Abstract
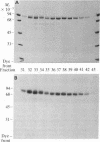
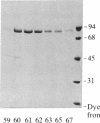
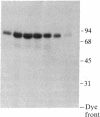
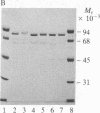
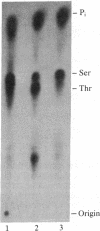
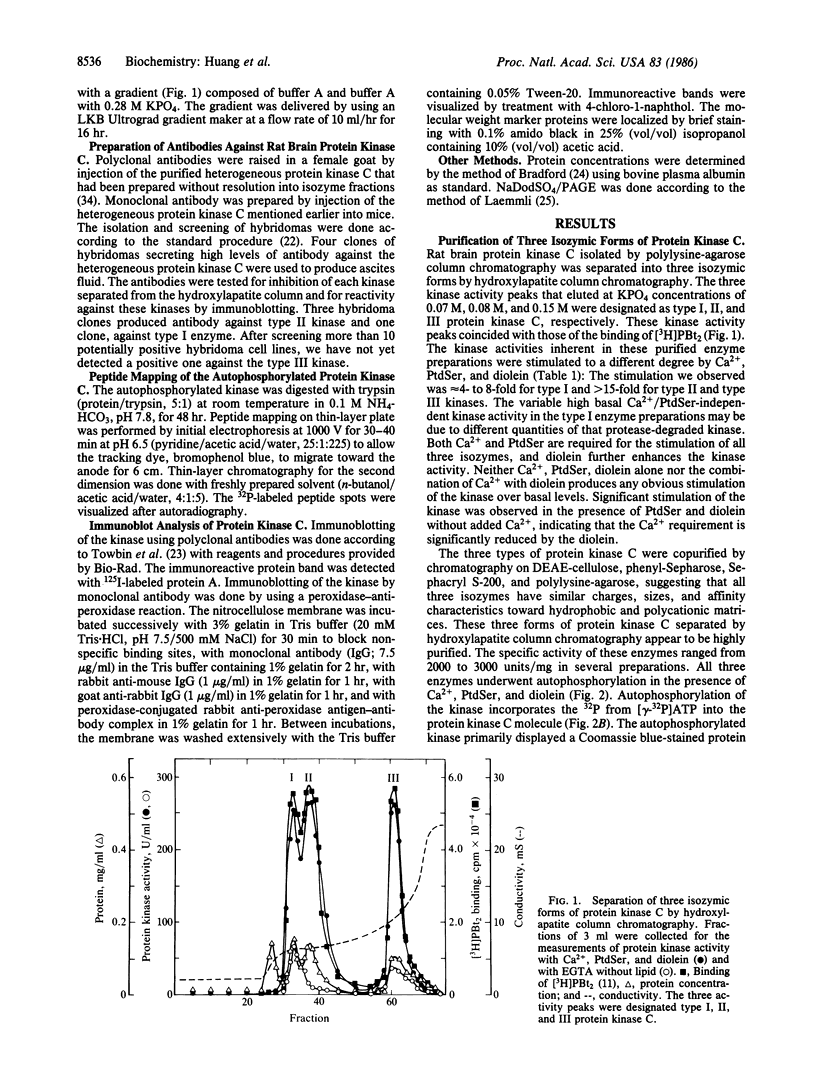
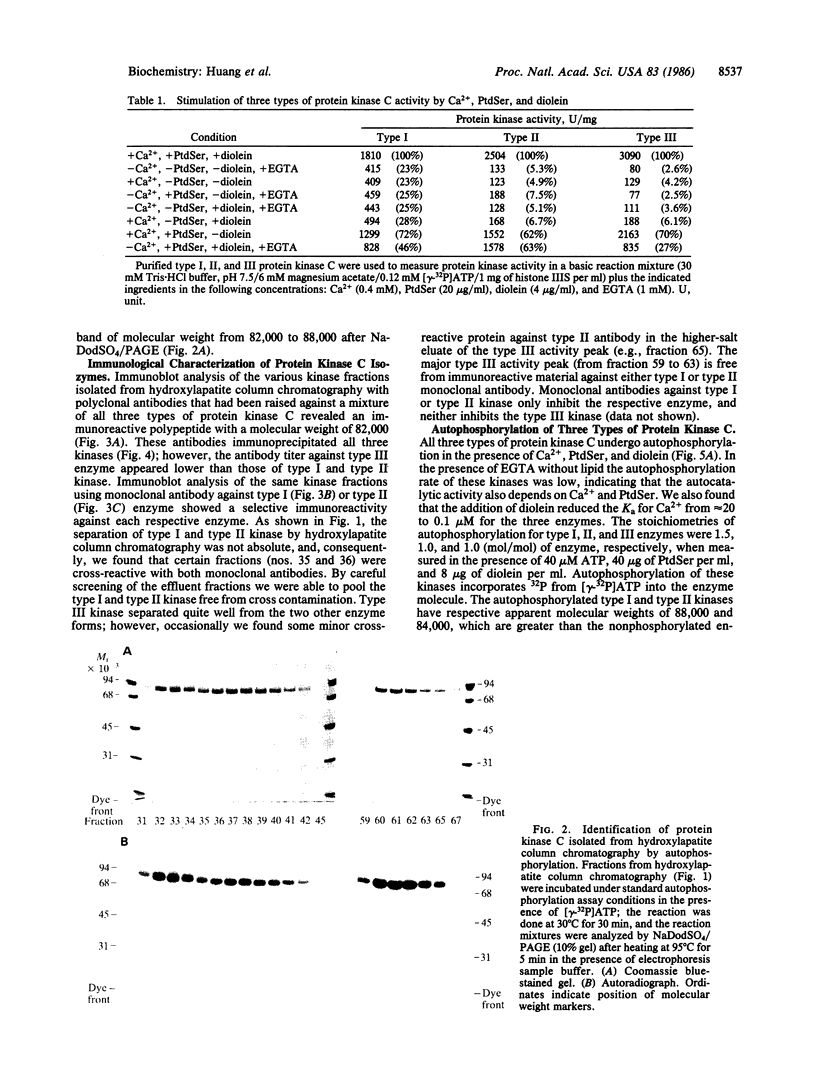
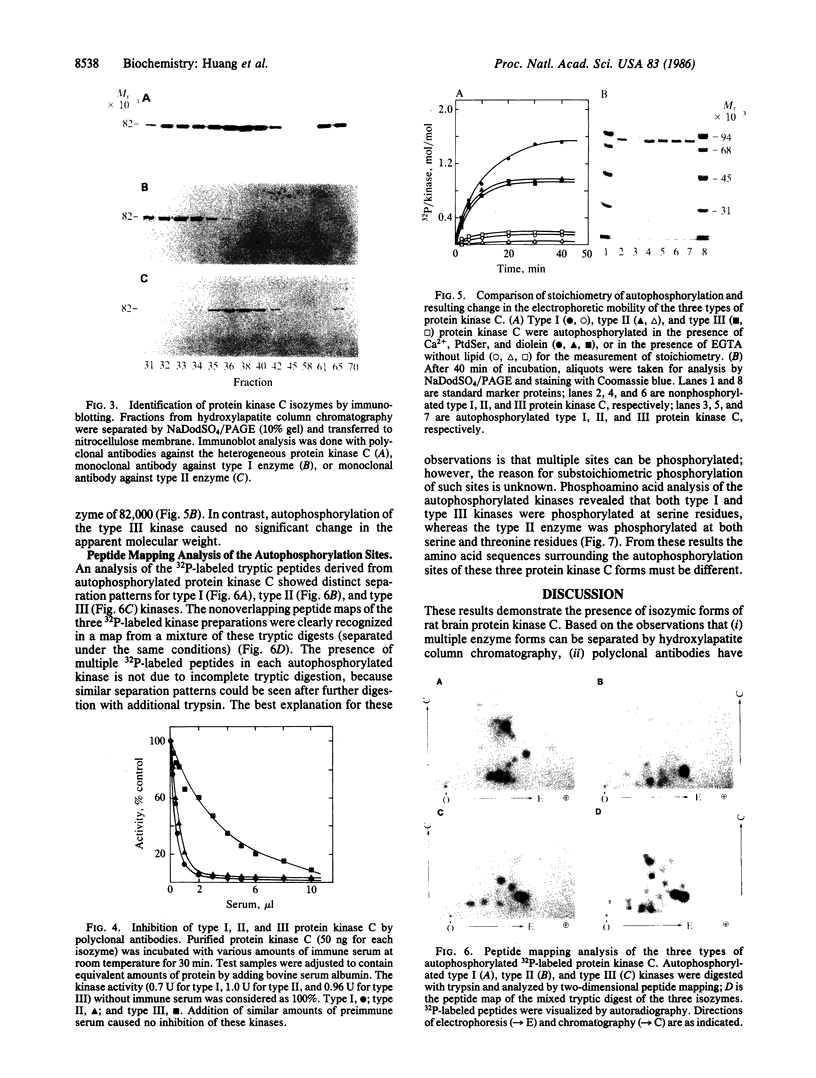
Three forms of rat brain Ca2+-activated and phospholipid-dependent protein kinase (EC 2.7.1.37) were separated by hydroxylapatite column chromatography. These enzymes, designated type I, II, and III protein kinase C, all have a molecular weight of 82,000, undergo autophosphorylation in the presence of Ca2+, phosphatidylserine, and diolein, and bind [3H]phorbol 12,13-dibutyrate. Autophosphorylation of these kinases resulted in an incorporation of 1-1.5 mol of 32P per mol of enzyme. Two-dimensional peptide mapping analysis revealed that these kinases had different sites of autophosphorylation. Phosphoamino acid analysis showed that type I and type III protein kinase C primarily autophosphorylated at a serine residue, whereas type II kinase autophosphorylated at both serine and threonine residues. In addition, polyclonal antibodies raised against a mixture of three types of the kinase preferentially inhibited type I and type II enzymes. Monoclonal antibodies against type I and type II kinase only recognized their respective enzymes but not the type III enzyme. These results demonstrate the presence of isozymic forms of protein kinase C in rat brain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Bechtel P. J., Krebs E. G. Mechanisms of control for cAMP-dependent protein kinase from skeletal muscle. Adv Cyclic Nucleotide Res. 1975;5:241–251. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Sul H. S., Walsh D. A. Phosphorylation and activation of the cardiac isoenzyme of phosphorylase kinase by the cAMP-dependent protein kinase. J Biol Chem. 1981 Aug 10;256(15):8030–8038. [PubMed] [Google Scholar]
- Huang K. P., Chan K. F., Singh T. J., Nakabayashi H., Huang F. L. Autophosphorylation of rat brain Ca2+-activated and phospholipid-dependent protein kinase. J Biol Chem. 1986 Sep 15;261(26):12134–12140. [PubMed] [Google Scholar]
- Huang K. P., Robinson J. C. A rapid and sensitive assay method for protein kinase. Anal Biochem. 1976 May 7;72:593–599. doi: 10.1016/0003-2697(76)90571-6. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Watanabe M., Hidaka H. N-(2-Aminoethyl)-5-isoquinolinesulfonamide, a newly synthesized protein kinase inhibitor, functions as a ligand in affinity chromatography. Purification of Ca2+-activated, phospholipid-dependent and other protein kinases. J Biol Chem. 1985 Mar 10;260(5):2922–2925. [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Jennissen H. P., Heilmeyer L. M., Jr Multiple forms of phosphorylase kinase in red and white skeletal muscle. FEBS Lett. 1974 May 15;42(1):77–80. doi: 10.1016/0014-5793(74)80283-8. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness T. L., Lai Y., Greengard P. Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J Biol Chem. 1985 Feb 10;260(3):1696–1704. [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J Biol Chem. 1985 Jul 25;260(15):9039–9046. [PubMed] [Google Scholar]
- Minakuchi R., Takai Y., Yu B., Nishizuka Y. Widespread occurrence of calcium-activated, phospholipid-dependent protein kinase in mammalian tissues. J Biochem. 1981 May;89(5):1651–1654. doi: 10.1093/oxfordjournals.jbchem.a133362. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzman R. C., Raynor R. L., Fritz R. B., Kuo J. F. Purification to homogeneity, characterization and monoclonal antibodies of phospholipid-sensitive Ca2+-dependent protein kinase from spleen. Biochem J. 1983 Feb 1;209(2):435–443. doi: 10.1042/bj2090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Inoue M., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977 Nov 10;252(21):7603–7609. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Takio K., Smith S. B., Krebs E. G., Walsh K. A., Titani K. Primary structure of the regulatory subunit of type II cAMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2544–2548. doi: 10.1073/pnas.79.8.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Sasagawa T., Ericsson L. H., Kumar S., Smith S. B., Krebs E. G., Walsh K. A. Amino acid sequence of the regulatory subunit of bovine type I adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1984 Aug 28;23(18):4193–4199. doi: 10.1021/bi00313a028. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Filburn C. R. Affinity chromatography of protein kinase C-phorbol ester receptor on polyacrylamide-immobilized phosphatidylserine. J Biol Chem. 1984 Oct 25;259(20):12311–12314. [PubMed] [Google Scholar]
- Wise B. C., Raynor R. L., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. I. Purification and general properties. J Biol Chem. 1982 Jul 25;257(14):8481–8488. [PubMed] [Google Scholar]
- Wolf M., Sahyoun N., LeVine H., 3rd, Cuatrecasas P. Protein kinase C: rapid enzyme purification and substrate-dependence of the diacylglycerol effect. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1268–1275. doi: 10.1016/0006-291x(84)91229-4. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Kerlavage A. R., Taylor S. S. Structural comparisons of cAMP-dependent protein kinases I and II from porcine skeletal muscle. J Biol Chem. 1979 Apr 10;254(7):2408–2412. [PubMed] [Google Scholar]