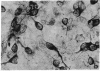
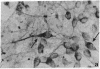
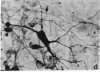
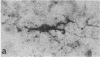
Abstract
Some cells from cultured embryonic mouse hypothalamus were found to express aromatic-L-amino acid decarboxylase (EC 4.1.1.28) activity and serotonin uptake and storage. These neuron-like cells differed from serotoninergic neurons in cultured embryonic mouse brain stem since they did not contain tryptophan hydroxylase. We studied the effect of the serotonin agonist 8-hydroxy-2-[di-(n-propyl)amino]tetralin on neuronal differentiation of hypothalamic cells from 12- to 15-day embryos. Repeated treatment of cultures with the serotonin agonist for 10 days resulted in an increased number of serotonin cells containing high levels of decarboxylase activity. Both the increase in cell numbers and the elevated decarboxylase activity could be suppressed by the addition of the serotonin antagonist metergoline to the culture medium. These data show that serotonin (or an agonist), acting on specific receptors, can initiate and amplify its own synthesis in embryonic hypothalamic neurons, as observed in the primitive hypothalamic nerve cell line F7 [De Vitry, F., Catelon, J., Dubois, M., Thibault, J., Barritault, D., Courty, J., Bourgoin, S. & Hamon, M. (1986) Neurochem. Int. 9, 43-53]. Such an autocrine-like mechanism may be active during nervous system development and may represent an example of learning at the cellular level.
Full text
PDF

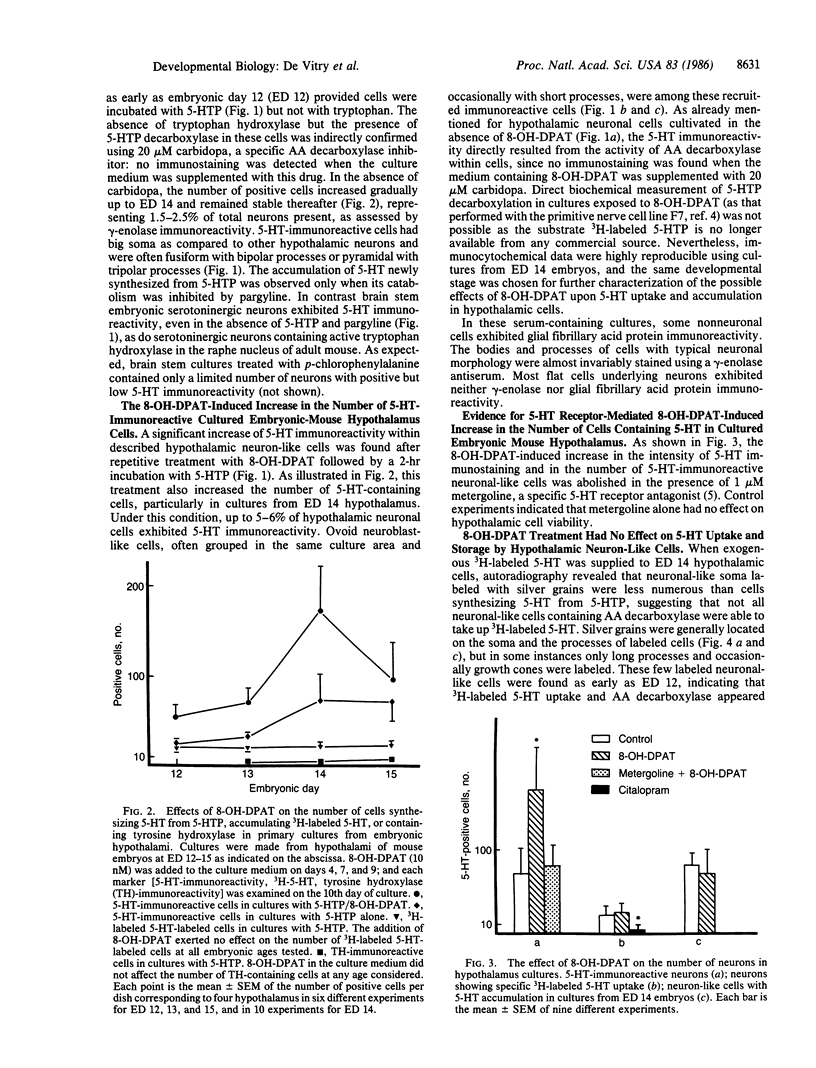

Images in this article
Selected References
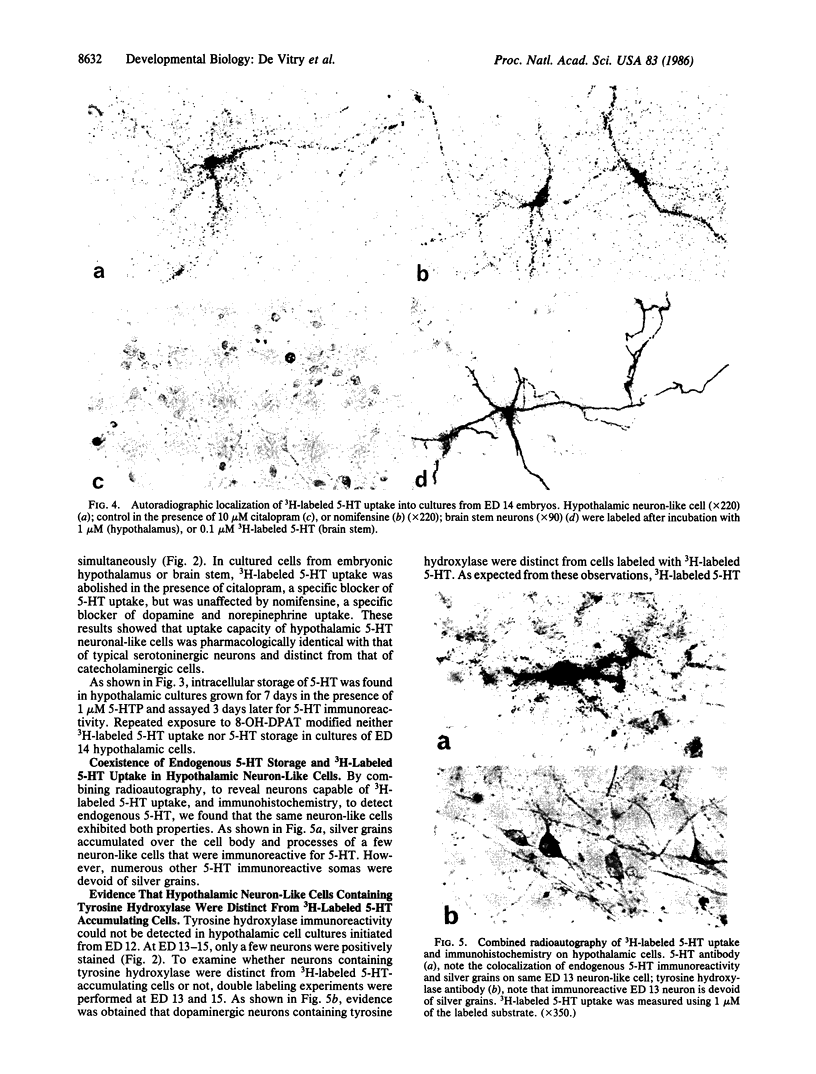
These references are in PubMed. This may not be the complete list of references from this article.
- Arluison M., Dietl M., Thibault J. Ultrastructural morphology of dopaminergic nerve terminals and synapses in the striatum of the rat using tyrosine hydroxylase immunocytochemistry: a topographical study. Brain Res Bull. 1984 Aug;13(2):269–285. doi: 10.1016/0361-9230(84)90128-x. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Indoleamine neurons and their processes in the normal rat brain and in chronic diet-induced thiamine deficiency demonstrated by uptake of 3H-serotonin. J Comp Neurol. 1977 Dec 15;176(4):467–493. doi: 10.1002/cne.901760402. [DOI] [PubMed] [Google Scholar]
- De Vitry F., Camier M., Czernichow P., Benda P., Cohen P., Tixier-Vidal A. Establishment of a clone of mouse hypothalamic neurosecretory cells synthesizing neurophysin and vasopressin. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3575–3579. doi: 10.1073/pnas.71.9.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vitry F., Picart R., Jacque C., Legault L., Dupouey P., Tixier-Vidal A. Presumptive common precursor for neuronal and glial cell lineages in mouse hypothalamus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4165–4169. doi: 10.1073/pnas.77.7.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L., Beaudet A., Watkins K. C. Serotonin nerve terminals in adult rat neocortex. Brain Res. 1975 Dec 26;100(3):563–588. doi: 10.1016/0006-8993(75)90158-4. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Ungerstedt U. Histochemical studies on the distribution of catecholamines and 5-hydroxytryptamine after intraventricular injections. Histochemie. 1968;13(1):16–28. doi: 10.1007/BF00303872. [DOI] [PubMed] [Google Scholar]
- Hamon M., Bourgoin S., Gozlan H., Hall M. D., Goetz C., Artaud F., Horn A. S. Biochemical evidence for the 5-HT agonist properties of PAT (8-hydroxy-2-(di-n-propylamino)tetralin) in the rat brain. Eur J Pharmacol. 1984 May 4;100(3-4):263–276. doi: 10.1016/0014-2999(84)90002-5. [DOI] [PubMed] [Google Scholar]
- Lauder J. M., Krebs H. Serotonin as a differentiation signal in early neurogenesis. Dev Neurosci. 1978;1(1):15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- Levitt P., Cooper M. L., Rakic P. Early divergence and changing proportions of neuronal and glial precursor cells in the primate cerebral ventricular zone. Dev Biol. 1983 Apr;96(2):472–484. doi: 10.1016/0012-1606(83)90184-7. [DOI] [PubMed] [Google Scholar]
- Sakumoto T., Sakai K., Jouvet M., Kimura H., Maeda T. Neurones de type APUD dans le système nerveux central: mise en évidence d'un groupe de neurones dans l'hypothalamus ventrolatéral du rat et du chat révélé par l'immunohistochimie de la sérotonine après administration de 5-hydroxytryptophane. C R Seances Acad Sci III. 1982 Nov 22;295(10):631–635. [PubMed] [Google Scholar]
- Teitelman G., Jaeger C. B., Albert V., Joh T. H., Reis D. J. Expression of amino acid decarboxylase in proliferating cells of the neural tube and notochord of developing rat embryo. J Neurosci. 1983 Jul;3(7):1379–1388. doi: 10.1523/JNEUROSCI.03-07-01379.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillet Y., Ravault J. P., Selve C., Evin G., Castro B., Dubois M. P. Conditions d'utilisation d'anticorps spécifiques pour la visualisation immunohistochimique de la sérotonine et de la mélatonine dans la glande pinéale du mouton. C R Acad Sci III. 1986;303(3):77–82. [PubMed] [Google Scholar]
- Yamamoto M., Steinbusch H. W., Jessell T. M. Differentiated properties of identified serotonin neurons in dissociated cultures of embryonic rat brain stem. J Cell Biol. 1981 Oct;91(1):142–152. doi: 10.1083/jcb.91.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vitry F., Delaunoy J. P., Thibault J., Buisson N., Lamande N., Legault L., Delasalle A., Dupouey P. Induction of oligodendrocyte-like properties in a primitive hypothalamic cell line by cholesterol, an eye derived growth factor and brain extract. EMBO J. 1983;2(2):199–203. doi: 10.1002/j.1460-2075.1983.tb01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]