Abstract
Background
Treatment with neoadjuvant chemotherapy (NAC) has made it possible for some women to be successfully treated with breast conservation therapy (BCT ) who were initially considered ineligible. Factors related to current practice patterns of NAC use are important to understand particularly as the surgical treatment of invasive breast cancer has changed. The goal of this study was to determine variations in neoadjuvant chemotherapy use in a large multi-center national database of patients with breast cancer.
Methods
We evaluated NAC use in patients with initially operable invasive breast cancer and potential impact on breast conservation rates. Records of 2871 women ages 18-years and older diagnosed with 2907 invasive breast cancers from January 2003 to December 2008 at four institutions across the United States were examined using the Breast Cancer Surgical Outcomes (BRCASO) database. Main outcome measures included NAC use and association with pre-operatively identified clinical factors, surgical approach (partial mastectomy [PM] or total mastectomy [TM]), and BCT failure (initial PM followed by subsequent TM).
Results
Overall, NAC utilization was 3.8%l. Factors associated with NAC use included younger age, pre-operatively known positive nodal status, and increasing clinical tumor size. NAC use and BCT failure rates increased with clinical tumor size, and there was significant variation in NAC use across institutions. Initial TM frequency approached initial PM frequency for tumors >30-40mm; BCT failure rate was 22.7% for tumors >40mm. Only 2.7% of patients undergoing initial PM and 7.2% undergoing initial TM received NAC.
Conclusions
NAC use in this study was infrequent and varied among institutions. Infrequent NAC use in patients suggests that NAC may be underutilized in eligible patients desiring breast conservation.
Introduction
The efficacy of neoadjuvant chemotherapy (NAC) in achieving successful breast conservation for women initially considered ineligible for breast conservation therapy (BCT) was demonstrated more than 15 years ago [1]. Current practice patterns of NAC use, however, are not well-described, particularly as the surgical treatment of invasive breast cancer has changed in the past 20 years. Partial mastectomy (PM) has become more common as the initial surgical procedure for management of the primary breast tumor [2,3]. This shift is largely due to the results of several randomized controlled trials and meta-analyses that established BCT to be as effective as total mastectomy (TM) with regard to overall patient survival [4,5] and the 1990 National Institutes of Health Consensus Conference [6] recommendation of BCT as an alternative to TM. More recently, TM rates appear to be increasing secondary to a variety of proposed factors, although this remains controversial [3,7-9]. Clinical conditions or patient-related factors associated with greater utilization of initial mastectomy include larger tumor size, multicentric breast cancer, family history of breast cancer, race, younger age, pre-operative breast magnetic resonance imaging (MRI) utilization, lower socioeconomic status, greater living distance from a radiation facility, patient preference, and provider preference [10-14].
Commonly accepted indications for NAC include the treatment of patients presenting with inflammatory breast cancer or T4 lesions (direct invasion of tumor into chest wall or skin) as well as treatment of women with initially operable tumors (T1c-3, N0-2, M0) who desire BCT but would be considered ineligible based on tumor size or tumor to breast size ratio [15,16]. A significant amount of clinical research has demonstrated the efficacy and safety of NAC in allowing patients initially considered ineligible for BCT to successfully undergo BCT without compromising patient overall survival. The National Surgical Adjuvant Breast and Bowel Project-18 (NSABP B-18) trial found NAC to be equally efficacious to post-operative chemotherapy in overall survival rates, while demonstrating a moderate increase in the percentage of women who were candidates for BCT, as did NSABP B-27 [1,17]. Similarly, investigators at the Milan Cancer Institute reported even greater success with NAC use, allowing for BCT in >50% of patients with large tumors (>5.0 cm) who were initially considered ineligible for BCT [18]. The results of these and other trials have led to endorsement of NAC by the National Comprehensive Cancer Network (NCCN) and an international panel of experts [19,20].
Under-utilization of effective treatments and other forms of unexplained clinical variation are recognized barriers to efficient and optimal health care. Studies have demonstrated wide variation in both regional and provider-level practice patterns that can result in decreased quality of care due to underuse, overuse, or misuse of health care services [21,22]. Comparison of NAC utilization between different health care organizations outside of clinical trials has not been well reported; therefore, with our study, we sought to examine the current pattern of NAC utilization among four geographically diverse healthcare institutions and identify factors associated with NAC use, particularly in the scenario of failed breast conservation.
Methods
Ethics Statement
Approval to conduct the study, with waiver of consent to collect patient and provider-level data, was obtained from the Institutional Review Board (IRB) of Fletcher Allen Health Care/University of Vermont, the Kaiser Permanente Colorado IRB, and the Group Health Cooperative IRB. The Marshfield Clinic Research Foundation’s IRB ceded approval to Kaiser Permanente Colorado’s IRB. Criteria for waiver of consent included minimal risk study (retrospective review of data already in existence) and only a de-identified data set that was shared and used for analysis with the Van Andel Research Institute, where the database is housed.
The source of data for this study is the Breast Cancer Surgical Outcomes (BRCASO) database. The BRCASO study was an American Recovery and Reinvestment Act (ARRA) funded comparative effectiveness study intended to evaluate and compare initial surgical treatment and outcomes of patients with newly diagnosed breast cancer from January 2003 to December 2008 [23]. The BRCASO database is a multi-institutional database of female patients (age >18 years) who underwent initial surgical treatment for breast cancer at four institutions including Fletcher Allen Health Care (affiliated with the University of Vermont) and three sites of the Cancer Research Network (CRN): Group Health Cooperative (Washington State), Kaiser Permanente of Colorado, and the Marshfield Clinic (Wisconsin). BRCASO study general eligibility criteria included female gender, pathologic confirmation of stage 0–IV invasive breast cancer, and initial surgical treatment (ie, PM or TM) performed by a study institution-employed surgeon. Women with conditions commonly associated with ineligibility for BCT including multifocal breast cancer, inflammatory breast cancer, and prior history of ipsilateral breast cancer or radiation therapy were excluded from analyses. We also excluded women with a pre-operative diagnosis of ductal carcinoma in situ (DCIS). Inclusion and exclusion criteria were designed to identify patients who would be candidates for BCT if tumor size prior to initial surgery permitted. Available data include that pertaining to surgical procedures, surgeon information, cancer information, whether or not neoadjuvant chemotherapy was given, and patient demographics and characteristics.
Each of the four study sites contributed their data to the BRCASO database. Data were collected both electronically and by extensive manual review of the medical records [23]. The three CRN affiliated study sites utilized electronically captured data elements from the Virtual Data Warehouse (VDW), a standardized source of data housed at each CRN site with standard formatting across sites. Data incorporated into the VDW includes patient information related to clinical care and health plan enrollment. One programmer familiar with the VDW wrote the programs for identifying patients and for collection of available and relevant data at the CRN sites, and the BRCASO database was prefilled with these data. Experienced chart abstractors at all sites underwent group training for this study in order to collect and report data in a standard fashion at each site. A detailed manual of instructions and definitions was available for abstractor use. Data that were specifically reviewed manually included surgical reports and pathology reports. Abstractors at each site reviewed charts from their own site and entered data directly into a database that was saved in the BRCASO database at the Van Andel Research Institute.
For this study evaluating NAC, we specifically evaluated the utilization of pre-operative systemic chemotherapy as it related to the clinician’s best estimate of tumor size before treatment initiation. The largest tumor diameter reported by radiology studies was used for analysis, and when not available, the tumor size reported by a clinician’s physical exam was used. All tumor size estimations used for analysis were obtained before chemotherapy and initial surgery. Likewise, preoperative tumor markers were analyzed, including estrogen and progesterone receptor status. Her2Neu receptor status was not analyzed, as it was not consistently available before surgery based on biopsy results alone.
NAC was selected according to the treating oncologist of each institution; specific treatment regimens were not analyzed. Patients receiving neoadjuvant endocrine therapy alone were not included in the analysis. We did not analyze the conversion rate for patients initially ineligible for BCT who then successfully underwent BCT after NAC, given the retrospective limitations. For study purposes, BCT failure was defined as patients undergoing TM after an initial PM for the same diagnostic event. PM requiring re-excision was not considered BCT failure if the final procedure was a PM.
Bivariate analyses relating demographic and clinical variables with NAC use were performed using Pearson’s Chi-square test. A Markov chain montecarlo (MCMC) approximation to Fisher’s exact test was used when cell counts were too small for the Chi-square test. Variables that were significant in the univariate analyses were included in a multivariable logistic regression model. P-values are reported for 2-sided tests and are not adjusted for multiple comparisons. All data analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
A total of 4527 patient records were reviewed in the BRCASO database and consisted of 4630 diagnostic events (including additional tumors in the contralateral breast). Of these patients, 2871 women with 2907 diagnostic events met study entry criteria and were included in statistical analyses. Subjects were excluded for undergoing initial surgery without confirmed pre-operative malignancy (n=393), pre-operative diagnosis of DCIS (n=936), pre-operative malignancy type unknown (n=16), known Stage IV breast cancer (n=6), confirmed breast cancer in an axillary node with no known primary (n=5), inflammatory breast cancer (n=75), pre-operatively known multifocal breast cancer (n=96), previous ipsilateral breast cancer (n=85), previous history of radiation therapy (n=98), and patients receiving endocrine-only neoadjuvant therapy (n=13). The remaining patients did not have identifiable clinical factors limiting eligibility for BCT. Of these patients, 111/2907 (3.8%) were treated with NAC.
Patient demographics according to utilization of NAC are shown in Table 1. Younger women (< age 35) were more likely to receive NAC (P<0.0001). Additional pre-operative variables associated with higher utilization rates of NAC included known positive nodal status (P<0.0001), increasing tumor size (P<0.0001), negative estrogen receptor (ER) status (P<0.0001), and negative progesterone receptor (PR) status (P<0.0001). There was no statistically significant difference in NAC use between invasive ductal carcinoma and invasive lobular carcinoma (P=0.1041). Utilization of NAC varied significantly across the four treatment facilities (P<0.0001).
Table 1. Patient Demographics according to utilization of neoadjuvant chemotherapy.
| Value | Initial surgery (N) | Neoadjuvant treatment (N) | Neoadjuvant treatment % | P-value | |
|---|---|---|---|---|---|
| Age | <35 | 22 | 5 | 22.73 | |
| N=2907 | 35-44 | 240 | 21 | 8.75 | |
| 45-54 | 670 | 50 | 7.46 | <0.0001 | |
| 55-64 | 794 | 22 | 2.77 | ||
| 65-74 | 616 | 10 | 1.62 | ||
| >75 | 565 | 3 | 0.53 | ||
| Ethnicity | African American | 60 | 3 | 5.00 | |
| N=2907 | Asian | 72 | 1 | 1.39 | |
| Hispanic | 54 | 2 | 3.70 | 0.3087 | |
| White/ Non-Hispanic | 2287 | 95 | 4.15 | ||
| Other/Unknown | 434 | 10 | 2.30 | ||
| Institution | 1 | 713 | 60 | 8.42 | |
| N=2907 | 2 | 1017 | 11 | 1.08 | <0.0001 |
| 3 | 922 | 23 | 2.49 | ||
| 4 | 255 | 17 | 6.67 | ||
| Preoperative tumor size | 0-10 mm | 646 | 6 | 0.93 | |
| N=2558 | >10-20mm | 1112 | 24 | 2.16 | |
| >20-30mm | 487 | 21 | 4.31 | <0.0001 | |
| >30-40 mm | 177 | 10 | 5.65 | ||
| >40-50 mm | 60 | 9 | 15.00 | ||
| >50 mm | 76 | 12 | 15.79 | ||
| Preoperative diagnosis | IDC | 2439 | 101 | 4.14 | |
| N=2907 | ILC | 282 | 7 | 2.48 | 0.1041 |
| Type Unknown | 186 | 3 | 1.61 | ||
| ER status | Positive | 2423 | 70 | 2.89 | <0.0001 |
| N=2887 | Negative | 464 | 41 | 8.84 | |
| PR status | Positive | 2223 | 62 | 2.79 | <0.0001 |
| N=2887 | Negative | 664 | 49 | 7.38 | |
| Preoperative nodal status | Negative | 2655 | 43 | 1.62 | <0.0001 |
| N=2907 | Positive | 252 | 68 | 26.98 | |
| Initial surgery | Partial mastectomy | 2202 | 60 | 2.72 | <0.0001 |
| N=2907 | Total mastectomy | 705 | 51 | 7.23 |
Abbreviations: BCT, breast conservation therapy; ER, estrogen receptor; PR, progesterone receptor; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma
A multivariable analysis of the patient, institution, and pre-operative tumor characteristics related to NAC use in the univariate analysis described above was performed (Table 2). Pre-operative positive nodal status, treating institution, pre-operative tumor size, and age were significantly associated with increased NAC utilization after controlling for other variables in the model (Table 2). Of note, ER and PR status were not independently significant.
Table 2. Multivariable analysis for association with neoadjuvant chemotherapy use.
| Value | OR | 95% CI | P-value | |
|---|---|---|---|---|
| Age | <35 | 6.77 | 1.33-34.46 | 0.021 |
| 35-44 | 3.68 | 1.32-10.29 | 0.013 | |
| 45-54 | 3.92 | 1.62-9.5 | 0.003 | |
| 55-64 | 2.31 | 0.90-5.96 | 0.083 | |
| 65-74 | ref | |||
| ≥75 | 0.44 | 0.11-1.88 | 0.269 | |
| Institution | 1 | ref | ||
| 2 | 0.08 | 0.03-0.22 | <0.0001 | |
| 3 | 0.61 | 0.33-1.10 | 0.101 | |
| 4 | 1.31 | 0.63-2.75 | 0.469 | |
| Preoperative tumor size | 0-10 mm | ref | ||
| >10-20 mm | 1.29 | 0.50-3.35 | 0.595 | |
| >20-30 mm | 1.8 | 0.67-4.85 | 0.243 | |
| >30-40 mm | 2.24 | 0.72-6.97 | 0.164 | |
| >40-50 mm | 6.27 | 1.77-22.22 | 0.004 | |
| >50 mm | 5.69 | 1.75-18.44 | 0.004 | |
| ER status | Positive | 0.81 | 0.36-1.84 | 0.612 |
| Negative | ref | |||
| PR status | Positive | 0.59 | 0.27-1.28 | 0.183 |
| Negative | ref | |||
| Preoperative nodal status | Negative | ref | ||
| Positive | 10.17 | 6.05-17.08 | <0.0001 | |
| Initial surgery | BCT | ref | ||
| Mastectomy | 1.17 | 0.66-2.08 | 0.589 |
Abbreviations: OR, odds ratio; CI, confidence interval; BCT, breast conservation therapy; ER, estrogen receptor; PR, progesterone receptor
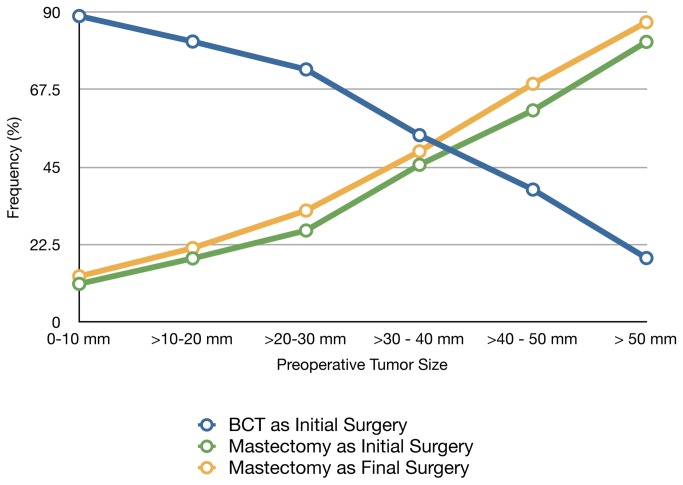
The frequencies of PM and TM as the initial or final surgical procedures were examined (Figure 1). An estimated clinical tumor size of 30–40 mm was noted to have an approximately 1:1 ratio for frequency of initial PM to initial TM. BCT was used more often for smaller tumors, while initial TM and final TM were more common for larger tumors. When these data were examined according to individual institution, the trend for increased initial TM (exceeding 50% in tumors >40 mm) was seen at each institution and was statistically significant (P<0.0001) (Table 3).
Figure 1. Frequency of Surgery Modalities according to Tumor Size.
As tumor size increases, the frequency of breast conservation therapy (BCT) decreases and the frequency of mastectomy as initial or final surgery increases. At a tumor size of >30 - 40 mm, an approximate 1:1 ratio exists between BCT and mastectomy.
Table 3. Percent of initial mastectomy and neoadjuvant therapy according to tumor size by institution.
|
Institution 1
|
Institution 2
|
Institution 3
|
Institution 4
|
P value
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-operative tumor size | Initial mastectomy | NAC | Initial mastectomy | NAC | Initial mastectomy | NAC | Initial mastectomy | NAC | Initial mastectomy | NAC |
| 0-10 mm | 7.3 | 2.4 | 16.4 | 0.0 | 16.2 | 0.0 | 8.6 | 2.9 | 0.0199 | 0.0135 |
| >10-20 mm | 9.2 | 5.8 | 21.2 | 0.3 | 27.2 | 0.7 | 24.1 | 3.6 | <0.0001 | <0.0001 |
| >20-30 mm | 21.5 | 8.3 | 35.4 | 1.1 | 27.9 | 2.6 | 21.6 | 13.5 | 0.0477 | 0.0005 |
| >30-40 mm | 34.1 | 11.4 | 58.6 | 1.4 | 40.4 | 7.7 | 63.6 | 0.0 | 0.0318 | 0.1068 |
| >40-50 mm | 57.1 | 21.4 | 76.2 | 4.7 | 50.0 | 15.0 | 80.0 | 40.0 | 0.2952 | 0.2050 |
| >50 mm | 88.5 | 3.9 | 81.8 | 0.0 | 78.3 | 43.4 | 60.0 | 20.0 | 0.4900 | <0.001 |
| P value | <0.0001 | 0.022 | <0.0001 | 0.045 | <0.0001 | <0.0001 | <0.001 | 0.002 | ||
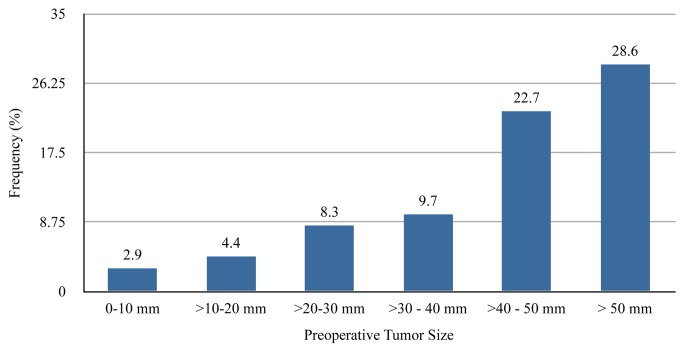
Abbreviations: NAC: neoadjuvant chemotherapyOverall, initial surgery was PM for 60 (2.7%) patients and and TM for 51 (7.2%) patients who received NAC (P<0.0001). Of the 60 patients whose initial surgery was PM, achievement of BCT occurred in 53 patients (88.3%) and 7 (11.7%) required further surgery (P=0.0905). Utilization of NAC increased with clinical tumor size, yet less than 16% of tumors >50 mm in clinical size were treated with NAC (Table 1). Larger tumors were noted to have an increasing initial TM rate compared to smaller tumors (Figure 1), with the initial TM rate >80% for tumors with clinically estimated size >50 mm. Increasing utilization of NAC for increasing tumor size was also noted when NAC use was analyzed according to each individual institution (Table 3). Interestingly, however, three of the four institutions had lower rates of NAC for tumors >50 mm compared to tumors 40-50 mm at each respective institution. The frequency of BCT failure also increased with increasing clinical tumor size, reaching 29% in tumors >50 mm in size (Figure 2).
Discussion
Clinical trials have demonstrated the benefit of NAC for increasing breast conservation rates without compromising overall survival compared to adjuvant chemotherapy [16-18,24-28]. We found that NAC use varied significantly across four large institutions, suggesting non-uniform practice patterns among the participating institutions, and potentially throughout the country. The data presented here also suggest that NAC may be relatively underutilized as a means of tumor down-staging as evidenced by BCT failure. Interestingly, the percentage of patients undergoing TM as a first procedure increased dramatically for tumor sizes >3.0 cm, rather than the 5.0 cm size traditionally evaluated in clinical trials examining the effectiveness of NAC in tumor downstaging [1,18]. A similar finding was reported in a previous single institution study [29]. The present study demonstrates that a marked increase in initial TM for patients with tumors >3.0 cm exists across multiple institutions. Our findings suggest that in addition to larger tumor size, factors such as younger age and positive nodal status are also associated with increased NAC use. NAC was used infrequently in patients aged 65 or older. Lower use in the elderly may reflect higher co-morbidity or less patient concern with breast preservation.
Common practice would suggest that NAC use would increase with increasing tumor size, as smaller tumors are more likely to be amenable to proceeding with BCT without requiring NAC. However, we did not observe a proportional increase in utilization of NAC with larger pre-operative tumor size, especially in tumors >4 cm in size (Table 1). When compared to the frequency of TM as the initial surgery, utilization of NAC is variable among the institutions, even for larger tumors. The rates of NAC use in tumors >5 cm were 3.9%, 0%, 43.5%, and 20% for the four institutions, despite frequencies of initial TM as the initial surgery of 88.5%, 81.8%, 78.3%, and 60%, respectively (Table 3). Data from these four institutions, all of which show a predilection for BCT in patients with smaller tumors, demonstrate potentially concerning variation in the utilization of NAC. At one institution, NAC use was extremely infrequent (1.1%). Two of the institutions had slightly more prevalent use (8.4% and 6.7%), but one of these surprisingly had lower utilization for tumor size >5 cm.
It is possible that many women who underwent TM as the initial procedure may have chosen TM as their treatment of choice, but we are unable to evaluate patient decision-making or education regarding potential NAC use in our study. Nevertheless, given that the majority of women with smaller breast tumors in this study chose initial BCT, it is not unreasonable to assume that women with larger tumors would also desire BCT if feasible. The large disparity between NAC use and BCT failure (compare Table 1 with Figure 2) suggests a potential opportunity to increase the rate of breast conservation through the greater use of NAC.
Figure 2. Frequency of BCT Failure by Tumor Size.
As tumor size increases, the rate of breast conservation therapy (BCT) failure increases. Frequencies are indicated above each bar.
Variation in NAC use among various treatment centers deserves further investigation into the possible causes. Legitimate variation could result from inherent differences in the patient population or presentation of disease. However, we sought to minimize these differences by careful selection of study inclusion and exclusion criteria. Despite strict selection criteria, variation in NAC use persisted across institutions. There are several potential driving factors that may account for this finding including discrepancies in available resources such as multidisciplinary breast clinics, timing of multidisciplinary tumor conferences relative to time of initial surgery, level of training, and local culture [30].
A major limitation of the present study is the retrospective nature of data collection, which limited the determination of other treatment decision-making factors. Individual patient or surgeon preference was not available for review. Women with comorbidities precluding chemotherapy, strong family history or genetic risk factors such as BRCA status, or tumors with an extensive intraductal component may account for some of the omission of NAC [7]. Patient awareness of NAC as an option before surgery and evaluation of how it may impact the success of their initial surgical therapy could not be evaluated. Similarly, prospective involvement of medical oncologists or utilization of multidisciplinary tumor conferences before treatment initiation were not evaluated as part of this study. Gene expression profile testing was not widely implemented during the timeframe of this study, and therefore, should not have impacted the results. HER2-neu status was not available, as it was not consistently tested during the study time period. Type of NAC used was not available, and specific information regarding accessibility and enrollment in clinical trials was not available. Despite these limitations, the findings of the present study suggest further investigation may be useful for optimizing patient treatment preferences and initial treatment strategy. Strategies to reduce clinical variation and match best initial treatment selection with patient desired outcomes are essential steps in quality improvement.
Management of invasive breast cancer requires an effective multi-disciplinary program. Early involvement of medical oncologists in treatment discussions may impact the utilization of NAC for newly diagnosed patients who desire BCT. Institutional variation in the use of NAC may represent an opportunity to enhance quality in overall breast cancer care. Understanding the factors that influence this variation and potential underuse of NAC may advance efforts to improve treatment outcomes and optimize patient treatment choices and patient satisfaction in breast cancer care. Further investigation into improving both patient and clinician education is crucial.
Funding Statement
This study was funded under the American recovery and Reinvestment Act of 2009 by National Institute of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB Jr, Fisher ER, Wickerham DL, Wolmark N, DeCillis A, Hoehn JL, Lees AW, Dimitrov NV (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15: 2483–2493. PubMed: 9215816. [DOI] [PubMed] [Google Scholar]
- 2. McCahill LE, Privette A, James T, Sheehey-Jones J, Ratliff J, Majercik D, Krag DN, Stanley M, Harlow S (2009) Quality measures for breast cancer surgery: initial validation of feasibility and assessment of variation among surgeons. Arch Surg 144: 455–463; discussion: 19451489. [DOI] [PubMed] [Google Scholar]
- 3. Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM (2010) Are mastectomy rates really increasing in the United States? J Clin Oncol 28: 3437–3441. doi: 10.1200/JCO.2009.27.6774. PubMed: 20548000. [DOI] [PubMed] [Google Scholar]
- 4. Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ, Menard C, Lippman ME, Lichter AS, Altemus RM (2003) Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer 98: 697–702. doi: 10.1002/cncr.11580. PubMed: 12910512. [DOI] [PubMed] [Google Scholar]
- 5. Jatoi I, Proschan MA (2005) Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol 28: 289-294. doi: 10.1097/01.coc.0000156922.58631.d7. PubMed: 15923803. [DOI] [PubMed] [Google Scholar]
- 6. NIH. consensus conference (1991) Treatment of early-stage breast cancer. JAMA 265: 391–395 [Google Scholar]
- 7. McGuire KP, Santillan AA, Kaur P, Meade T, Parbhoo J, Mathias M, Shamehdi C, Davis M, Ramos D, Cox CE (2009) Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 16: 2682–2690. doi: 10.1245/s10434-009-0635-x. PubMed: 19653046. [DOI] [PubMed] [Google Scholar]
- 8. James T, McCahill L, Ratliff J, Ashikaga T, Single R, Sheehey-Jones J, Messier N, Stanley M, Krag D, Harlow S (2009) Quality assessment of neoadjuvant therapy use in breast conservation: barriers to implementation. Breast J 15: 524–526. doi: 10.1111/j.1524-4741.2009.00771.x. PubMed: 19624412. [DOI] [PubMed] [Google Scholar]
- 9. Damle S, Teal CB, Lenert JJ, Marshall EC, Pan Q, McSwain AP (2011) Mastectomy and contralateral prophylactic mastectomy rates: an institutional review. Ann Surg Oncol 18: 1356–1363. doi: 10.1245/s10434-010-1434-0. PubMed: 21125335. [DOI] [PubMed] [Google Scholar]
- 10. Nattinger AB, Kneusel RT, Hoffmann RG, Gilligan MA (2001) Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst 93: 1344–1346. doi: 10.1093/jnci/93.17.1344. PubMed: 11535710. [DOI] [PubMed] [Google Scholar]
- 11. Gilligan MA, Kneusel RT, Hoffmann RG, Greer AL, Nattinger AB (2002) Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Med Care 40: 181–189. doi: 10.1097/00005650-200203000-00002. PubMed: 11880791. [DOI] [PubMed] [Google Scholar]
- 12. Gomez SL, France AM, Lee MM (2004) Socioeconomic status, immigration/acculturation, and ethnic variations in breast conserving surgery, San Francisco Bay area. Ethn Dis 14: 134–140. PubMed: 15002933. [PubMed] [Google Scholar]
- 13. Temple WJ, Russell ML, Parsons LL, Huber SM, Jones CA, Bankes J, Eliasziw M (2006) Conservation surgery for breast cancer as the preferred choice: a prospective analysis. J Clin Oncol 24: 3367–3373. doi: 10.1200/JCO.2005.02.7771. PubMed: 16849750. [DOI] [PubMed] [Google Scholar]
- 14. McCahill LE, Privette AR, Hart MR, James TA (2009) Are mastectomy rates a reasonable quality measure of breast cancer surgery? Am J Surg 197: 216–221. doi: 10.1016/j.amjsurg.2007.12.056. PubMed: 18614141. [DOI] [PubMed] [Google Scholar]
- 15. Mieog JS, van der Hage JA, van de Velde CJ (2007) Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 94: 1189–1200. doi: 10.1002/bjs.5894. PubMed: 17701939. [DOI] [PubMed] [Google Scholar]
- 16. Moreno-Aspitia A (2012) Neoadjuvant therapy in early-stage breast cancer. Crit Rev Oncol Hematol 82: 187‑199. doi: 10.1016/j.critrevonc.2011.04.013. PubMed: 21616677. [DOI] [PubMed] [Google Scholar]
- 17. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26: 778–785 [DOI] [PubMed]
- 18. Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, Zambetti M (1998) Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol 16: 93-100. PubMed: 9440728. [DOI] [PubMed] [Google Scholar]
- 19. Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, Colleoni M, Denkert C, Eiermann W, Jackesz R, Makris A, Miller W, Pierga JY, Semiglazov V, Schneeweiss A, Souchon R, Stearns V, Untch M, Loibl S (2007) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol 18: 1927–1934. doi: 10.1093/annonc/mdm201. PubMed: 17998286. [DOI] [PubMed] [Google Scholar]
- 20. Kaufmann M, Morrow M, von Minckwitz G, Harris JR, Biedenkopf Expert Panel Members (2010) Locoregional treatment of primary breast cancer: consensus recommendations from an International Expert Panel. Cancer 116: 1184–1191. doi: 10.1002/cncr.24874. PubMed: 20087962. [DOI] [PubMed] [Google Scholar]
- 21. Foster JA, Abdolrasulnia M, Doroodchi H, McClure J, Casebeer L (2009) Practice patterns and guideline adherence of medical oncologists in managing patients with early breast cancer. J Natl Compr Canc Netw 7: 697–706. PubMed: 19635225. [DOI] [PubMed] [Google Scholar]
- 22. Chassin MR, Galvin RW (1998) The urgent need to improve health care quality. JAMA 280: 1000–1005. doi: 10.1001/jama.280.11.1000. PubMed: 9749483. [DOI] [PubMed] [Google Scholar]
- 23. Aiello Bowles EJ, Feigelson HS, Barney T, Broecker K, Sterrett A, Bischoff K, Engel J, Gundersen G, Sheehey-Jones J, Single R, Onitilo A, James TA, McCahill LE (2012) Improving quality of breast cancer surgery through development of a national breast cancer surgical outcomes (BRCASO) research database. BMC Cancer 12: 136. doi: 10.1186/1471-2407-12-136. PubMed: 22472011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB Jr, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16: 2672‑2685. PubMed: 9704717. [DOI] [PubMed] [Google Scholar]
- 25. Peintinger F, Symmans WF, Gonzalez-Angulo AM, Boughey JC, Buzdar AU, Yu TK, Hunt KK, Singletary SE, Babiera GV, Lucci A, Meric-Bernstam F, Kuerer HM (2006) The safety of breast-conserving surgery in patients who achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer 107: 1248‑1254. doi: 10.1002/cncr.22111. PubMed: 16862596. [DOI] [PubMed] [Google Scholar]
- 26. Thomas A, Ohlinger R, Hauschild M, Mustea A, Blohmer JU, Kümmel S (2006) Options and limits of surgery after pre-operative chemotherapy in breast cancer. Anticancer Res 26: 1677–1682. PubMed: 16617561. [PubMed] [Google Scholar]
- 27. Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B, Winer EP (2008) Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 26: 814–819. doi: 10.1200/JCO.2007.15.3510. PubMed: 18258991. [DOI] [PubMed] [Google Scholar]
- 28. Roth SL, Audretsch W, Bojar H, Lang I, Willers R, Budach W (2010) Retrospective study of neoadjuvant versus adjuvant radiochemotherapy in locally advanced noninflammatory breast cancer: survival advantage in cT2 category by neoadjuvant radiochemotherapy. Strahlenther Onkol 186: 299–306. doi: 10.1007/s00066-010-2143-0. PubMed: 20495968. [DOI] [PubMed] [Google Scholar]
- 29. James TA, McCahill LE (2010) Variant mastectomy rates: implications for quality of care in breast cancer surgery. J Clin Oncol 28: 2364; author reply: 20547985. [DOI] [PubMed] [Google Scholar]
- 30. Caldon LJ, Walters SJ, Ratcliffe J, Reed MW (2007) What influences clinicians’ operative preferences for women with breast cancer? An application of the discrete choice experiment. Eur J Cancer 43: 1662–1669. doi: 10.1016/j.ejca.2007.04.021. PubMed: 17555955. [DOI] [PubMed] [Google Scholar]