Abstract
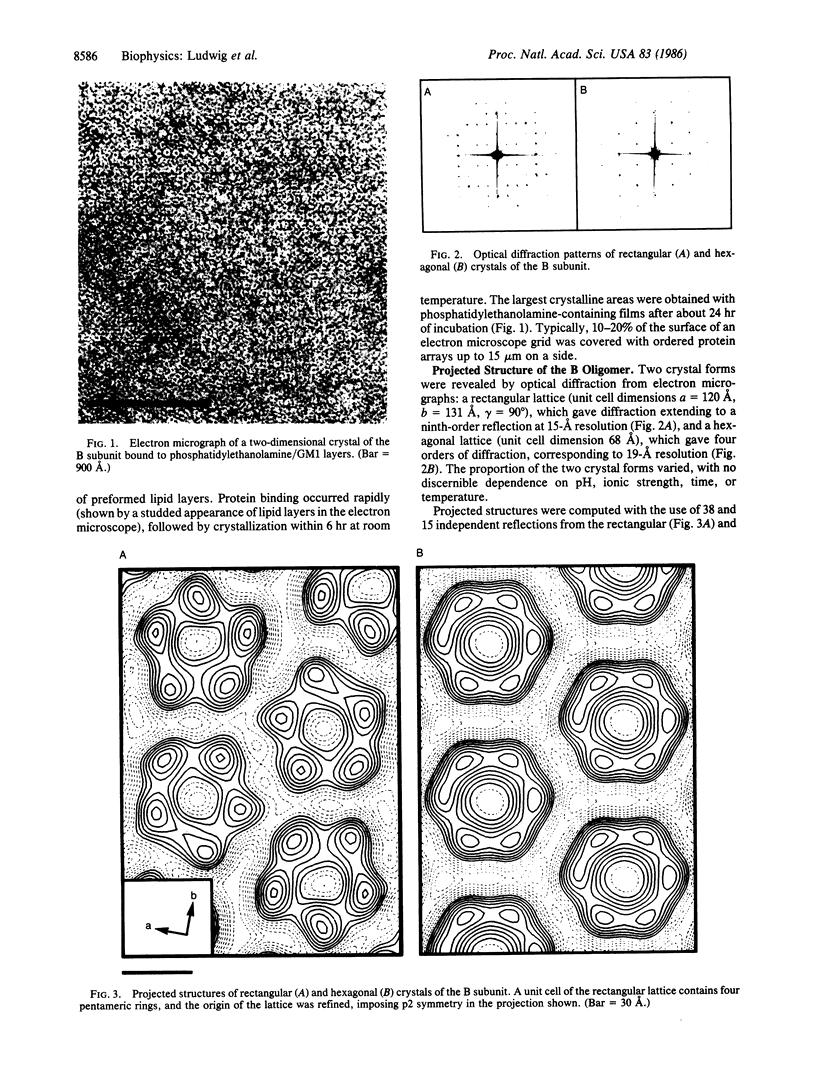
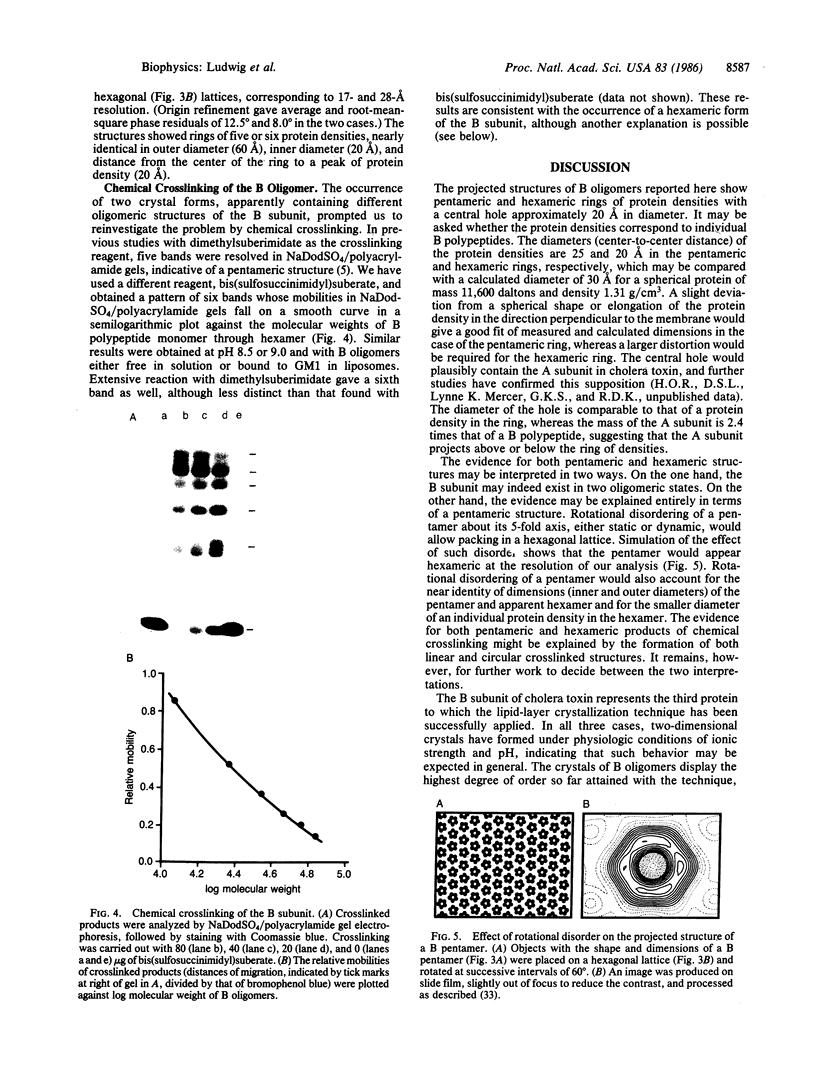
The B subunit of cholera toxin forms two-dimensional crystals when bound to its membrane receptor, ganglioside GM1, in phospholipid layers. A rectangular crystal lattice gives diffraction extending to 15-A resolution in negative stain, and image-processing of electron micrographs reveals a ring of five protein densities. The diameter of the central hole and the outer diameter of the ring are about 20 and 60 A, respectively. These data are consistent with a pentameric, doughnut-shaped structure of the B subunit that lies flat on a membrane surface. A hexagonal crystal lattice is obtained as well, and results of image processing and chemical crosslinking allow two interpretations: the B subunit may exist in both pentameric and hexameric forms or, more likely, the hexagonal lattice may represent a disordered or liquid crystalline form, in which a pentamer undergoes rotational averaging about its 5-fold axis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A., Henderson R., Unwin P. N. Three-dimensional structure determination by electron microscopy of two-dimensional crystals. Prog Biophys Mol Biol. 1982;39(3):183–231. doi: 10.1016/0079-6107(83)90017-2. [DOI] [PubMed] [Google Scholar]
- Bennett V., Cuatrecasas P. Mechanism of activation of adenylate cyclase by Vibrio cholerae enterotoxin. J Membr Biol. 1975 Jun 3;22(1):29–52. doi: 10.1007/BF01868162. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- DE S. N. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature. 1959 May 30;183(4674):1533–1534. doi: 10.1038/1831533a0. [DOI] [PubMed] [Google Scholar]
- DUTTA N. K., PANSE M. V., KULKARNI D. R. Role of cholera a toxin in experimental cholera. J Bacteriol. 1959 Oct;78:594–595. doi: 10.1128/jb.78.4.594-595.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wolf M. J., Fridkin M., Epstein M., Kohn L. D. Structure-function studies of cholera toxin and its A and B protomers. Modification of tryptophan residues. J Biol Chem. 1981 Jun 10;256(11):5481–5488. [PubMed] [Google Scholar]
- De Wolf M. J., Fridkin M., Kohn L. D. Tryptophan residues of cholera toxin and its A and B protomers. Intrinsic fluorescence and solute quenching upon interacting with the ganglioside GM1, oligo-GM1, or dansylated oligo-GM1. J Biol Chem. 1981 Jun 10;256(11):5489–5496. [PubMed] [Google Scholar]
- De Wolf M., Van Dessel G., Lagrou A., Hilderson H. J., Dierick W. Structural features of the binding site of cholera toxin inferred from fluorescence measurements. Biochim Biophys Acta. 1985 Nov 29;832(2):165–174. doi: 10.1016/0167-4838(85)90328-0. [DOI] [PubMed] [Google Scholar]
- Duffy L. K., Lai C. Y. Involvement of arginine residues in the binding site of cholera toxin subunit B. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1005–1010. doi: 10.1016/0006-291x(79)91979-x. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman M., Neoh S. H., LaRue M. K., Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J Immunol. 1974 Jul;113(1):145–150. [PubMed] [Google Scholar]
- Fishman P. H., Moss J., Osborne J. C., Jr Interaction of choleragen with the oligosaccharide of ganglioside GM1: evidence for multiple oligosaccharide binding sites. Biochemistry. 1978 Feb 21;17(4):711–716. doi: 10.1021/bi00597a024. [DOI] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Multiple roles of erythrocyte supernatant in the activation of adenylate cyclase by Vibrio cholerae toxin in vitro. J Infect Dis. 1976 Mar;133 (Suppl):55–63. doi: 10.1093/infdis/133.supplement_1.s55. [DOI] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Kassis S., Hagmann J., Fishman P. H., Chang P. P., Moss J. Mechanism of action of cholera toxin on intact cells. Generation of A1 peptide and activation of adenylate cyclase. J Biol Chem. 1982 Oct 25;257(20):12148–12152. [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosky A., Markel D. E., Peterson J. W. Covalent structure of the beta chain of cholera enterotoxin. J Biol Chem. 1977 Oct 25;252(20):7257–7264. [PubMed] [Google Scholar]
- Lai C. Y. Determination of the primary structure of cholera toxin B subunit. J Biol Chem. 1977 Oct 25;252(20):7249–7256. [PubMed] [Google Scholar]
- Ludwig D. S., Holmes R. K., Schoolnik G. K. Chemical and immunochemical studies on the receptor binding domain of cholera toxin B subunit. J Biol Chem. 1985 Oct 15;260(23):12528–12534. [PubMed] [Google Scholar]
- Markel D. E., Hejtmancik K. E., Peterson J. W., Kurosky A. Structure, function, and antigenicity of cholera toxin. J Supramol Struct. 1979;10(2):137–149. doi: 10.1002/jss.400100204. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Sattler J., Schwarzmann G., Staerk J., Ziegler W., Wiegandt H. Studies of the ligand binding to cholera toxin, II. The hydrophilic moiety of sialoglycolipids. Hoppe Seylers Z Physiol Chem. 1977 Feb;358(2):159–163. doi: 10.1515/bchm2.1977.358.1.159. [DOI] [PubMed] [Google Scholar]
- Sattler J., Wiegandt H. Studies of the subunit structure of choleragen. Eur J Biochem. 1975 Sep 1;57(1):309–316. doi: 10.1111/j.1432-1033.1975.tb02302.x. [DOI] [PubMed] [Google Scholar]
- Sigler P. B., Dryan M. E., Kiuefer H. C., Finkelstein R. A. Cholera toxin crystals suitable for x-ray diffraction. Science. 1977 Sep 23;197(4310):1277–1279. doi: 10.1126/science.197.4310.1277-a. [DOI] [PubMed] [Google Scholar]
- Tayot J. L., Holmgren J., Svennerholm L., Lindblad M., Tardy M. Receptor-specific large-scale purification of cholera toxin on silica beads derivatized with lysoGM1 ganglioside. Eur J Biochem. 1981 Jan;113(2):249–258. doi: 10.1111/j.1432-1033.1981.tb05060.x. [DOI] [PubMed] [Google Scholar]
- Tomasi M., Montecucco C. Lipid insertion of cholera toxin after binding to GM1-containing liposomes. J Biol Chem. 1981 Nov 10;256(21):11177–11181. [PubMed] [Google Scholar]
- Tsuji T., Honda T., Miwatani T., Wakabayashi S., Matsubara H. Analysis of receptor-binding site in Escherichia coli enterotoxin. J Biol Chem. 1985 Jul 15;260(14):8552–8558. [PubMed] [Google Scholar]
- Uzgiris E. E., Kornberg R. D. Two-dimensional crystallization technique for imaging macromolecules, with application to antigen--antibody--complement complexes. Nature. 1983 Jan 13;301(5896):125–129. doi: 10.1038/301125a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wisnieski B. J., Bramhall J. S. Photolabelling of cholera toxin subunits during membrane penetration. Nature. 1981 Jan 22;289(5795):319–321. doi: 10.1038/289319a0. [DOI] [PubMed] [Google Scholar]