Abstract
L-amino acid oxidase (LAAO) has important biological roles in many organisms, thus attracting great attention from researchers to establish its detection methods. In this study, a new quantitative in-gel determination of LAAO activity based on ferric-xylenol orange (FeIIIXO) formation was established. This method showed that due to the conversion of FeII to FeIII by H2O2 and subsequent formation of FeIIIXO complex halo in agar medium, the logarithm of H2O2 concentration from 5 to 160 µM was linearly correlated to the diameter of purplish red FeIIIXO halo. By extracting the LAAO-generated H2O2 concentration, the LAAO activity can be quantitatively determined. This FeIIIXO agar assay is highly sensitive to detect H2O2 down to micromolar range. More importantly, it is easy to handle, cheap, reproducible, convenient and accurate. Coupled with SDS-PAGE, it can directly be used to determine the number and approximate molecular weight of LAAO in one assay. All these features make this in-gel FeIIIXO assay useful and convenient as a general procedure for following enzyme purification, assaying fractions from a column, or observing changes in activity resulting from enzyme modifications, hence endowing this method with broad applications.
Introduction
L-amino acid oxidases (LAAOs; EC 1.4.3.2) function in catalyzing the transformation of L-amino acids to the corresponding a-keto acids with the release of ammonium and hydrogen peroxide (H2O2) [1], [2]. Ever since the first discovery of LAAO from the bacterium Proteus vulgaris [3], LAAOs have been isolated from diverse organisms including snake venoms [4], insect drugs [5], sea hare [6], fungi [7], bacteria [8], [9] and algae [10]. LAAOs show broad biological activities including apoptosis, cytotoxicity, edema, hemolysis, hemorrhage, platelet aggregation, parasite-killing activity and antimicrobial activity, all of which are believed to be associated with the H2O2 production [11], [12]. LAAO activity has been characterized by quantifying the substances that are either consumed or generated in the redox reaction [13]–[15]. Among them, H2O2, one of the oxidative reaction products, is considered as an ideal substance for the detection of LAAO activity. The quantitative detection of H2O2 is mostly done by measuring the chemiluminescence due to the addition of horseradish peroxidase (HRP) and its substrate. There exists the commercially available kit (Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit, Invitrogen, USA), with detection limit down to micromolar level. [16]. However, most of the HRP substrates are mutagenic, carcinogenic or extremely toxic compounds, and HRP itself is easily inactivated and very expensive. Recently, we established a Prussian blue agar assay for quantitatively determining the LAAO activity [17]. In brief, iron (III) and potassium hexacyanoferrate (III) in the assay can be oxidized to yield the blue precipitate of Prussian blue where the H2O2 produced by LAAO activity acts as electron donor. Although the Prussian blue agar assay is cheaper and more convenient than the HRP-based assays, there are still several drawbacks that may limit its further application. Firstly, its quantitative detection limit is only down to about 0.5 mM level of H2O2. Secondly, potassium hexacyanoferrate (III) itself is safe, but under the peracidic condition it may degrade and release extremely toxic CN−. Thirdly, Prussian blue is a complicate class of chemical compounds containing Prussian blue, Prussian brown, Prussian white and Berlin green, which is sensitive to pH condition. Therefore, extremely careful pH adjustment in the Prussian agar preparation is required to produce reproducible result of color formation. It is desirable to develop an assay combining the advantages of HRP-based and Prussian blue-based measurement.
Like Prussian blue, xylenol orange (XO, 3, 3′-Bis[N,N-bis(carboxymethyl) aminomethyl]-o-cresolsulfonephthalein) is one of the most important color materials. Considering that ferrous ion (FeII) can be oxidized to ferric ion (FeIII) in the presence of H2O2, previous studies have demonstrated that XO can be applied to measure H2O2 by spectrophotometrically analyzing the purplish red complex (ferric-xylenol orange, FeIIIXO) that is formed by FeIII and XO [18]. This XO-based assay in solution can detect down to micromolar level of H2O2 [18], thus in theory providing higher H2O2 sensitivity, compared to Prussian blue agar assay. The purpose of this study is to describe a new application of the FeIIIXO formation for quantitatively determining the LAAO activity by in-gel visualization and measurement of H2O2. This new FeIIIXO assay is not only comparative to the HRP-involved assay in terms of sensitivity, but also bears similar benefits of Prussian blue agar assay, including ease of handling and cost-effectiveness. Moreover, it can be directly used for in-gel determination of the number and molecular weight of LAAO on the SDS-PAGE after visualization of the purplish red FeIIIXO complex.
Results
FeIIIXO complex formation can be used to detect the concentration of hydroperoxides [18]. FeII can be oxidized by H2O2 to FeIII which will sequentially coordinate with XO to yield purplish red FeIIIXO complex as shown below.
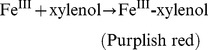 |
These reactions are fast, sensitive and reproducible. The concentration of H2O2 can usually be determined by spectrophotometrically measuring purplish red color of FeIIIXO complex in solution [19]. In the present study, we, for the first time, tried to adapt the FeIIIXO assay in agar gel to extracting the concentration of standard H2O2 or H2O2 produced by LAAO activity through directly measuring the diameter of the purplish red halo.
The formation of FeIIIXO complex requires acidic condition where 25 mM H2SO4 is usually appropriate for pH capacity of spectrophotometrical FeIIIXO assay in solution [20]. However, in our solid FeIIIXO agar assay, 3–10 mM H2SO4 were proper for acidic condition and gave purplish red halos with almost saturated diameter driven by 40 µM H2O2 (Figure S1). No purplish red zone of FeIIIXO was formed if the agar medium was devoid of H2SO4. On the other hand, further increase of H2SO4 concentration in assay agar resulted in the decrease of the size of purplish red halo. Since the color of purplish red zone of FeIIIXO on assay agar with 3 mM H2SO4 was blurred (data not shown), the proper concentration of H2SO4 for FeIIIXO agar assay is 6 mM up to 10 mM, giving final pH of 3.5 down to 2.3. After pouring, the FeIIXO agar plates consistently gave shallow orange red color as XO (pH indicator) itself will show orange red under acidic condition.
FeSO4 and XO are the two major elements in FeIIXO agar. To investigate the effect of the molar ratio of FeSO4 to XO on the FeIIIXO agar assay, FeSO4 with different concentrations from 0 to 0.4 mM were added to assay medium while XO was fixed at 0.15 mM. As shown in Figure S2, at 40 µM H2O2 condition, the diameter of purplish red halo increased with higher concentration of FeSO4 and appeared to a maximum when the FeSO4 concentration reached 0.25 mM. Further increase of FeSO4 concentrations from 0.25 mM to 0.4 mM cannot obviously enlarge the purplish red zone, indicating that the FeIIIXO was saturated when the molar ratio of FeSO4 to XO reached 5∶3.
Since FeIIXO agar medium was pH-sensitive, we also investigated the effect of pH on the color development of FeIIIXO agar. The background solutions with different pH values ranging from 1 to 14 were prepared by mixing 6N HCl with 6N NaOH as required. As indicated in row 1 of Figure 1, the background solutions with pH from 3 to 11 did not cause noticeable color change of FeIIIXO agar, remaining the original orange red. However, both lower pH and higher pH did make the color change of the agar medium. The background solutions with pH≤2 yielded lemon yellow halos, most likely due to the color presentation of XO as a pH indicator under peracidic condition. The lower the pH, the bigger and stronger the lemon yellow halos. On the other hand, when the background pH was above 12, the purplish red halos were generated even without H2O2 treatment (row 1 of Figure 1). There are two possible reasons: (1) as a pH indicator, XO will present a color of purplish red when pH≥12; (2) when pH≥12, FeII could easily be oxidized to FeIII by the oxidant like oxygen and subsequently form purplish red FeIIIXO with XO. The higher the pH from 12 to 14, the bigger and stronger the purplish red halos. Similarly, 20 µM H2O2 solutions with different pH values from 1 to 14 yielded different resultant halos (row 2 in Figure 1). Both the peracidic (pH≤2) and peralkaline (pH≥12) conditions had remarkable influence on the color development of assay agar under H2O2 pressure, while almost uniform sizes of purplish red halos were observed under 20 µM H2O2 with pH from 3 to 11. On the other hand, the 14 standard 20 µM H2O2 solutions with the same pH of 7.5 expectedly emerged consistent and reproducible purplish red halos with uniform size (row 3 in Figure 1). Similarly, the 14 oxidization reactions of L-Leu by LAAO from the LAAO-producer Psudoalteromonas sp. R3 (R3-LAAO) with the same pH of 7.5 also gave uniform and reproducible purplish red halos caused by the released H2O2 (row 5 in Figure 1). However, the oxidation solutions of L-Leu by R3-LAAO with different pH values adjusted to 1∼14 with HCl or NaOH after reactions yielded different resultant halos (row 4 in Figure 1). The pH values both ≤3 and ≥12 all obviously inhibited the formation of purplish red FeIIIXO. In contrast, the adjusted pH ranging from 4 to 11 had no obvious inhibition. All these findings indicate that the proper pH of detection solution is 4 up to 11.
Figure 1. Dependence of detection solution pH for detection of standard H2O2 or H2O2 caused by LAAO activity in FeIIIXO agar assay.
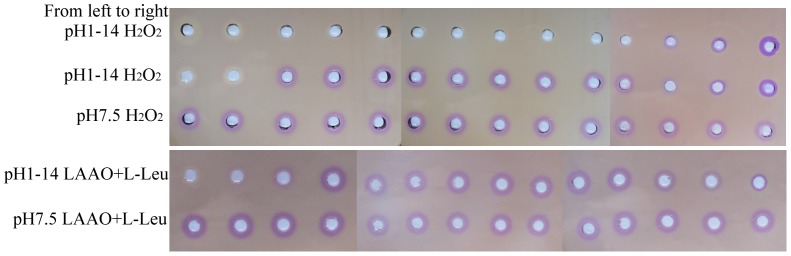
Row 1: 14 background solutions with different pH values from 1 to 14 (from left to right) prepared by a mixture of 6 N HCl and 6 N NaOH; row 2: 14 standard 20 µM H2O2 solutions with different pH values ranging from 1 to 14 (from left to right) adjusted by either HCl or NaOH; row 3: 14 standard 20 µM H2O2 solutions with uniform pH of 7.5; row 4: 14 individual oxidization reactions of L-Leu by LAAO from Psudoalteromonas sp. R3 (R3-LAAO) with different pH values from 1 to 14 (from left to right) adjusted by either HCl or NaOH after oxidation; row 5: 14 individual oxidization reactions of L-Leu by R3-LAAO with uniform pH of 7.5.
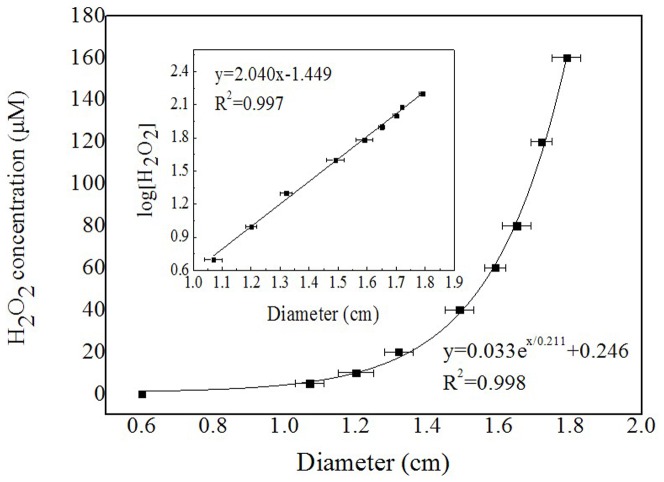
To quantify the LAAO activity, we first prepared a series of standard H2O2 solutions with different concentrations ranging from 0.5 µM to 250 µM with the same pH of 7.5 for the FeIIIXO agar assay. As displayed in Table 1, all the tested H2O2 gave purplish red zones, but with different sizes. The higher the H2O2 concentrations from 0.5 µM to 160 µM, the bigger the diameters of purplish red halos, indicating that FeIIIXO agar assay is extremely sensitive and the diameters of the purplish red halos are positively associated with H2O2 concentrations over 0.5 µM to 160 µM. However, further increase of H2O2 concentration to 200 µM and even up to 250 µM did not obviously make the purplish red halo bigger, probably due to the saturation of FeIIIXO agar by H2O2. The statistical analysis of mean difference of the purplish red halo diameters under different H2O2 concentrations showed that the increase of the halo diameter was extremely significant (P<0.001) with the increase of H2O2 concentration from 0 to 160 µM, but not significant (P>0.05) with the further increase of H2O2 concentration from 160 µM to 250 µM (Supplementary Table S1). To extract the correlation between the H2O2 concentrations ranging from 0 to 160 µM and diameters of purplish red halos, the data in Table 1 were plotted as displayed in Figure 2. The distribution can be fitted with an exponential equation y = 0.033e×/0.241+0.246 (R2 = 0.998), where x is the diameter and y the H2O2 concentration. Further plotting in Figure 2 inset showed that the change in diameter of the halo was a function of logarithm of the H2O2 concentration in a range of 5 µM to 160 µM with linear fit under an equation y = 2.049×−1.460 (R2 = 0.997), where x is the diameter and y the logarithm of H2O2 concentration.
Table 1. The diameters of the purplish red halos under different concentrations of H2O2.
| H2O2 concentration (µM) | log[H2O2] | Diameter (cm) |
| 5 | 0.70 | 1.07±0.03 |
| 10 | 1.00 | 1.20±0.02 |
| 20 | 1.30 | 1.32±0.02 |
| 40 | 1.60 | 1.49±0.03 |
| 60 | 1.78 | 1.59±0.03 |
| 80 | 1.90 | 1.65±0.01 |
| 120 | 2.08 | 1.72±0.03 |
| 160 | 2.20 | 1.79±0.01 |
| 200 | 2.30 | 1.79±0.01 |
| 250 | 2.40 | 1.79±0.01 |
Figure 2. Correlation between the detected H2O2 concentration and the corresponding diameter of purplish red FeIIIXO halo.
The distribution can perfectly be fitted with an exponential equation y = 0.033e×/0.241+0.246 (R2 = 0.998), where x is the diameter of the purplish red halo and y the H2O2 concentration. Further plotting in inset displayed that the change in diameter of the purplish red halo was a function of logarithm of the H2O2 concentration in the range of 5 µM to 160 µM with linear fit under an equation y = 2.049×−1.460 (R2 = 0.997), where x is the diameter of the purplish red halo and y the logarithm of H2O2 concentration.
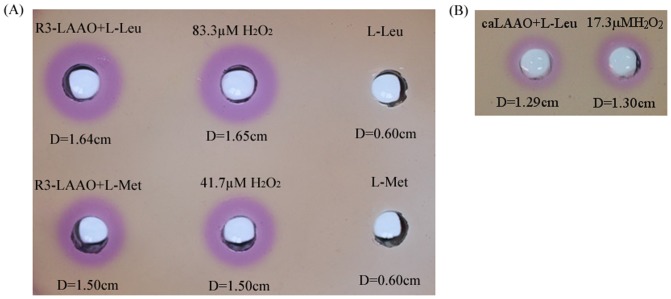
To evaluate the LAAO activity determined as H2O2 concentration fashion using the above extracted equation, R3-LAAO was used to oxidize the substrates L-Leu and L-Met, respectively, in separate reactions. After oxidization, the reaction solution was diluted 50 times and then subjected to FeIIIXO agar assay. The results in Figure 3A showed that R3-LAAO with both L-Leu and L-Met as substrates can yield purplish red halos with diameters of 1.64 cm and 1.50 cm, respectively, which correspond to the H2O2 concentrations of 83.3 µM and 41.7 µM, respectively, on the basis of the above extracted equation in Figure 2. In contrast, without R3-LAAO, both L-Leu and L-Met (negative controls in right holes) did not give purplish red halos. To confirm the reliability of the extracted results, the standard H2O2 with concentrations of 83.3 µM and 41.7 µM were applied to FeIIIXO agar assay and finally yielded the purplish red halos with diameters of 1.65 cm and 1.50 cm, respectively, all agreeing with our calculated concentrations. Therefore, the R3-LAAO activities with L-Leu and L-Met as substrates were 0.833 U/mL and 0.417 U/mL, respectively. All these findings indicate that the FeIIIXO agar assay is feasible to sensitively detect the H2O2 produced by LAAO activity and the extracted equation is reliable to quantitatively determine the LAAO activity. To verify this method, another enzyme source, the commercial Crotalus adamanteus venom LAAO (caLAAO) was also used to oxidize L-Leu and applied to FeIIIXO agar assay after 400 times dilution. Figure 3B showed that the oxidization solution of L-Leu by caLAAO gave a purplish red halo with 1.29 cm diameter which corresponds to 17.3 µM H2O2 based on the extracted equation. When 17.3 µM standard H2O2 was applied to FeIIIXO agar assay, a purplish red zone with 1.30 cm diameter appeared. To further confirm its reliability, the oxidization solution of L-Leu by caLAAO was serially diluted by 100 times, 200 times, 300 times and 400 times, and subsequently subjected to FeIIIXO agar assay. The results (Figure S3) showed that all detection solutions (in left holes) with different dilutions yielded purplish red halos with diameter of 1.60 cm, 1.46 cm, 1.38 cm and 1.30 cm, respectively, which correspond to the H2O2 concentration of 63.3 µM, 34.5 µM, 23.0 µM and 17.5 µM, respectively, representing the original H2O2 concentration in oxidization solution of 6.33 mM, 6.90 mM, 6.90 mM and 7.00 mM, respectively. As expected, the standard H2O2 (in right holes) with different concentrations of 63.3 µM, 34.5 µM, 23.0 µM and 17.5 µM gave purplish red halos with diameters of 1.60 cm, 1.46 cm, 1.38 cm and 1.30 cm, respectively, all agreeing with our calculated results. All these findings indicate that FeIIIXO agar assay is very reliable for quantitatively detecting the LAAO activity.
Figure 3. Characterization of LAAO activities from Pseudoalteromonas sp. R3 (R3-LAAO) with L-Leu and L-Met as substrates, respectively (A) and from Crotalus adamanteus venom (caLAAO) with L-Leu as substrate (B) based on the FeIIIXO agar assay.
On the basis of the diameters of the formed purplish red FeIIIXO halos, the concentrations of H2O2 produced by LAAO activities were calculated with the equations in Figure 2. The corresponding standard H2O2 solutions as indicated above the corresponding holes were used to confirm the accuracy of FeIIIXO agar assay. The diameters of the purplish red halos were marked below the holes. Without R3-LAAO, both L-Leu and L-Met (negative controls) did not give purplish red FeIIIXO halos (right holes).
Compared with Prussian blue agar assay, whose quantitative detection limit of H2O2 concentration is around 500 µM [17], the FeIIIXO agar assay is much more sensitive (around 100 times higher). To further compare their sensitivity, R3-LAAO was used to oxidize L-Cys, L-Glu, L-Asp, L-Val, L-Ala, L-Ser, L-Gly and L-Pro, in separate reactions, and subsequently the generated H2O2 was measured with both Prussian blue agar assays and FeIIIXO agar assay. Results showed that no clear color was developed in Prussian blue agar plate (Figure 4A), suggesting that R3-LAAO has no obvious oxidization activity to those substrates. However, FeIIIXO agar assay resulted in clear purplish red halos with L-Glu, L-Asp, L-Val, L-Ala and L-Ser as substrates (Figure 4B), indicating that R3-LAAO has activity to those substrates with an order of L-Asp>L-Glu>L-Val>L-Ser>L-Ala. All these findings indicate that the FeIIIXO agar assay is much more sensitive than the Prussian blue agar assay.
Figure 4. Comparison of detection sensitivity between Prussian blue agar assay (A) and FeIIIXO agar assay (B).
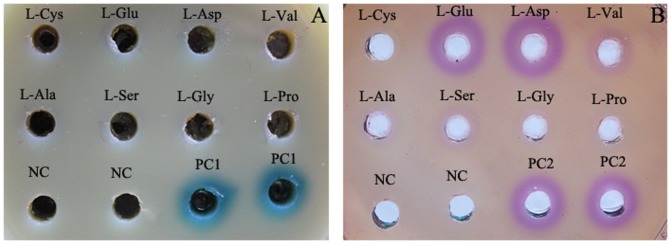
LAAO produced by Pseudoalteromonas sp. R3 (R3-LAAO) was used to oxidize the different L-amino acids including L-Cys, L-Glu, L-Asp, L-Val, L-Ala, L-Ser, L-Gly and L-Pro, in separate reactions, and subsequently the produced H2O2 in oxidization solution was measured with both Prussian blue agar assay [17] and FeIIIXO agar assay. For the Prussian blue agar assay, the standard H2O2 with concentration of 10 mM was used as the positive control (PC1); in contrast, for the FeIIIXO agar assay, the standard H2O2 with concentration of 50 µM was treated as the positive control (PC2). For both assays, the reaction solution without L-amino acid was used as the negative control (NC).
Different methods have been developed to determine the LAAO number and its molecular weight [16], [17] since it is important for the characterization of the LAAO sequence. To achieve this purpose, FeIIIXO agar assay was coupled with SDS-PAGE to determine the number and molecular weight of R3-LAAO. In brief, several replicates of R3-LAAO sample without heating to maintain its activity were electrophoresed on SDS-PAGE. After electrophoresis, different lanes of SDS-PAGE were sliced out for different treatments and subsequently put together on FeIIXO agar for color development. It was found in Figure S4 that the duplicate sample lanes (lanes 1 and 2) without Coomassie brilliant blue (CBB) staining clearly yielded a purplish red band on FeIIXO agar (below SDS-PAGE), indicating that R3-LAAO in SDS-PAGE bears only one active unit. Similarly, the two sample replicates (lanes 3 and 4) with CBB staining also gave a clear purplish red band at the same migration position on FeIIXO agar, revealing that R3-LAAO used in this assay is resistant to SDS and β-mercaptoethanol. As expected, the duplicate lanes 3 and 4 additionally showed CBB-stained protein bands in SDS-PAGE since R3-LAAO sample was precipitated from fermentation crude of Psudoalteromonas sp. R3 [17]. To determine which protein band bears the LAAO activity, all the CBB-stained protein bands near the purplish red band on FeIIXO agar were sliced out from a CBB-stained lane-4 replicate lane, and put on FeIIXO agar (lane 5). It was found that even after long time exposure to multiple steps treatment, the sliced target protein band with LAAO activity in SDS-PAGE still clearly yielded a purplish red band on FeIIXO agar, indicating that LAAO is very stable in this SDS-PAGE coupled FeIIIXO agar assay. According to the molecular weights of CBB-stained standard protein marker (lane M), the corresponding protein band with LAAO activity from Psudoalteromonas sp. R3 was estimated to be around 65 kDa in size on SDS-PAGE, which is in agreement with the one achieved by SDS-PAGE coupled Prussian blue agar assay [17].
Discussion
The diameters of the purplish red halos of FeIIIXO complex driven by H2O2 is a linear function of logarithm of H2O2 concentration from 5 µM to 160 µM, thus allowing this assay to quantitatively determine the LAAO activity with a similar sensitivity as the HRP-involved methods. Although HRP is H2O2 sensitive probe, the assay is complex and expensive. Moreover, the solution of HRP is unstable and needs to be used right after it is ready. Besides, it needs detection instrument. In contrast, FeIIIXO agar assay does not rely on any detection instrument, and it is simple, stable and cost-effective. Compared with Prussian blue agar assay [17], FeIIIXO agar assay is more environmentally friendly. More importantly, it gives two orders of magnitude improvement in sensitivity (5 µM vs. 0.5 mM). Considering its high sensitivity and convenience, this FeIIIXO agar assay can be used to differentiate the mutants with slight difference in LAAO activity from a mutant library with altered expression of LAAO, saving a great number of workload for the investigation of the involved regulation mechanisms underlying the LAAO production.
As reported [20], acidic condition is critical to the proper fabrication of FeIIXO agar before assay. Under non-acidic condition, the entire FeIIXO agar medium will soon become purplish red even in the absence of H2O2 (data not shown). Most probably, FeII in medium is unstable and will be easily oxidized by oxygen to FeIII which will sequentially coordinate with XO to form purplish red FeIIIXO complex. During the fabrication of FeIIXO agar, H2SO4 should be added to medium before FeSO4. Otherwise, the whole mixture of assay medium will also immediately turn to purplish red (data not shown). Another reason for acidic condition in FeIIXO agar medium is that it can help to avoid the hydrolysis of the iron [20]. Besides the fabrication of FeIIXO agar, the acidic capacity or low pH in agar medium is also required for the formation of FeIIIXO complex driven by H2O2. As shown in Figure S1, no clear purplish red halo formation is observed on the FeIIXO agar treated with 40 µM H2O2 if if FeIIXO agar is lack of H2SO4 to give final pH 6. However, extreme acidic condition (pH 1.8 in the presence of 13 mM H2SO4) will make the FIIXO agar medium become lemon yellow, and obscure the color development of FeIIIXO complex. It is most likely that XO is responsible for the lemon yellow color of the agar medium under pH 1.8. Based on the above observation, a final pH value between 2.3 and 3.5 of the agar medium by supplying 6 mM to 10 mM H2SO4 is recommended for proper color development of the assay. We also show that the pH of the detection solution is crucial to the success of FeIIIXO agar assay. Proper color development of FeIIIXO agar is only observed when the detection solution has a pH between 4 and 11. When the pH is ≤2, XO itself will give strong lemon orange and thus mask the color development of purplish red FeIIIXO complex caused by H2O2. When the pH is ≥12, there are two more sources responsible for the purplish red color development in addition to FeIIIXO complex formation due to H2O2. First, XO itself will show purplish red color at this pH; second, under peralkaline condition, other oxidants, such as oxygen, can also easily convert FeII to FeIII, which will subsequently react with XO to form purplish red FeIIIXO. Therefore, the detection of purplish red FeIIIXO formed by H2O2 is not possible at pH above 12. Fortunately, the fermentation solutions from LAAO-producing microorganisms or direct LAAO enzymatic reaction solutions usually have pH values in the range of 4 to 11, thus giving this method broad applicability.
It has been reported that the complex FeIIIXO has a 1∶1 stoichiometry [20]. However, in our method, the diameter of purplish red FeIIIXO halo reaches equilibrium when the molar ratio of FeSO4 to XO in medium is close to 2∶1. XO and FeIII can form FeIII 2XO, FeIIIXO and FeIIIXO2 since XO is a bi-functional metallochromic reagent, mainly depending on the molar ratio of iron ion to XO [21]. The 1∶2 complex (FeIIIXO2) will form if XO is in excess. When the molar ratio of iron to XO approaches to 1∶1, the 1∶1 complex (FeIIIXO) becomes predominant. In contrast, FeIII 2XO will predominate in the presence of excess iron, which is attributed to XO's two isolated iminodiacetate groups that can bind metal ions. This is in agreement with our observation.
Coupled with SDS-PAGE, FeIIIXO agar assay can be directly used to determine the numbers and approximate molecular weights of LAAO protein in one assay, giving crucial advantages over conventional spectrophotometric or fluorometric measurement. Without heating, the LAAO used in this study can tolerate SDS and β-mercaptoethanol, and maintain its activity even after long time exposure to the CBB-staining procedure and de-staining solution with glacial acetic acid. It is clear that knowing exactly the numbers and molecular weights of LAAO can benefit further purification and characterization of this enzyme. In particular, the sliced target band with LAAO activity can directly be analyzed with different techniques, such as protein sequencing and LC-MS/MS analysis.
With agar in medium, our FeIIIXO agar assay can be performed based on the visual measurement rather than the spectrophotometric or fluorometric colorimetry, thus providing it with broad advantages of simplicity and cost-effectiveness. To push the visual threshold detection for trace H2O2 caused by LAAO activity, adjustment of the acidic condition of FeIIXO agar and near 2∶1 molar ratio of FeSO4 to XO are highly necessary. Besides, the addition of D-sorbitol to FeIIXO agar medium can also increase the sensitivity of this FeIIIXO agar assay [24]. Combining all the above conditions, this in-gel method serves ideally as a sensitive procedure for quantitative determination of LAAO activity in following enzyme purification, assaying fractions from a column, or observing changes in activity resulting from enzyme modifications.
Materials and Methods
Chemicals and reagents
All chemicals are of at least analytical grade and used without further purification. H2O2 was purchased from Shanghai Lingfeng Chemical Reagent CO., LTD (China). Xylenol orange [o-cresosulfonphthalein-3, 3′-bis (methyliminodiacetate) sodium salt] and ferrous sulfate were purchased from SANGON BIOTECH (Shanghai, China), and D-sorbitol supplied by Biosharp CO., LTD (China).
LAAO from the marine bacterial Psudoalteromonas sp. R3 (R3-LAAO) was harvested as reported [17]. LAAO solution from Crotalus adamanteus venom (caLAAO) was purchased from Worthington Biochemical Corporation, USA.
FeIIIXO agar assay
Unless otherwise stated, FeIIIXO agar assay was performed as follows: (1) prepare solution of ferrous-XO (FeIIXO) with 0.25 mM FeSO4, 6 mM H2SO4, 0.15 mM XO, 0.1 mM D-sorbitol and 1.5% agar; (2) dissolve the mixture completely at 100°C for 5 min and pour into glass Petri dish to make agar plate; (3) make circular wells on agar plate with a hole puncher whose diameter is 6 mm; (4) add 50 µL detection solutions containing standard H2O2 or H2O2 produced by LAAO activity to each well and wait for 60 min at room temperature for color change; (5) visualize the FeIIIXO formation and measure the size of purplish red halo.
Stereospecific oxidation of amino acid by LAAO activity
The stereospecific oxidation reaction was performed with 10 mM of each amino acid in 10 mL of R3-LAAO solution harvested from Psudoalteromonas sp. R3 culture supernatant [17] or caLAAO solution (mixture of 1 µL caLAAO with 10 mL of 0.1 M PBS buffer with pH 7.5, Worthington Biochemical Corporation, USA). Unless otherwise described, the pH of reaction mixture was adjusted to about 7.5. The reaction mixture was incubated at 37°C for 30 min. After oxidation, 50 µL detection solutions were subjected to either FeIIIXO agar assay with appropriate dilution or Prussian blue agar assay without dilution [17].
Determination of LAAO activity using FeIIIXO agar assay
Unless otherwise stated, the determination of the LAAO activity includes the following steps: (1) 50 µL standard H2O2 solutions with different concentrations ranging from 0.5 µM to 250 µM with uniform pH 7.5 were subjected to FeIIIXO agar assay; (2) after assay, the diameters of the formed purplish red FeIIIXO halos were measured and the correlation equations between H2O2 concentrations and purplish red halo diameters were extracted using Origin software; (3) 50 µL oxidization solutions of L-amino acid by LAAO were applied to FeIIIXO agar assay and the diameters of the generated purplish red halo were recorded; (4) the concentration of H2O2 produced by LAAO activity was calculated based on the extracted correlation equations between H2O2 concentrations and purplish red halo diameters; (5) the LAAO activity was determined with the fashion of the produced H2O2 concentration. One unit (U) is defined as the amount of enzyme that catalyses the formation of 1 mM H2O2/h at 37°C.
SDS-polyacrylamide gel (SDS-PAGE) coupled FeIIIXO agar assay
Unless otherwise described, SDS-PAGE coupled FeIIIXO agar assay consists of the following three steps: (1) SDS-PAGE electrophoresis. The several replicates of detection samples containing LAAO were mixed with 4-fold sample loading buffer (1.0 M Tris-HCl, pH 6.8, 10% SDS, 20% β-mercaptoethanol, 50% glycerol, 1% bromophenol blue). Without heating, 20 µL resultant mixtures were separately applied to each well of normal SDS-PAGE with 5% stacking gel and 12% separation gel, as described by Laemmli [22]. Gel was run at a constant current of 4 mA until the dye reached the end of the gel; (2) FeIIIXO agar assay using LAAO-contained gel. After SDS-PAGE electrophoresis, the entire gel was washed once with distilled water and cut into two pieces. One piece was directly put on FeIIXO agar for the color change; the other with the sample replicates was first stained with Coomassie brilliant blue (CBB) [23] and then also put on FeIIXO agar aside for the color development after three times wash with distilled water; (3) targeting of the protein with LAAO activity. After visualization of purplish red band on FeIIXO agar (below SDS-PAGE), the protein bands directly above the formed purplish red band area were cut out from the sample replicate of CBB stained SDS-PAGE and put on FeIIXO agar to determine the target band with LAAO activity which caused the formation of purplish red. If necessary, the standard protein ladder was used for the determination of molecular weight of target LAAO.
Supporting Information
Statistical analysis of dependent variable diameters of purplish red halos from H2O2 with different concentrations by ANOVA.
(DOC)
Effect of acidic condition derived from H2SO4 with different concentrations in FeIIXO agar medium on the formation of purplish red FeIIIXO complex caused by 40 µM H2O2.
(TIF)
Effect of FeSO4 in FeIIXO agar medium on the formation of purplish red FeIIIXO caused by 40 µM H2O2. Xylenol orange (XO) was fixed at 0.15 mM.
(TIF)
Reliability of FeIIIXO agar assay for determination of Crotalus adamanteus LAAO (caLAAO) activity. The oxidization solutions of L-Leu by caLAAO were serially diluted by 100 times, 200 times, 300 times and 400 times, and 50 µL diluted solutions were subjected to FeIIIXO agar assay (left hole). On the basis of the diameters of the formed purplish red FeIIIXO halos, the concentrations of H2O2 produced by LAAO activities were calculated with the equations in Figure 2. The corresponding standard H2O2 solutions (right hole) as indicated above the corresponding halos were used to confirm the accuracy of FeIIIXO agar assay. The diameters of the purplish red halos were marked below the holes.
(TIF)
SDS-PAGE coupled in-gel FeIIIXO agar assay for the characterization of LAAO from Pseudoalteromonas sp. R3 (R3-LAAO). After electrophoresis, different lanes of SDS-PAGE with replicated samples were sliced out for different treatments and subsequently put together on FeIIXO agar for color development. Lane M: standard protein marker stained with Coomassie brilliant blue (CBB); Lanes 1 and 2: duplicate LAAO samples from Pseudoalteromonas sp. R3 (R3-LAAO) [17] without CBB staining; lanes 3 and 4: two replicates of lane-1 and lane-2 with CBB staining; lane 5: the sliced protein bands from a lane-4 replicate as indicated by arrow directly above the formed purplish red band area. The results showed that R3-LAAO in SDS-PAGE had only one active protein band to form purplish red band on FeIIXO agar (below SDS-PAGE) and its molecular weight was around 65 kDa.
(TIF)
Acknowledgments
We thank Prof. Yili Huang at Zhejiang University and Dr. Jianxun Lin at Columbia University for critical reading and comments on this manuscript.
Funding Statement
This work was supported by Natural Science Foundation of Zhejiang Province, China (Y5100153) (http://www.zjnsf.gov.cn/),Science and Technology Planning Project of Zhejiang Province, China (Welfare Technology Applied Research Project of Zhejiang Province, Grant No.:2011C23007) (http://www.zjkjt.gov.cn/), and Natural Science Foundation of ZJUT (20100213) (http://zjut.edu.cn/) to ZY. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yu Z, Qiao H (2012) Advances in non-snake venom L-amino acid oxidase. Appl Biochem Biotechnol 167: 1–13. [DOI] [PubMed] [Google Scholar]
- 2. Lucas-Elio P, Gomez D, Solano F, Sanchez-Amat A (2006) The antimicrobial activity of Marinocine, synthesized by Marinomonas mediterranea, is due to hydrogen peroxide generated by its lysine oxidase activity. J Bacteriol 188: 2493–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stumpf PK, Green DE (1944) L-Amino acid oxidase of Proteus vulgaris . The Journal of Biological Chemistry 153: 387–399. [Google Scholar]
- 4. Bregge-Silva C, Nonato MC, de Albuquerque S, Ho PL, Junqueira de Azevedo IL, et al. (2012) Isolation and biochemical, functional and structural characterization of a novel L-amino acid oxidase from Lachesis muta snake venom. Toxicon 60 (7) 1263–76. [DOI] [PubMed] [Google Scholar]
- 5. Ahn MY, Ryu KS, Lee YW, Kim YS (2000) Cytotoxicity and L-amino acid oxidase activity of crude insect drugs. Arch Pharm Res 23 (5) 477–481. [DOI] [PubMed] [Google Scholar]
- 6. Yang H, Johnson PM, Ko KC, Kamio M, Germann MW, et al. (2005) Cloning, characterization and expression of escapin, a broadly antimicrobial FAD-containing L-amino acid oxidase from ink of the sea hare Aplysia californica . J Exp Biol 208: 3609–3622. [DOI] [PubMed] [Google Scholar]
- 7. Davis MA, Askin MC, Hynes MJ (2005) Amino acid catabolism by an areA-regulated gene encoding an L-amino acid oxidase with broad substrate specificity in Aspergillus nidulans . Appl Environ Microbiol 71 (7) 3551–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gomez D, Espinosa E, Bertazzo M, Lucas-Elio P, Solano F, et al. (2008) The macromolecule with antimicrobial activity synthesized by Pseudoalteromonas luteoviolacea strains is an L-amino acid oxidase. Appl Microbiol Biotechnol 79: 925–930. [DOI] [PubMed] [Google Scholar]
- 9. Huang YL, Li M, Yu Z, Qian PY (2011) Correlation between pigmentation and larval settlement deterrence by Pseudoalteromonas sp. sf57. Biofouling 27 (3) 287–293. [DOI] [PubMed] [Google Scholar]
- 10. Vallon O, Bulté L, Kuras R, Olive J, Wollman FA (1993) Extensive accumulation of an extracellular L-amino-acid oxidase during gametogenesis of Chlumydomonas reinhardtii . Eur J Biochem 215: 351–360. [DOI] [PubMed] [Google Scholar]
- 11. Du XY, Clemetson KJ (2002) Snake venom L-amino acid oxidases. Toxicon 40: 659–665. [DOI] [PubMed] [Google Scholar]
- 12. Skarner RC (1970) L-amino acid oxidase a bactericidal system. Nature 225: 1072–1073. [DOI] [PubMed] [Google Scholar]
- 13. Timmer B, Olthuis W, van den Berg A (2005) Ammonia sensors and their applications—a review. Sensor Actuat B 107: 666–677. [Google Scholar]
- 14. Singh S, Gogoi BK, Bezbaruah RL (2009) Optimization of medium and cultivation conditions for L-amino acid oxidase production by Aspergillus fumigatus . Can J Microbiol 55 (9) 1096–1102. [DOI] [PubMed] [Google Scholar]
- 15. Okubo BM, Silva ON, Migliolo L, Gomes DG, Porto WF, et al. (2012) Evaluation of an antimicrobial L-amino acid oxidase and peptide derivatives from Bothropoides mattogrosensis Pitviper venom. PLoS ONE 7 (3) e33639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rau JE, Fischer U (2011) In-gel detection of L-amino acid oxidases based on the visulisation of hydrogen peroxide production. J Microbiol Methods 85 (3) 228–229. [DOI] [PubMed] [Google Scholar]
- 17. Yu Z, Zhou N, Zhao C, Qiu J (2013) In-gel determination of L-amino acid oxidase activity based on the visualization of Prussian blue-forming reaction. PLoS ONE 8 (2) e55548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hermes-Lima M, Willmore WG, Storey KB (1995) Quantification of lipid peroxidation in tissue extracts based on Fe(III)xylenol orange complex formation. Free Radical Biology & Medicine 19: 271–280. [DOI] [PubMed] [Google Scholar]
- 19. Gay C, Collins J, Gebicki JM (1999) Hydroperoxide assay with the ferric–xylenol orange complex. Anal Biochem 273 (2) 149–155. [DOI] [PubMed] [Google Scholar]
- 20. Gay C, Collins J, Gebicki JM (1999) Determination of iron in solutions with the ferric-xylenol orange complex. Anal Biochem 273 (2) 143–148. [DOI] [PubMed] [Google Scholar]
- 21. Mizuguchi H, Yotsuyanagi T (2001) Visual threshold detection of trace metal ions using a bi-functional metallochromic reagent. Anal Sci 17 (Suppl) i1687–i1689. [Google Scholar]
- 22. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 23. Kang D, Gho YS, Suh M, Kang C (2002) Highly sensitive and fast protein detection with Coomassie brilliant blue in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Bull Korean Chem Soc 23: 1511–1512. [Google Scholar]
- 24. Gay C, Gebicki JM (2000) A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal Biochem 284 (2) 217–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical analysis of dependent variable diameters of purplish red halos from H2O2 with different concentrations by ANOVA.
(DOC)
Effect of acidic condition derived from H2SO4 with different concentrations in FeIIXO agar medium on the formation of purplish red FeIIIXO complex caused by 40 µM H2O2.
(TIF)
Effect of FeSO4 in FeIIXO agar medium on the formation of purplish red FeIIIXO caused by 40 µM H2O2. Xylenol orange (XO) was fixed at 0.15 mM.
(TIF)
Reliability of FeIIIXO agar assay for determination of Crotalus adamanteus LAAO (caLAAO) activity. The oxidization solutions of L-Leu by caLAAO were serially diluted by 100 times, 200 times, 300 times and 400 times, and 50 µL diluted solutions were subjected to FeIIIXO agar assay (left hole). On the basis of the diameters of the formed purplish red FeIIIXO halos, the concentrations of H2O2 produced by LAAO activities were calculated with the equations in Figure 2. The corresponding standard H2O2 solutions (right hole) as indicated above the corresponding halos were used to confirm the accuracy of FeIIIXO agar assay. The diameters of the purplish red halos were marked below the holes.
(TIF)
SDS-PAGE coupled in-gel FeIIIXO agar assay for the characterization of LAAO from Pseudoalteromonas sp. R3 (R3-LAAO). After electrophoresis, different lanes of SDS-PAGE with replicated samples were sliced out for different treatments and subsequently put together on FeIIXO agar for color development. Lane M: standard protein marker stained with Coomassie brilliant blue (CBB); Lanes 1 and 2: duplicate LAAO samples from Pseudoalteromonas sp. R3 (R3-LAAO) [17] without CBB staining; lanes 3 and 4: two replicates of lane-1 and lane-2 with CBB staining; lane 5: the sliced protein bands from a lane-4 replicate as indicated by arrow directly above the formed purplish red band area. The results showed that R3-LAAO in SDS-PAGE had only one active protein band to form purplish red band on FeIIXO agar (below SDS-PAGE) and its molecular weight was around 65 kDa.
(TIF)