Abstract
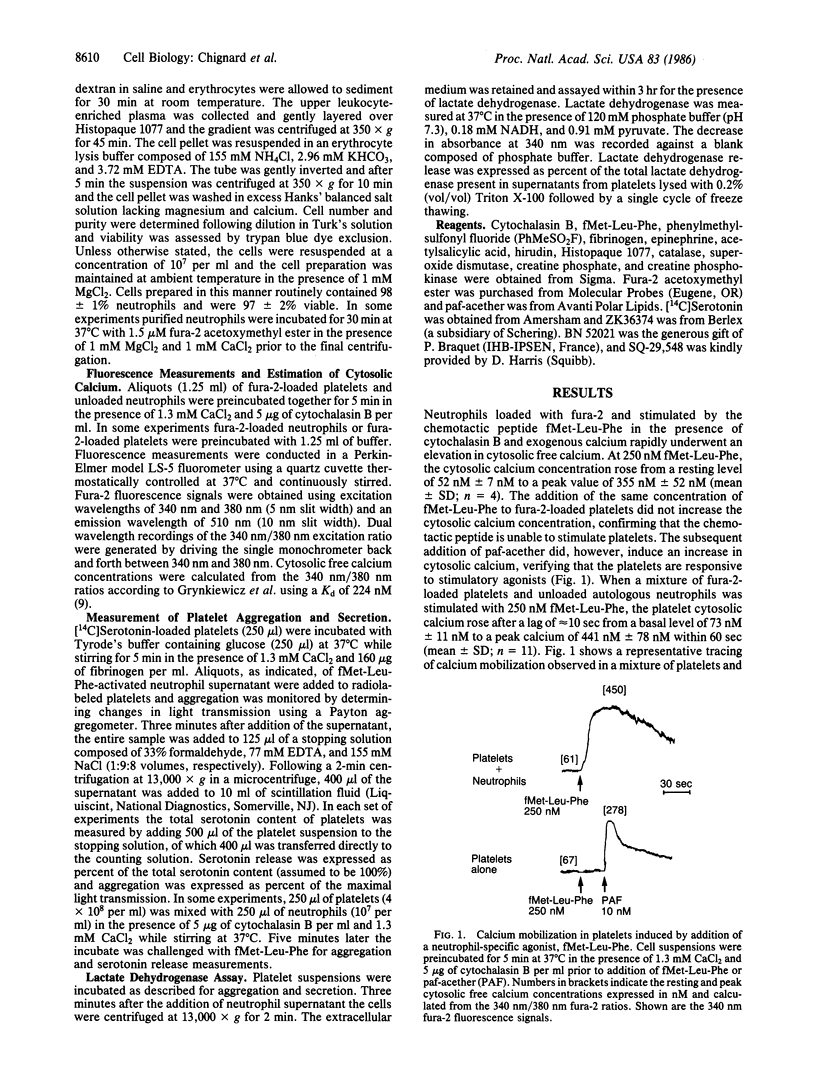
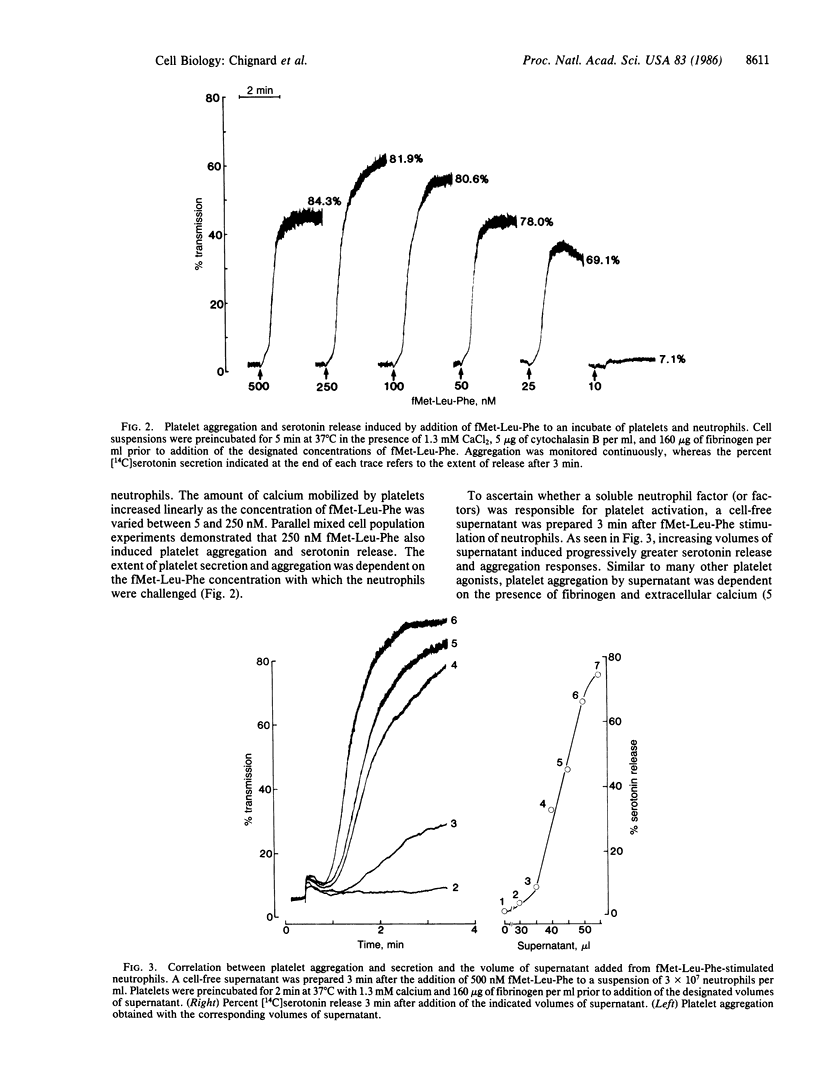
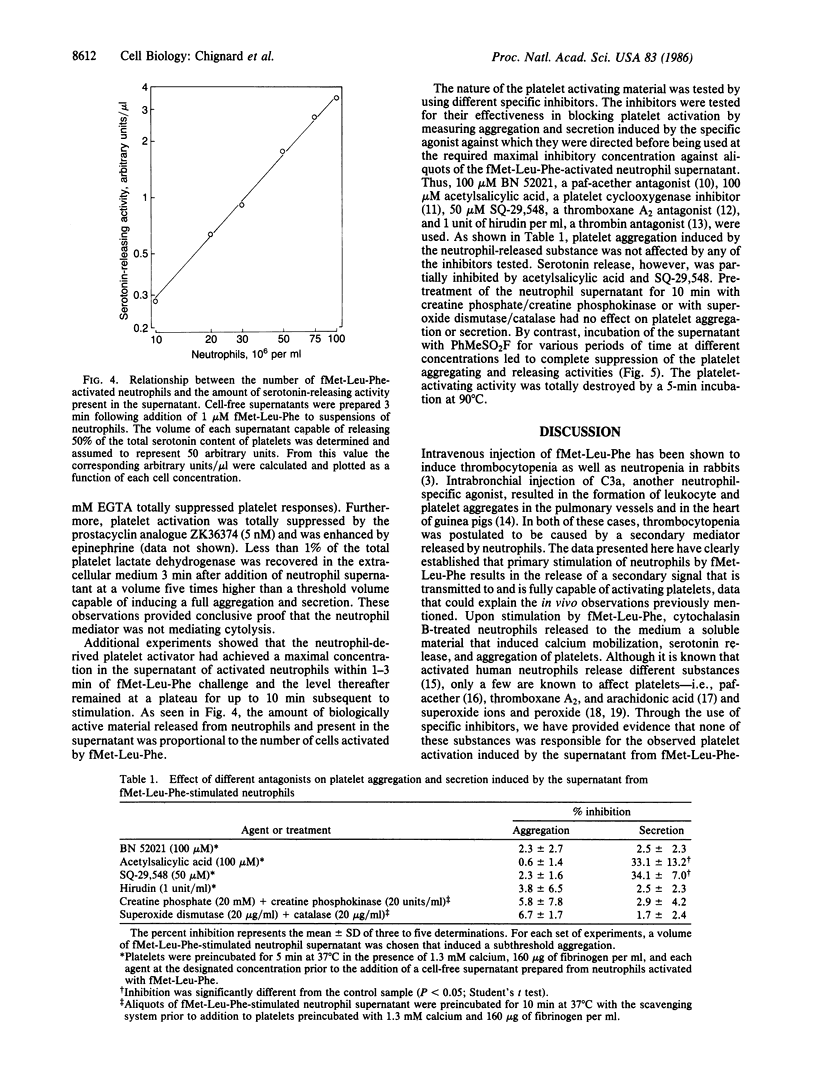
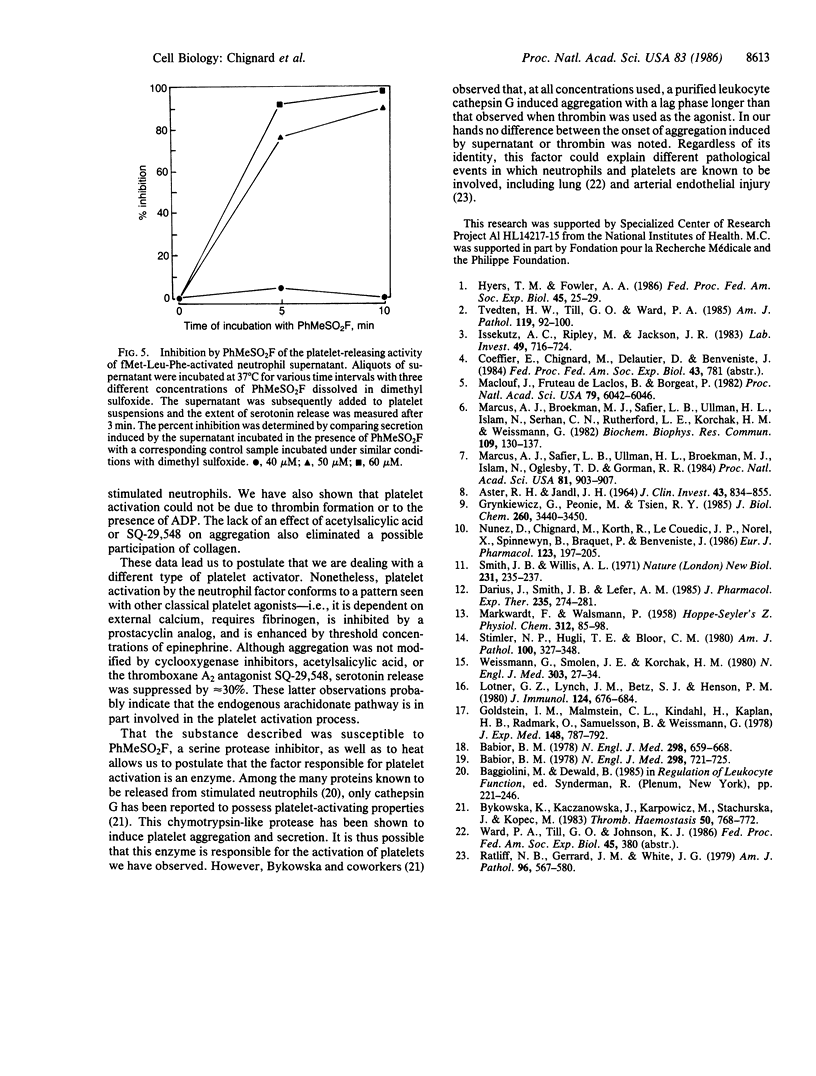
Human neutrophils and platelets were loaded with the intracellular calcium indicator fura-2. The chemotactic peptide N-formyl-Met-Leu-Phe (fMet-Leu-Phe) induced a rapid elevation of cytosolic free calcium in cytochalasin B-treated neutrophils but failed to increase the cytosolic calcium in platelets. On the other hand, when unloaded neutrophils were incubated together with autologous fura-2-loaded platelets, fMet-Leu-Phe stimulated a 6-fold increase in platelet cytosolic calcium subsequent to a brief lag. Parallel experiments demonstrated that the addition of fMet-Leu-Phe to neutrophil/platelet incubates also elicited platelet aggregation and serotonin release. Platelet activation showed a positive correlation with the concentration of fMet-Leu-Phe added to the mixed cell population. Cell-free supernatants prepared from fMet-Leu-Phe-stimulated neutrophils were capable of inducing platelet calcium mobilization, aggregation, and secretion. The amount of platelet-activating material present in the supernatant was proportional to the number of activated neutrophils. Preincubation of platelets with BN 52021, acetylsalicylic acid, SQ-29,548, or hirudin did not modify the aggregation response induced by the supernatant collected from fMet-Leu-Phe-activated neutrophils, suggesting that the material was not 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (paf-acether), arachidonic acid, thromboxane A2, or thrombin. Pretreatment of the neutrophil supernatant with an ADP (creatine phosphate/creatine phosphokinase) or a superoxide/peroxide (superoxide dismutase/catalase) scavenging system also had no effect on aggregation or secretion, indicating that these substances did not participate in platelet activation. The biological activity present in the neutrophil supernatant was destroyed by heat and inactivated by treatment with phenylmethylsulfonyl fluoride, indicating that it is a protein and most probably an enzyme with serine protease activity. These data provide the direct observation of secondary signal transmission to platelets following primary activation of neutrophils. We propose the name neutrophilin for the neutrophil-derived mediator.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Bykowska K., Kaczanowska J., Karpowicz M., Stachurska J., Kopeć M. Effect of neutral proteases from blood leukocytes on human platelets. Thromb Haemost. 1983 Dec 30;50(4):768–772. [PubMed] [Google Scholar]
- Darius H., Smith J. B., Lefer A. M. Beneficial effects of a new potent and specific thromboxane receptor antagonist (SQ-29,548) in vitro and in vivo. J Pharmacol Exp Ther. 1985 Nov;235(2):274–281. [PubMed] [Google Scholar]
- Goldstein I. M., Malmsten C. L., Kindahl H., Kaplan H. B., Rådmark O., Samuelsson B., Weissmann G. Thromboxane generation by human peripheral blood polymorphonuclear leukocytes. J Exp Med. 1978 Sep 1;148(3):787–792. doi: 10.1084/jem.148.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hyers T. M., Fowler A. A. Adult respiratory distress syndrome: causes, morbidity, and mortality. Fed Proc. 1986 Jan;45(1):25–29. [PubMed] [Google Scholar]
- Issekutz A. C., Ripley M., Jackson J. R. Role of neutrophils in the deposition of platelets during acute inflammation. Lab Invest. 1983 Dec;49(6):716–724. [PubMed] [Google Scholar]
- Lotner G. Z., Lynch J. M., Betz S. J., Henson P. M. Human neutrophil-derived platelet activating factor. J Immunol. 1980 Feb;124(2):676–684. [PubMed] [Google Scholar]
- MARKWARDT F., WALSMANN P. Die Reaktion zwischen Hirudin und Thrombin. Hoppe Seylers Z Physiol Chem. 1958;312(1-3):85–98. [PubMed] [Google Scholar]
- Maclouf J., de Laclos B. F., Borgeat P. Stimulation of leukotriene biosynthesis in human blood leukocytes by platelet-derived 12-hydroperoxy-icosatetraenoic acid. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6042–6046. doi: 10.1073/pnas.79.19.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Broekman M. J., Safier L. B., Ullman H. L., Islam N., Sherhan C. N., Rutherford L. E., Korchak H. M., Weissmann G. Formation of leukotrienes and other hydroxy acids during platelet-neutrophil interactions in vitro. Biochem Biophys Res Commun. 1982 Nov 16;109(1):130–137. doi: 10.1016/0006-291x(82)91575-3. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B., Ullman H. L., Broekman M. J., Islam N., Oglesby T. D., Gorman R. R. 12S,20-dihydroxyicosatetraenoic acid: a new icosanoid synthesized by neutrophils from 12S-hydroxyicosatetraenoic acid produced by thrombin- or collagen-stimulated platelets. Proc Natl Acad Sci U S A. 1984 Feb;81(3):903–907. doi: 10.1073/pnas.81.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez D., Chignard M., Korth R., Le Couedic J. P., Norel X., Spinnewyn B., Braquet P., Benveniste J. Specific inhibition of PAF-acether-induced platelet activation by BN 52021 and comparison with the PAF-acether inhibitors kadsurenone and CV 3988. Eur J Pharmacol. 1986 Apr 16;123(2):197–205. doi: 10.1016/0014-2999(86)90660-6. [DOI] [PubMed] [Google Scholar]
- Ratliff N. B., Gerrard J. M., White J. G. Platelet-leukocyte interactions following arterial endothelial injury. Am J Pathol. 1979 Aug;96(2):567–580. [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Willis A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971 Jun 23;231(25):235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- Stimler N. P., Hugli T. E., Bloor C. M. Pulmonary injury induced by C3a and C5a anaphylatoxins. Am J Pathol. 1980 Aug;100(2):327–348. [PMC free article] [PubMed] [Google Scholar]
- Tvedten H. W., Till G. O., Ward P. A. Mediators of lung injury in mice following systemic activation of complement. Am J Pathol. 1985 Apr;119(1):92–100. [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]



