Abstract
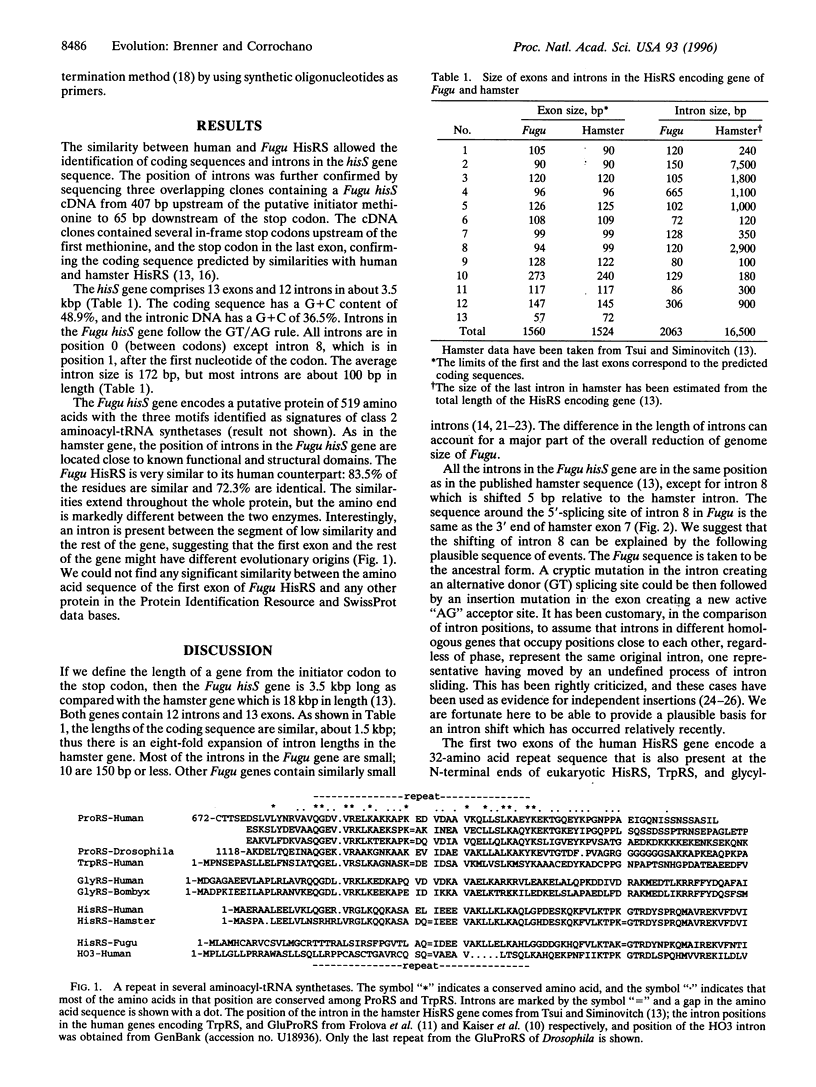
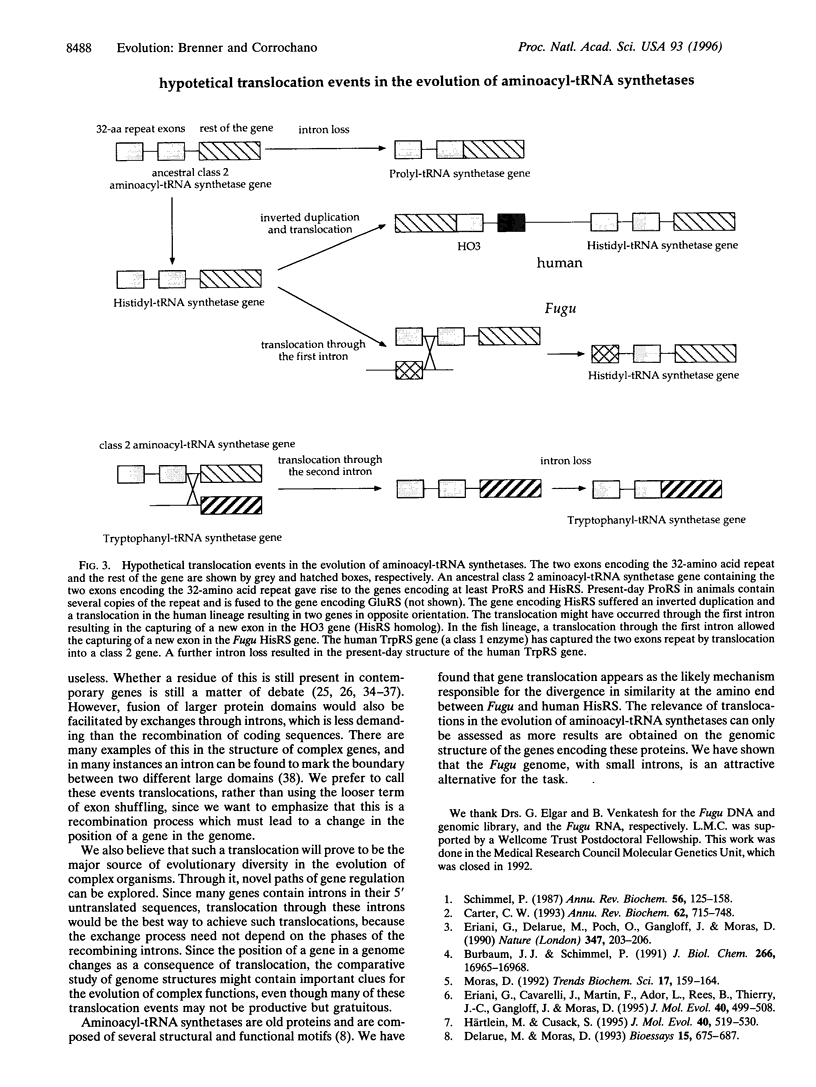
We have characterized hisS, the gene encoding the histidyl-tRNA synthetase (HisRS) from the tetraodontoid fish Fugu rubripes. The hisS gene is about 3.5 kbp long and contains 13 exons and 12 introns of 172 bp, on average. The Fugu hisS gene encodes a putative protein of 519 amino acids with the three motifs identified as signatures of class 2 aminoacyl-tRNA synthetases. A model for the shifting of intron 8 between Fugu and hamster is proposed based on the successive appearance of a cryptic splicing site followed by an insertion mutation that created a new acceptor site. In addition, sequence comparisons suggest that the hisS gene has undergone a translocation through the first intron. As a result, the Fugu HisRS has an N-terminal sequence markedly different from that in the human and hamster enzymes. We propose that similar events have been responsible for variations at the N-terminal end of other aminoacyl-tRNA synthetases. Our analysis suggests that this involves exchanges through introns of two exons encoding an ancestral 32-amino acid motif.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amaar Y. G., Baillie D. L. Cloning and characterization of the C. elegans histidyl-tRNA synthetase gene. Nucleic Acids Res. 1993 Sep 11;21(18):4344–4347. doi: 10.1093/nar/21.18.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnez J. G., Harris D. C., Mitschler A., Rees B., Francklyn C. S., Moras D. Crystal structure of histidyl-tRNA synthetase from Escherichia coli complexed with histidyl-adenylate. EMBO J. 1995 Sep 1;14(17):4143–4155. doi: 10.1002/j.1460-2075.1995.tb00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale S., Abdulla S., Elgar G., Buck D., Berks M., Micklem G., Durbin R., Bates G., Brenner S., Beck S. Comparative sequence analysis of the human and pufferfish Huntington's disease genes. Nat Genet. 1995 May;10(1):67–76. doi: 10.1038/ng0595-67. [DOI] [PubMed] [Google Scholar]
- Brenner S., Elgar G., Sandford R., Macrae A., Venkatesh B., Aparicio S. Characterization of the pufferfish (Fugu) genome as a compact model vertebrate genome. Nature. 1993 Nov 18;366(6452):265–268. doi: 10.1038/366265a0. [DOI] [PubMed] [Google Scholar]
- Burbaum J. J., Schimmel P. Structural relationships and the classification of aminoacyl-tRNA synthetases. J Biol Chem. 1991 Sep 15;266(26):16965–16968. [PubMed] [Google Scholar]
- Carter C. W., Jr Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu Rev Biochem. 1993;62:715–748. doi: 10.1146/annurev.bi.62.070193.003435. [DOI] [PubMed] [Google Scholar]
- Cerini C., Kerjan P., Astier M., Gratecos D., Mirande M., Sémériva M. A component of the multisynthetase complex is a multifunctional aminoacyl-tRNA synthetase. EMBO J. 1991 Dec;10(13):4267–4277. doi: 10.1002/j.1460-2075.1991.tb05005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrochano L. M. A test of human cDNA synthesis by the polymerase chain reaction. Genet Anal Tech Appl. 1991 Jun;8(4):134–135. doi: 10.1016/1050-3862(91)90030-u. [DOI] [PubMed] [Google Scholar]
- Delarue M., Moras D. The aminoacyl-tRNA synthetase family: modules at work. Bioessays. 1993 Oct;15(10):675–687. doi: 10.1002/bies.950151007. [DOI] [PubMed] [Google Scholar]
- Dibb N. J., Newman A. J. Evidence that introns arose at proto-splice sites. EMBO J. 1989 Jul;8(7):2015–2021. doi: 10.1002/j.1460-2075.1989.tb03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. The multiplicity of domains in proteins. Annu Rev Biochem. 1995;64:287–314. doi: 10.1146/annurev.bi.64.070195.001443. [DOI] [PubMed] [Google Scholar]
- Dorit R. L., Schoenbach L., Gilbert W. How big is the universe of exons? Science. 1990 Dec 7;250(4986):1377–1382. doi: 10.1126/science.2255907. [DOI] [PubMed] [Google Scholar]
- Elgar G., Rattray F., Greystrong J., Brenner S. Genomic structure and nucleotide sequence of the p55 gene of the puffer fish Fugu rubripes. Genomics. 1995 Jun 10;27(3):442–446. doi: 10.1006/geno.1995.1075. [DOI] [PubMed] [Google Scholar]
- Eriani G., Cavarelli J., Martin F., Ador L., Rees B., Thierry J. C., Gangloff J., Moras D. The class II aminoacyl-tRNA synthetases and their active site: evolutionary conservation of an ATP binding site. J Mol Evol. 1995 May;40(5):499–508. doi: 10.1007/BF00166618. [DOI] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Fett R., Knippers R. The primary structure of human glutaminyl-tRNA synthetase. A highly conserved core, amino acid repeat regions, and homologies with translation elongation factors. J Biol Chem. 1991 Jan 25;266(3):1448–1455. [PubMed] [Google Scholar]
- Frolova L. Y., Grigorieva A. Y., Sudomoina M. A., Kisselev L. L. The human gene encoding tryptophanyl-tRNA synthetase: interferon-response elements and exon-intron organization. Gene. 1993 Jun 30;128(2):237–245. doi: 10.1016/0378-1119(93)90568-n. [DOI] [PubMed] [Google Scholar]
- Härtlein M., Cusack S. Structure, function and evolution of seryl-tRNA synthetases: implications for the evolution of aminoacyl-tRNA synthetases and the genetic code. J Mol Evol. 1995 May;40(5):519–530. doi: 10.1007/BF00166620. [DOI] [PubMed] [Google Scholar]
- Kaiser E., Hu B., Becher S., Eberhard D., Schray B., Baack M., Hameister H., Knippers R. The human EPRS locus (formerly the QARS locus): a gene encoding a class I and a class II aminoacyl-tRNA synthetase. Genomics. 1994 Jan 15;19(2):280–290. doi: 10.1006/geno.1994.1059. [DOI] [PubMed] [Google Scholar]
- Long M., Rosenberg C., Gilbert W. Intron phase correlations and the evolution of the intron/exon structure of genes. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12495–12499. doi: 10.1073/pnas.92.26.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. J., Stevens D. J., Luzzatto L., Brenner S., Aparicio S. Genomic structure and sequence of the Fugu rubripes glucose-6-phosphate dehydrogenase gene (G6PD). Genomics. 1995 Apr 10;26(3):587–591. doi: 10.1016/0888-7543(95)80179-p. [DOI] [PubMed] [Google Scholar]
- Mattick J. S. Introns: evolution and function. Curr Opin Genet Dev. 1994 Dec;4(6):823–831. doi: 10.1016/0959-437x(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Moras D. Structural and functional relationships between aminoacyl-tRNA synthetases. Trends Biochem Sci. 1992 Apr;17(4):159–164. doi: 10.1016/0968-0004(92)90326-5. [DOI] [PubMed] [Google Scholar]
- Nada S., Chang P. K., Dignam J. D. Primary structure of the gene for glycyl-tRNA synthetase from Bombyx mori. J Biol Chem. 1993 Apr 15;268(11):7660–7667. [PubMed] [Google Scholar]
- Nagel G. M., Doolittle R. F. Phylogenetic analysis of the aminoacyl-tRNA synthetases. J Mol Evol. 1995 May;40(5):487–498. doi: 10.1007/BF00166617. [DOI] [PubMed] [Google Scholar]
- O'Hanlon T. P., Raben N., Miller F. W. A novel gene oriented in a head-to-head configuration with the human histidyl-tRNA synthetase (HRS) gene encodes an mRNA that predicts a polypeptide homologous to HRS. Biochem Biophys Res Commun. 1995 May 16;210(2):556–566. doi: 10.1006/bbrc.1995.1696. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Logsdon J. M., Jr The recent origins of introns. Curr Opin Genet Dev. 1991 Dec;1(4):470–477. doi: 10.1016/s0959-437x(05)80194-7. [DOI] [PubMed] [Google Scholar]
- Raben N., Borriello F., Amin J., Horwitz R., Fraser D., Plotz P. Human histidyl-tRNA synthetase: recognition of amino acid signature regions in class 2a aminoacyl-tRNA synthetases. Nucleic Acids Res. 1992 Mar 11;20(5):1075–1081. doi: 10.1093/nar/20.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben N., Nichols R., Dohlman J., McPhie P., Sridhar V., Hyde C., Leff R., Plotz P. A motif in human histidyl-tRNA synthetase which is shared among several aminoacyl-tRNA synthetases is a coiled-coil that is essential for enzymatic activity and contains the major autoantigenic epitope. J Biol Chem. 1994 Sep 30;269(39):24277–24283. [PubMed] [Google Scholar]
- Rogers J. H. The role of introns in evolution. FEBS Lett. 1990 Aug 1;268(2):339–343. doi: 10.1016/0014-5793(90)81282-s. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. Aminoacyl tRNA synthetases: general scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu Rev Biochem. 1987;56:125–158. doi: 10.1146/annurev.bi.56.070187.001013. [DOI] [PubMed] [Google Scholar]
- Stoltzfus A., Spencer D. F., Zuker M., Logsdon J. M., Jr, Doolittle W. F. Testing the exon theory of genes: the evidence from protein structure. Science. 1994 Jul 8;265(5169):202–207. doi: 10.1126/science.8023140. [DOI] [PubMed] [Google Scholar]
- Tolstrup A. B., Bejder A., Fleckner J., Justesen J. Transcriptional regulation of the interferon-gamma-inducible tryptophanyl-tRNA synthetase includes alternative splicing. J Biol Chem. 1995 Jan 6;270(1):397–403. doi: 10.1074/jbc.270.1.397. [DOI] [PubMed] [Google Scholar]
- Tsui F. W., Siminovitch L. Isolation, structure and expression of mammalian genes for histidyl-tRNA synthetase. Nucleic Acids Res. 1987 Apr 24;15(8):3349–3367. doi: 10.1093/nar/15.8.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]




