced chemiluminescence and their densities were quantified [7].
2.7 Determination of malondialdehyde
The amount of malondialdehyde (MDA), a well-established lipid peroxidation marker, was measured using the commercial kit from Oxford Biomedical Research Inc. (Oxford, MI, USA) following the manufacturer's protocol. The quantity of MDA (μM) in each sample was measured using 40 μg of each whole lung lysate.
2.8 Data evaluation
The results are expressed as mean ± standard deviation. Student’s t-tests were performed to compare quantitative data obtained from two different groups. A value of P < 0.05 was considered statistically significant.
3. Results
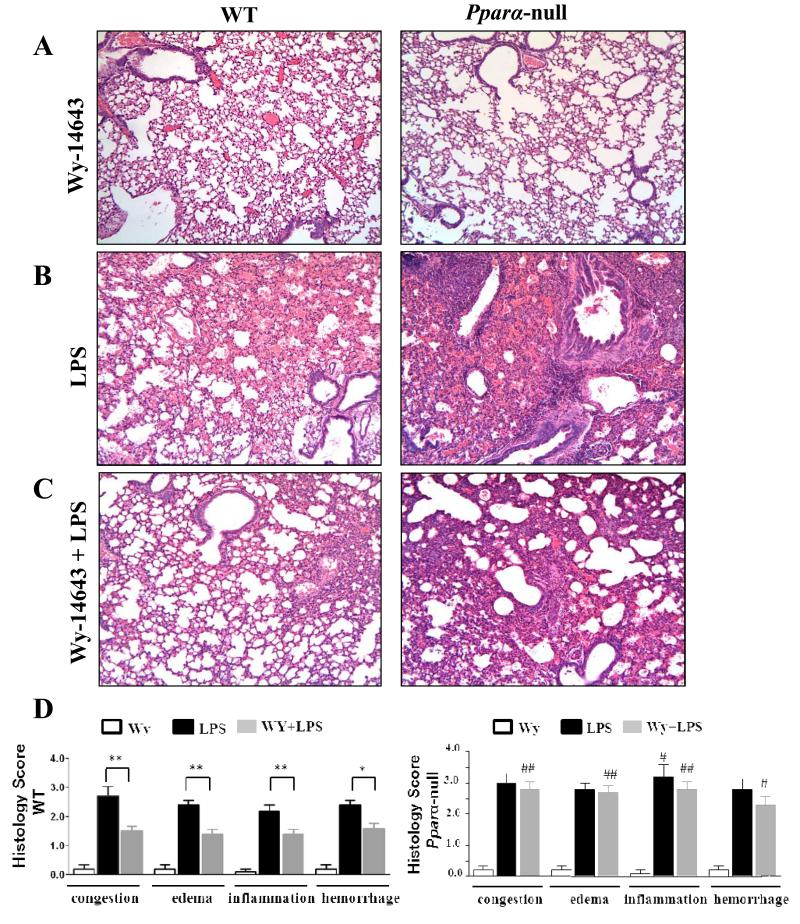
Histological analysis was performed on lung specimens from Ppara-null and WT mice to determine the effects of PPARα activation on LPS-induced ALI. Without the administration of LPS, no obvious differences were observed between Ppara-null and WT mice pretreated with the PPARα activator Wy-14643 (Fig. 1A). When LPS was administered without Wy-14643 pretreatment, alveolar hemorrhagic edema with infiltrations of inflammatory cells was found in the lungs of both mouse groups (Fig. 1B and D). However, the amount of neutrophil infiltrates in perivascular and alveolar areas in Ppara-null mice was significantly higher compared to that of WT mice (Fig. 1B and D). While a significant reduction in inflammatory cells infiltration and alveolar hemorrhagic edema were observed in lung sections from WT group mice with LPS and pretreated with Wy-14643, the LPS-mediated histologic damage in Ppara-null mice was not alleviated by pretreatment of Wy-14643 (Fig. 1C and D). Accordingly, the levels of congestion, edema, neutrophil infiltrates and alveolar hemorrhages in the Ppara-null mice with Wy-14643 and LPS were significantly higher than those of the WT mice (Fig. 1D).
Figure 1.
Histopathological changes from lung tissue, showing the effects of LPS with Wy-14643 on WT and PPARα null mice. (A-C) Hematoxylin-eosin (H&E) staining with magnification ×200: (A) WT and Pparα-null mice pretreated with only Wy-14643, (B) WT and Pparα-null mice after intraperitoneal LPS administration, (C) WT and Pparα-null mice with pretreatment of Wy-14643 and intraperitoneal LPS injection, (D) Histology scores of WT and Pparα-null mice. * P < 0.05 different between LPS and LPS with pretreatment of WY-14643; ** P < 0.01 different between LPS and LPS with pretreatment of WY-14643; # P < 0.05 different from the corresponding WT group; ## P < 0.01 different from the corresponding WT group.
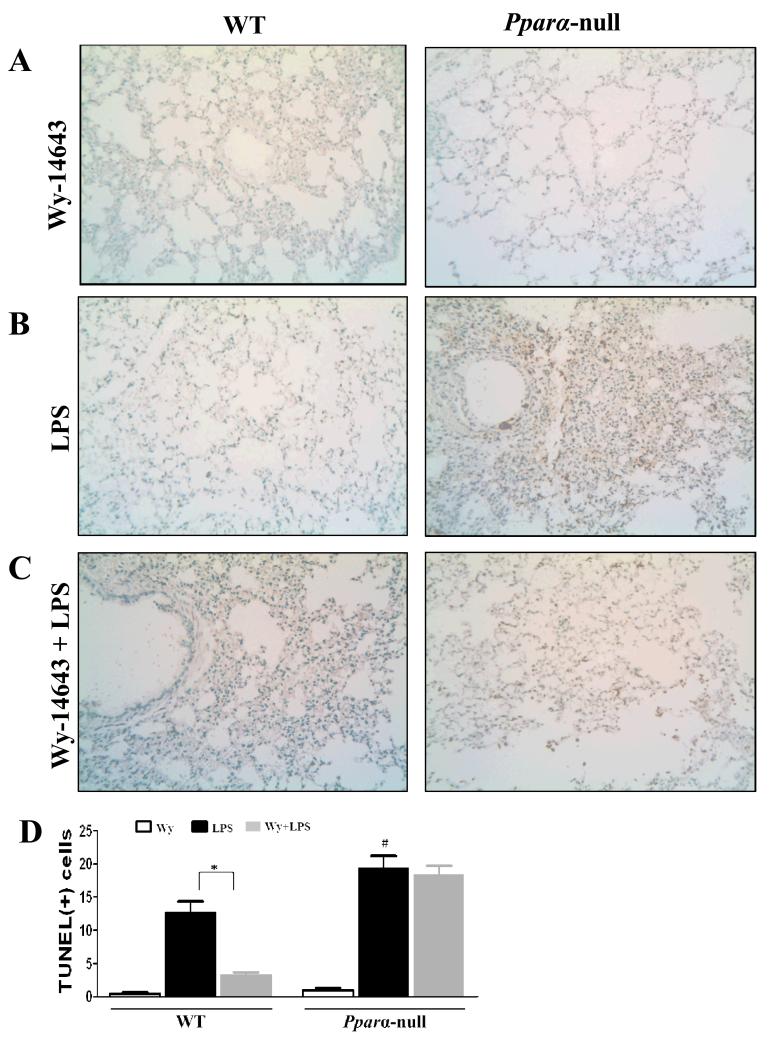
To determine whether pretreatment with Wy-14643 would differentially protect the mouse groups treated with LPS, TUNEL analysis was performed to detect apoptotic cells in lung tissue from the Ppara-null and WT mice (Fig. 2). While there were extremely few apoptotic cells in both mouse groups treated with Wy-14643 (Fig. 2A and D), many more TUNEL-positive pneumocytes and vascular endothelial cells were observed in LPS-exposed Ppara-null mice without Wy-14643 pretreatment than in the corresponding WT mice (Fig. 3B and D). Furthermore, Wy-14643 pretreatment significantly decreased the numbers of apoptotic cells in the LPS-exposed WT mice compared to the corresponding Ppara-null mice (Fig. 3C and D).
Figure 2.
Evaluation of apoptosis using TUNEL assays from lung sections in WT and Pparα-null mice with Wy-14643 and/or LPS. (A-C) TUNEL staining with magnification ×200: (A) WT and Pparα-null mice pretreated with Wy-14643, (B) WT and Pparα-null mice after intraperitoneal LPS administration, (C) WT and Pparα-null mice with pretreatment of Wy-14643 and intraperitoneal LPS injection, (D) Semiquantitative analysis of TUNEL-positive cells. Data are expressed as mean ± SEM of 3-4 mice/group. * P < 0.05 for WT after LPS administration compared to WT with Wy-14643; # P < 0.05 different from the corresponding WT group.
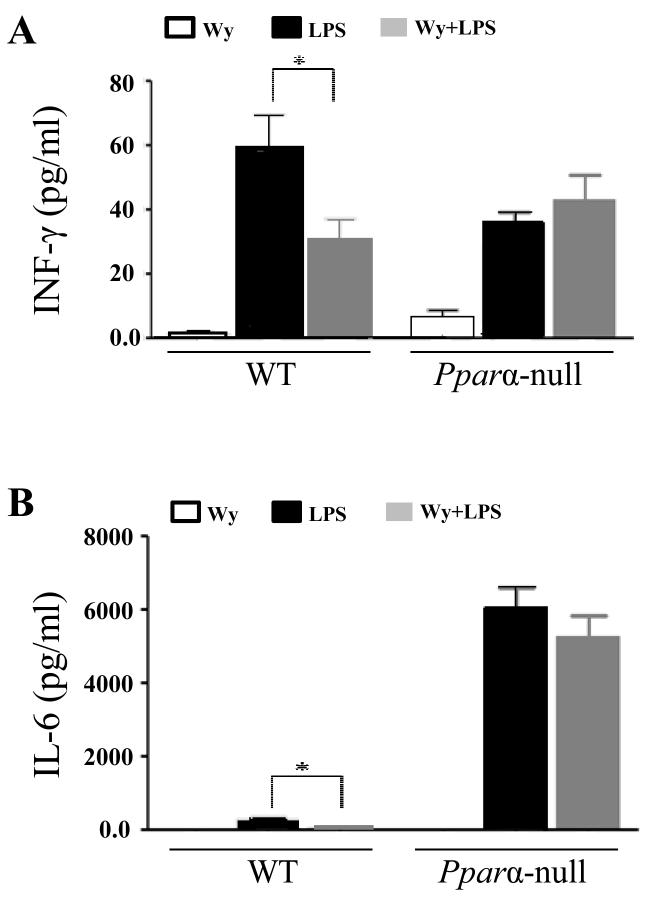
Figure 3.
Changes of serum levels of IFN-γ and IL-6 in response to LPS and Wy-14643 in WT and PPARα null mice. (A) IFN-γ levels and (B) IL-6 levels. Data are expressed as mean ± SEM of 3-4 mice/group. * P < 0.05 different between LPS and LPS with pretreatment of WY-14643.
To elucidate the mechanisms underlying the differential effects of Wy-14643 pretreatment on Ppara-null and WT mice, we analyzed the serum levels of two prominent pro-inflammatory cytokines, namely IFN-γ and IL-6. Compared to the LPS-exposed WT mice, serum levels of IFN-γ and IL-6 were significantly decreased in WT mice pretreated with Wy-14643 (Fig. 3A and B, respectively). In contrast, the cytokine levels were not significantly changed in LPS-exposed Ppara-null mice upon pretreatment with Wy-14643 (Fig. 3).
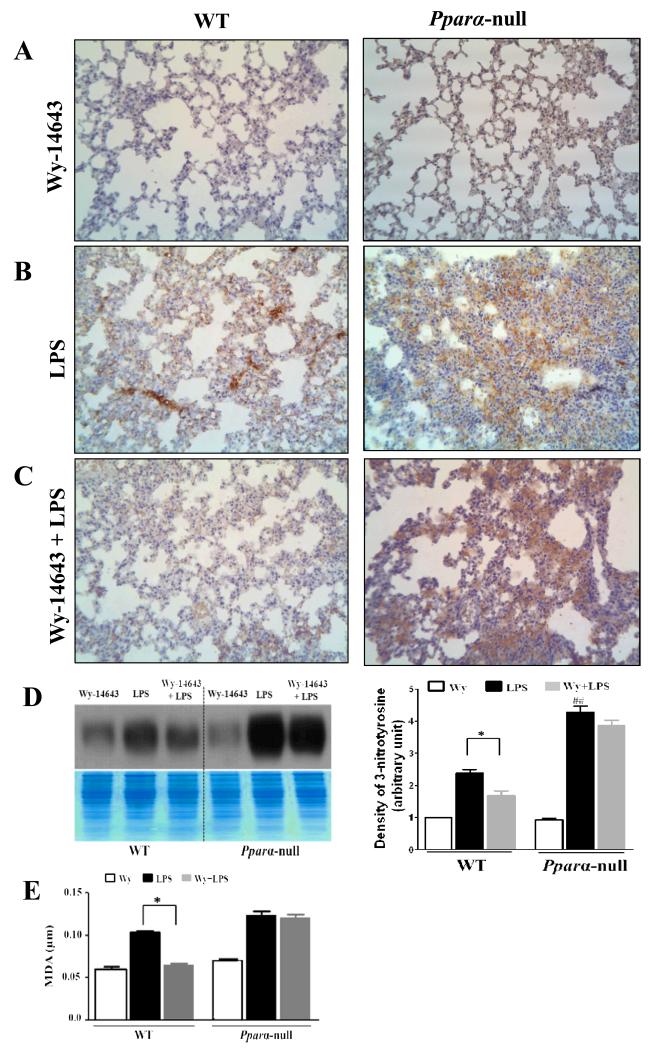
We have reported that nitrosative/oxidative stress plays a critical role in LPS-mediated liver damage [7]. To evaluate peroxynitrite and nitrative stress in the LPS-exposed lungs of Ppara-null and WT mice, we performed immunohistochemical analysis to detect 3-NT in formalin-fixed lung tissues (Fig. 4A-D). No significant differences were observed between the two groups treated with Wy-14643 alone (Fig. 4A). The highest levels of 3-NT staining were detected in sections from the lungs of Ppara-null mice exposed to LPS (Fig. 4B). However, pretreatment with Wy-14643 did not decrease the levels of 3-NT immunostaining in the lungs of Ppara-null mice (Fig. 4C). In contrast, the level of 3-NT staining was reduced in LPS-exposed WT mice pretreated with Wy-14643 compared to the corresponding WT mice that were not pretreated (Fig. 4B and C). Immunoblot analysis also showed a significant reduction of the increased 3-NT levels in the lungs of LPS-exposed WT mice pretreated with Wy-14643, compared to the corresponding Ppara-null mice (Fig. 4D). We also evaluated the effects of Wy-14643 pretreatment on the LPS-induced lipid peroxidation by measuring the levels of MDA in Ppara-null and WT mice. MDA levels were increased in LPS-exposed mice of both genotypes (Fig. 4E). Wy-14643 pretreatment significantly decreased the level of MDA in LPS-exposed WT mice, whereas lipid peroxidation was not reduced in the corresponding Ppara-null mice in response to Wy-14643 pretreatment (Fig. 4E).
Figure 4.
Evaluation of 3-nitrotyrosine levels and lipid peroxdiation in WT and PPARα null mice with Wy-14643 and/or LPS. (A-C) Immunohistochemical analysis of 3-nitrotyrosine, Magnification ×200: (A) WT and Pparα-null mice pretreated with Wy-14643, (B) WT and Pparα-null mice after intraperitoneal LPS administration, (C) WT and Pparα-null mice with pretreatment of Wy-14643 and intraperitoneal LPS injection, (D) Immunoblot analysis for 3-nitrotyrosine levels (top) compared to Coomassie-stained gels for similar protein loading per sample (bottom), (E) MDA as a marker for lipid peroxidation. Data are expressed as mean ± SEM of 3-4 mice/group. * P < 0.05 different between groups of LPS and LPS with pretreatment of WY-14643.
4. Discussion
The activation of PPARα plays a crucial role in the up-regulation of genes encoding proteins responsible for the adequate maintenance of healthy proteomes [10]. Both oral and dietary administrations of Wy-14643 were found to up-regulate genes encoding for various heat-stress inducible chaperone proteins and proteasome components in WT mice; this upregulation was PPARα-dependent, as these genes were not upregulated in the corresponding Ppara-null mice [10]. Based on our results, it is expected that WT mice containing the intact PPARα would prevent protein aggregation and/or efficiently remove damaged proteins via activation of these critical PPARα-dependent genes/proteins in critical situation such as ALI. In contrast, Ppara-null mice may have a deficiency in the maintenance of cellular proteomes and the elimination of damaged proteins due to the absence of PPARα. Hence, it is likely that the absence of PPARα and the critical genes/proteins needed for maintaining healthy proteomes in the Ppara-null mice may aggravate tissue damage, particularly when exposed to LPS or other toxic agents such as carbon tetrachloride, cadmium, or paraquat, as has been demonstrated [10].
ALI can be induced by various conditions including severe sepsis, severe bacterial pneumonia, and trauma. Among these, Gram-negative bacteria-induced sepsis is one of the leading causes of ALI [11]. LPS constitutes the main component of the outer membranes of Gram-negative bacteria, and when LPS is released into the circulation during sepsis, interconnected inflammatory cascades are activated in the lung, ultimately resulting in ALI. Thus, LPS has been implicated as the most important antigen contributing to the development of ALI. In this study, we aimed to study the role of PPARα and the beneficial effects of its agonist Wy-14643 on LPS-induced ALI with systemic inflammation, which is similar to ALI in clinical settings.
This study clearly demonstrated that pretreatment with Wy-14643 significantly reduced the degree of ALI in LPS-exposed WT mice, but not in Ppara-null mice. The histopathological findings of this study showed that inflammatory infiltrates, alveolar hemorrhagic edema and the number of apoptotic cells were significantly reduced in the lungs of LPS-exposed WT mice pretreated with Wy14643. Our previous study indicated that the absence of PPARα promotes the infiltration of inflammatory cells into hepatic tissue exposed to systemic administration of LPS [7]. Accordingly, a marked reduction of lung inflammation was observed in the present study as a result of the chemical activation of PPARα, suggesting the involvement of PPARα in ALI and systemic inflammation. In addition, several reports have indicated that the activation of PPARα reduced the number of leukocytes in bronchoalveolar lavage in ALI [8,12]. These observations are consistent with our study, which additionally showed that Wy-14643 pretreatment reduced the levels of serum proinflammatory cytokines including IFN-γ and IL-6. These cytokines are closely associated with the infiltration of acute inflammatory cells in ALI [13,14]. Increased levels of IFN-γ have been shown to exacerbate ALI by initiating the oxidative burst of reactive oxygen species production from neutrophils, and by increasing IL-6 production by alveolar macrophages [15]. Recently, it has been reported that the activation of PPARα represses IFN-γ production by human T cells, as PPARα is associated with modulating the accessibility of the IFNG gene locus [16]. Further, it has been demonstrated that the activation of PPARα can suppress the phosphorylation (activation) of STAT1 in neuronal inflammation [6]. Our previous results also showed that the lack of PPARα activates STAT1, leading to the increased levels of IFN-γ [7]. Additionally, the in vivo results of the present study clearly indicate that the activation of PPARα significantly reduced the IFN-γ and IL-6 levels, contributing to a decrease in inflammatory cell infiltration and inflammatory cascade in LPS-induced lung damage.
Peroxynitrite (ONOO−), which is produced by a reaction between NO and superoxide, has also been implicated in the initiation and progression of ALI [17]. Further, ONOO− is known to promote potentially noxious chemical reactions, including tyrosine nitration and lipid peroxidation. It has been also established that type II pnuemocytes undergo cell death following ONOO− administration [18]. Our findings that the levels of 3-nitrotyrosine (a footprint of peroxynitrite) and lipid peroxidation are decreased by Wy-14643 during LPS-induced ALI are consistent with the cytoprotective role of PPARα [10].
In summary, we found that the activation of PPARα by Wy-14643 ameliorated ALI in a mouse model of systemic LPS administration. Pretreatment with Wy-14643 significantly decreased the infiltration of inflammatory cells, apoptotic cells, and proinflammatory cytokine levels, while it also downregulated nitrosative/nitrative stress and lipid peroxidation. These results highlight the protective effects of PPARα and its agonists, which may be potential therapeutic targets for ALI and similar systematic inflammatory diseases.
Acknowledgements
This study was supported by a National Research Foundation grant funded by the Korean Government (800-20120365) and the Intramural Program of National Institute on Alcohol Abuse and Alcoholism.
Abbreviations
- ALI
acute lung injury
- PPARα
peroxisome proliferator-activated receptor-α
- LPS
lipopolysaccharide
- STAT1
signal transducer and activator of transcription 1
- MDA
malondialdehyde
Footnotes
Conflict of Interest: The authors declare no conflicts of interest
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev. Respir. Med. 2010;4:773–783. doi: 10.1586/ers.10.71. [DOI] [PubMed] [Google Scholar]
- 3.Francis GA, Fayard E, Picard F, Auwerx J. Nuclear receptors and the control of metabolism. Annu. Rev. Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- 4.Delerive P, De Bosscher K, Vanden Berghe W, Fruchart JC, Haegeman G, Staels B. DNA binding-independent induction of IkappaBalpha gene transcription by PPARalpha. Mol. Endocrinol. 2002;16:1029–1039. doi: 10.1210/mend.16.5.0826. [DOI] [PubMed] [Google Scholar]
- 5.Larter CZ, Yeh MM, Van Rooyen DM, Brooling J, Ghatora K, Farrell GC. Peroxisome proliferator-activated receptor-alpha agonist, Wy 14,643, improves metabolic indices, steatosis and ballooning in diabetic mice with non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2012;27:341–350. doi: 10.1111/j.1440-1746.2011.06939.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Joe EH, Jou I. lipopolysaccharide-activated rat glia. Neuroreport. 2005;16:829–833. doi: 10.1097/00001756-200505310-00010. [DOI] [PubMed] [Google Scholar]
- 7.Yoo SH, Park O, Henderson LE, Abdelmegeed MA, Moon KH, Song BJ. Lack of PPARalpha exacerbates lipopolysaccharide-induced liver toxicity through STAT1 inflammatory signaling and increased oxidative/nitrosative stress. Toxicol. Lett. 2011;202:23–29. doi: 10.1016/j.toxlet.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer MB, Pose A, Ott J, Hecker M, Behnk A, Schulz R, Weissmann N, Gunther A, Seeger W, Mayer K. Peroxisome proliferator-activated receptor-alpha reduces inflammation and vascular leakage in a murine model of acute lung injury. Eur. Respir. J. 2008;32:1344–1353. doi: 10.1183/09031936.00035808. [DOI] [PubMed] [Google Scholar]
- 9.Murakami K, McGuire R, Cox RA, Jodoin JM, Bjertnaes LJ, Katahira J, Traber LD, Schmalstieg FC, Hawkins HK, Herndon DN, Traber DL. Heparin nebulization attenuates acute lung injury in sepsis following smoke inhalation in sheep. Shock. 2002;18:236–241. doi: 10.1097/00024382-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SP, Howroyd P, Liu J, Qian X, Bahnemann R, Swanson C, Kwak MK, Kensler TW, Corton JC. The transcriptional response to a peroxisome proliferator-activated receptor alpha agonist includes increased expression of proteome maintenance genes. J. Biol. Chem. 2004;279:52390–52398. doi: 10.1074/jbc.M409347200. [DOI] [PubMed] [Google Scholar]
- 11.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr., Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am. J. Respir. Crit. Care Med. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 12.Delayre-Orthez C, Becker J, Guenon I, Lagente V, Auwerx J, Frossard N, Pons F. PPARalpha downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respir. Res. 2005;6:91. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 14.Shenkar R, Coulson WF, Abraham E. Hemorrhage and resuscitation induce alterations in cytokine expression and the development of acute lung injury. Am. J. Respir. Cell Mol. Biol. 1994;10:290–297. doi: 10.1165/ajrcmb.10.3.8117448. [DOI] [PubMed] [Google Scholar]
- 15.Miller DL, Welty-Wolf K, Carraway MS, Ezban M, Ghio A, Suliman H, Piantadosi CA. Extrinsic coagulation blockade attenuates lung injury and proinflammatory cytokine release after intratracheal lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 2002;26:650–658. doi: 10.1165/ajrcmb.26.6.4688. [DOI] [PubMed] [Google Scholar]
- 16.Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, Akkermann R, Stanczyk FZ, Prat A, Steinman L, Dunn SE. Peroxisome proliferator-activated receptor (PPAR)alpha and -gamma regulate IFNgamma and IL-17A production by human T cells in a sex-specific way. Proc. Natl. Acad. Sci. U S A. 2012;109:9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad IY, Pataki G, Hu P, Galliani C, Beckman JS, Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J. Clin. Invest. 1994;94:2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandari V, Johnson L, Smith-Kirwin S, Vigliotta G, Funanage V, Chander A. Hyperoxia and nitric oxide reduce surfactant components (DSPC and surfactant proteins) and increase apoptosis in adult and fetal rat type II pneumocytes. Lung. 2002;180:301–317. doi: 10.1007/s00408-002-0102-y. [DOI] [PubMed] [Google Scholar]