Abstract
Objective
To compare the presence or absence of meal replacements (MRs) and an energy density (ED) intervention to facilitate weight loss maintenance.
Design and Methods
238 overweight primary care patients (mean BMI= 39.5 kg/m2) began the study; 132 completed the 12-week weight loss phase. Participants were randomly assigned to one of four maintenance conditions formed by crossing the presence or absence of MRs (MR+/MR−) and of the ED program (ED+/ED−) during a subsequent 9-month maintenance phase. Follow-ups assessments occurred 1 and 2 years after treatment termination.
Results
Participants initially lost 6.1 kg. Analyses of variance based on weight change from the beginning of the maintenance phase to the 2-year follow-up produced a significant interaction. All groups except ED+/MR− regained substantial weight during follow-up; the ED+/MR− group regained significantly less weight than the control group at both follow-up assessments. No significant effects of treatment were found for several variables that were expected to mediate these outcomes.
Conclusions
Because weight losses achieved in lifestyle change programs for obesity are rarely maintained, the superior outcome achieved by the ED+/MR− condition is notable. Nonetheless, methodological issues and inability to identify a potential mediator of this outcome makes replication of this finding essential.
Keywords: obesity, maintenance, weight loss, energy density, meal replacements
Introduction
Despite intensive efforts to improve long-term weight control (1), weight loss maintenance continues to be an elusive goal in lifestyle change programs. In regard to energy intake, Lowe(2) proposed three possible reasons for this. First, lifestyle change programs place roughly equal emphasis on scores of diverse lifestyle changes but evidence suggests that specific attributes of the food environment (e.g., the availability of high-calorie foods) and of food itself (e.g., portioning and energy density) play a disproportionately large role in appetite and weight control (3). This suggests that it may be advantageous to make modification of participants’ personal food environments a major focus of treatment. Second, changes in self-control skills (e.g., self-monitoring, stimulus control, boosting motivation), though useful for weight loss, apparently cannot be reliably maintained, resulting in weight regain when treatment ends. Third, because obese individuals are particularly sensitive to the influence of food cues in the environment (5–9), they might benefit the most from an approach that directly produces and sustains nutritional changes in their personal food environments (2, 3). In this study two nutrition-focused strategies for enhancing the maintenance of weight loss were tested. The first was the use of meal replacements (MRs); past research has suggested their usefulness for weight loss (10) and maintenance (11). The second strategy was training in a reduced energy density eating (ED) approach to food selection and preparation. This approach (i.e., reducing the number of calories in a given weight or volume of food) is a promising strategy for weight loss maintenance because it promotes consumption of a satisfying volume of food while minimizing caloric intake (3, 12, 13). In the current study, participants also were instructed to make changes in their food purchases and food preparation so the ED of the foods in their personal food environment (2) was reduced in ways that would be sustainable after treatment ended.
To evaluate the effect of the MR and ED interventions specifically for weight loss maintenance, all participants first completed a 12-week behavioral weight loss program. Participants were randomized into one of four maintenance conditions before the weight loss phase but both participants and their group leaders were kept blind about their assignment until week 13. In this manner participants were able to remain in the same group for the full 12-month treatment period. The 9-month maintenance conditions were: (a) lifestyle modification only (i.e. the absence of both MRs and the ED program, designated as MR−/ED−), (b) lifestyle modification plus MRs (MR+/ED−), (c) lifestyle modification plus the ED intervention (MR−/ED+), or (d) lifestyle modification plus MRs and the ED intervention (MR+/ED+). The study tested the hypothesis that the latter three experimental conditions would produce superior weight loss maintenance at 1- and 2-year follow-ups.
Methods and procedures
Participants
A total of 238 overweight primary care patients (65.8% African American, 89% women, mean BMI= 39.5 kg/m2) were recruited by physicians in primary care offices. Participants had to be between the ages of 18–70 years, have a body mass index over 30 kg/m2 (or 27 kg/m2 with a comorbid medical condition), and have regular access to a telephone. Exclusion criteria included lactose intolerance, serious psychiatric disorders and medical conditions and drug regimens know to affect body weight or appetite.
The 132 participants who completed the 12-week weight loss phase were randomly assigned to one of four weight maintenance groups formed by crossing two factors: 1) the presence or absence of ongoing use of one meal and one snack replacement per day (MR+; n= 66 and MR−; n = 66) and 2) the presence or absence of a reduced energy density eating (ED) program (ED+; n=72 and ED−; n = 60). Informed consent was obtained and the study was approved by Drexel University’s Institutional Review Board.
Intervention
Weight Loss Phase
All participants were instructed to: 1) follow a 12-week weight loss diet using two meal replacements per day, plus a controlled meal and planned snacks, 2) read weekly lifestyle change modules from the LEARN manual (14), and 3) implement treatment recommendations via weekly 15-minute phone calls with a Weight Control Specialist (WCS). WCSs had graduate degrees in clinical psychology or nutrition. Participants were instructed to follow a balanced 1200–1500 kcal, MR-supplemented diet (using Slim Fast®); MR shakes and bars were provided free of charge. The WCS also reviewed appropriate use of MRs, answered questions, and provided support to motivate participants to continue adhering to LEARN recommendations. During the weight loss phase, all participants weighed themselves at least weekly on digital scales that were provided to them and reported their weight during each phone call with their WCS.
Maintenance Phase
Participants were randomly assigned to weight loss maintenance interventions formed by crossing two factors: 1) continued use of MRs or not; and 2) introduction of a reduced energy density eating (ED) program or not. During this phase the 15-minute phone calls and written educational modules continued, but gradually decreased in frequency from weekly for the first 3 months, to bi-weekly for the next 3 months, and then monthly for the final three months. MRs continued to be provided without charge to the MR+ conditions until treatment ended.
MR−/ED− Condition (n = 33)
In this condition, the focus was on learning to incorporate conventional foods into the diet (to achieve a maintenance level of caloric intake following LEARN manual guidelines for macronutrient composition of the diet).
MR+/ED− Condition (n=27)
Participants in this condition were told that MRs would constitute a significant part of their weight maintenance program. They were taught how to build in MRs to replace one meal and one snack per day. A rationale for the continued, long-term use of MRs to support weight loss maintenance was provided.
MR−/ED+ Condition (n= 33)
Participants in this condition were given the book Volumetrics (15) and individual modules that complemented the material in the book (12, 13). The discontinuation of MRs was explained as a necessary transition to learning how to maintain lost weight using regular foods. The ED condition emphasized purchasing and preparing foods lower in energy density, mostly by reducing fat content and/or increasing water content of foods throughout the 9-month maintenance phase. Regular homework assignments aimed at having participants systematically prepare or purchase foods that were reduced in energy density to permanently replace ingredients or foods that had been a regular part of their diet. The goal was to change the energy density of as many foods as possible in participants’ personal food environments (2) in ways that could be sustained. These environments included their car, workplace and any other contexts where they regularly spent time. Participants were also encouraged to begin meals with a food low in energy density (e.g., broth-based soups).
MR+/ED+ Condition (n= 39)
This condition combined the use of MRs and the ED condition as described above.
The same physical activity prescription was used across all conditions. All participants were encouraged to gradually increase their levels of structured physical activity (usually brisk walking) so that they were engaging in it at least 150 minutes per week.
Study design and measures
Assessments were conducted at baseline (prior to the start of the weight loss phase) and at months 3, 12, 24 and 36. Height was measured with a stadiometer at the baseline assessment and weight was measured with a digital scale at each assessment point.
24-hour food recalls
Dietary recalls were collected at each assessment (except month 36) and analyzed by the the Diet Assessment Center at Pennsylvania State University. The 24-hour recall has become the best available method for dietary assessment (16 – 18). The total energy and energy density of the diet (kcals/total weight of food and beverages) was analyzed; solid foods and beverages were analyzed separately (13).
Blood lipids and blood pressure
Fasting cholesterol, triglycerides, and HDL- and LDL-cholesterol were assessed. Blood pressure was also measured (using the final two of three measurements - 19).
Hemoglobin A1c
Hemoglobin A1c reflects the glycemic history over the previous 2 to 3 months and is positively impacted by weight loss, physical activity, improved diet composition.
Waist circumference
Waist circumference was measured (at the umbilicus) because it conveys information about health risks associated with obesity beyond body mass itself.
Eating Inventory
Cognitive restraint measures the efforts toward control of food intake and Disinhibition assesses tendencies toward overeating (20). The ability of these subscales to predict various aspects of eating behavior has been demonstrated (21, 22); Cognitive Restraint has been subdivided into flexible and rigid eating control subscales (22).
Weight Efficacy Life-Style Questionnaire (WEL)
The Weight Efficacy Life-Style Questionnaire (23) is a well-supported, 20-item scale that measures perceived self-efficacy in controlling eating in five types of situations (23, 24).
Physical activity measure
The Short Physical Activity History questionnaire (25) was used, with two modifications (a bout of activity was defined as 20 minutes and respondents were told to compare themselves to others of the same age and sex). Its reliability and validity have been supported (26).
Home food environment measure
Each participant completed a home food environment survey, which is a checklist of the foods stored in their refrigerator and cabinets (26). Similar questionnaires have demonstrated acceptable test-retest and inter-rater reliability (26, 27).
Statistical analysis
Analysis of variance and chi-square were used to compare treatment groups (treated as a 4-level between-subjects factor in these analyses) on (a) baseline demographic characteristics and body size (i.e., age, race, ethnicity, gender, BMI) and (b) outcome measures (weight change, blood pressure, cholesterol, glycemic control, food pantry, energy density of the diet, eating behavior, and physical activity) at the end of the initial 3-month weight loss phase (at the time of randomization to a maintenance condition). Participants who did not complete the initial weight loss phase were not randomized to a maintenance intervention.
The effect of the two maintenance interventions on change in outcomes during the maintenance phase was examined using linear and nonlinear mixed models. As a first step, unconditional models were specified for each outcome variable to test for linear and exponential trends over time, and to evaluate the variance components associated with slopes to determine assignment as fixed versus random effects (all slopes were treated as random effects). Given our 2×2 factorial design (MR−/+ and ED−/+), two binary variables representing the effect of the two maintenance conditions were then added simultaneously to the best fitting model from step 1, along with their interaction. This approach allowed us to test for main effects of the ED and MR conditions, as well as to test for an interaction (which, if not significant, was not retained in the final model). These analyses included the following covariates: the value of the outcome at baseline and the time of randomization, gender, race (Caucasian vs. non-Caucasian), and weight loss from baseline to 3-months. As a final step, the linear mixed models were used to estimate least squares (LS) means for each outcome, for each cell of the 2 ×2 design at 12-, 24-, and 36-months. The four means were compared against each other in post-hoc tests (see Tables 2 & 3).
TABLE 2.
Weight change from end of initial weight loss phase to 12, 24, and 36 month follow-up
| Condition | Weight Change (kg) | Standard Error |
|---|---|---|
| 12-Month Follow-up | ||
| Control | 1.33 a | 0.45 |
| MR | 0.46 a | 0.46 |
| ED | 0.29 a | 0.51 |
| MR+ED | 0.86 a | 0.39 |
| 24-Month Follow-up | ||
| Control | 3.20 a | 0.89 |
| MR | 1.38 ab | 0.90 |
| ED | 0.41 b | 1.03 |
| MR+ED | 2.70 ab | 0.76 |
| 36-Month Follow-up | ||
| Control | 5.06 a | 1.41 |
| MR | 2.30 ab | 1.42 |
| ED | 0.52 b | 1.63 |
| MR+ED | 4.55 ab | 1.21 |
Note: Tables contain LS means. Within each follow-up period, means with the same superscript do not differ at p< .05.
TABLE 3.
Nutritional composition of the diet at 3, 12, 24, and 36 months by condition
| MR−/ED− (Control) | MR−/ED+ | MR+/ED− | ED+MR | |
|---|---|---|---|---|
| 3 Months | ||||
| Energy density (kcal/g) | 0.67 ± 0.03 | 0.67 ± 0.03 | 0.65 ± 0.03 | 0.65 ± 0.02 |
| Kcal | 1095.7 ± 28.0 | 1074.0 ± 28.6 | 1069.9 ± 28.7 | 1048.3 ± 25.9 |
| % kcal fat | 31.5 ± 0.6 | 31.1 ± 0.6 | 32.0 ± 0.6 | 31.6 ± 0.6 |
| % kcal carb. | 51.4 ± 0.8 | 51.1 ± 0.8 | 50.7 ± 0.8 | 50.4 ± 0.8 |
| % kcal protein | 21.3 ± 0.4 | 21.9 ± 0.4 | 21.8 ± 0.4 | 22.3 ± 0.4 |
| 12 Months | ||||
| Energy density (kcal/g) | .79 ± 0.03 | .83 ± 0.03 | .76 ± 0.03 | .80 ± 0.02 |
| Kcal | 1148.2 ± 35.1 | 1187.4 ± 36.8 | 1128.9 ± 34.5 | 1168.1 ± 30.6 |
| % kcal fat | 33.1 ± 0.7 | 32.4 ± 0.8 | 32.0 ± 0.7 | 31.4 ± 0.7 |
| % kcal carb. | 48.08 ± 0.8 | 48.8 ± 0.9 | 49.5 ± 0.8 | 50.4 ± 0.7 |
| % kcal protein | 20.7 ± 0.5 | 20.4 ± 0.5 | 20.9 ± 0.5 | 20.5 ± 0.4 |
| 24 Months | ||||
| Energy density (kcal/g) | .95 ± 0.05 | 1.05 ± 0.06 | .90 ± 0.09 | 1. 00 ± 0.05 |
| Kcal | 1218.2 ± 76.6 | 1338.5 ± 81.2 | 1207.5 ± 74.2 | 1327.8 ± 65.1 |
| % kcal fat | 35.1 ± 1.6 | 34.2 ± 1.7 | 32.1 ± 1.6 | 31.2 ± 1.4 |
| % kcal carb. | 43.5 ± 1.8 | 45.8 ± 1.9 | 47.9 ± 1.7 | 50.3 ± 1.5 |
| % kcal protein | 19.9 ± 1.0 | 18.4 ± 1.1 | 19.7 ± 1.0 | 18.1 ± 0.9 |
Note: All values are modeled means ± SEM.
Results
Characteristics of Sample
Descriptive information about the participants enrolled for the initial weight loss phase and those retained for the experimental phase is reported in Table 1.
TABLE 1.
Participant characteristics at baseline and start of weight loss maintenance (WLM) phase
| Baseline (n= 238) | Start of WLM Phase (n = 132) | |
|---|---|---|
| Age (yrs) | 45.5 ±11.8 | 48.3 ± 11.4 |
| Baseline BMI (kg/m2) | 39.5 ± 6.6 | 38.2 ± 6.1 |
| Baseline WC (cm) | 112.8 ± 13.7 | 110.2 ± 12.9 |
| Weight loss (kg) | n/a | 6.1 ± 3.7 |
| Female (%) | 89.0 | 86.5 |
| Race (%) | ||
| Caucasian | 21.9 | 27.8 |
| African American | 65.8 | 62.4 |
| American Indian | 0.4 | 0 |
| Asian | 0.4 | 0.8 |
| Other | 11.5 | 9.0 |
| Ethnicity (%) | ||
| Hispanic | 6.7 | 6.8 |
| Non-Hispanic | 93.3 | 93.2 |
Participants were randomly assigned to ED− (n = 60) or ED+ (n =72), and the same participants were assigned to MR− (n = 66) or MR+ (n = 66). Treatment groups did not differ at baseline according to age, BMI, race, ethnicity, or gender (all p-values > 0.05).
Participants who completed the initial weight loss phase (n = 132) were significantly older than those who did not (n = 104; M ± SEM = 48.4 ± 1.0 vs. 41.9 ± 1.1 years, respectively, p < 0.001), had significantly lower BMIs at baseline (M ± SEM = 38.2 ± 0.5 vs. 41.2 ± 0.7 kg/m2, p < 0.001) and were significantly more likely to be Caucasian than African-American (p < 0.05). There were no significant differences in gender (p > 0.10). The percentages of participants that completed the weight loss phase who attended the end of treatment assessment, 12-month follow-up assessment and 24-month follow-up assessment were 65.9% (n=87), 62.1% (n=82) and 67.4% (n=89), respectively. The percentage of participants who contributed no follow-up data was 20.5% (n = 27). This degree of missingness is within acceptable limits for HLM analyses (28).
Weight Loss Maintenance by Condition
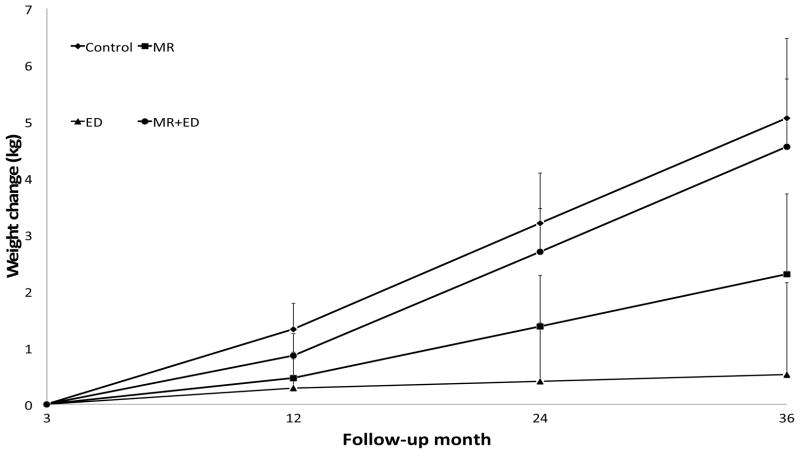
Weight loss at randomization (3-months) did not significantly differ between conditions (M ± SEM = 5.8 ± 0.6 kg in the MR−/ED− group, 5.9 ± 0.7 kg in MR−/ED+, 6.7 ± 0.6 kg in MR+/ED−, and 6.2 ± 0.6 kg in MR+/ED+; p > 0.80). Weight change during the maintenance phase (through 36-month follow-up) was characterized by a positive linear trend of weight regain over time (p < 0.001). A statistically significant interaction between the experimental conditions (MR−/+ and ED−/+) and time was detected for change in weight (p < 0.05). As shown in Table 2, the four conditions did not significantly differ at end of treatment (12-months) but at both 24- and 36-month follow-ups the ED group experienced significantly less weight regain than the control group (see Figure 1).
Figure 1.
Change in weight from end of weight loss phase to 36-month follow-up, by condition. At both 24- and 36-month follow-ups, the ED group experienced significantly less weight regain than the control group. (Error bars are standard errors of the means; for the sake of clarity, only the top half of error bars are included.)
For the remaining results, equivalence of experimental groups at time of randomization was confirmed except where noted.
Waist Circumference, Blood Pressure, Cholesterol, and Glycemic Control
Waist circumference decreased slightly early in the maintenance phase and then began to increase from 24-months to 36-months (p = .036); group had no effect (p’s > .20).
Systolic blood pressure increased during maintenance (p > 0.05), but there was no effect of treatment group (p > 0.05); no effects for diastolic blood pressure were found.
At randomization, total cholesterol (p < 0.001) and LDL cholesterol (p < 0.05) were higher in ED+ vs. ED−. There was a statistically significant effect of the ED intervention on total cholesterol (p < 0.05) and LDL cholesterol (p < 0.05); ED and ED+MR groups tended to experience little change or a decrease in these variables over the maintenance phase, whereas control and MR participants showed an increase. The ED+/MR− group exhibited a small decrease in HDL cholesterol during maintenance whereas the other groups showed a small increase (p < 0.05). When weight change during maintenance was controlled, the effect of group assignment on total cholesterol and LDL became nonsignificant but the effect of group assignment on HDL was slightly magnified. No significant group or time effects were found for triglycerides, the ratio of total cholesterol to HDL cholesterol or HbA1c.
Nutritional Composition of the Diet
The pattern of results was similar across method of energy density so only total energy density (foods plus beverages) results are reported (see Table 3). Both caloric intake and energy density increased significantly over the maintenance phase but no group effects were found.
Percent of calories from fat did not change significantly between groups or over time. Percent of calories from carbohydrate (p < 0.01) and protein (p < 0.001) decreased over time. The MR condition experienced less of a decline in percent of calories from carbohydrates (p < .05). There was no effect of group on change in percent of calories from protein (p > 0.10).
The number of high fat and low fat foods in the home did not change with time (p’s > 0.10) and no between group differences were detected (p’s > 0.10).
Eating Behavior
Cognitive restraint (p < 0.001) decreased over time but there was no change in disinhibition (p > 0.30); no effect of group was found for either measure.
Physical Activity
Physical activity increased early in the maintenance phase and then decreased later in the maintenance phase (p = 0.049); group had no effect (p’s > 0.10).
Discussion
This study combined elements of an effectiveness design (recruitment of primary care patients, relaxed inclusion/exclusion criteria, absence of run-in period to exclude poorly motivated applicants) and an efficacy design (manualized interventions, thoroughly trained interventionists) to examine the benefits for weight loss maintenance of adding meal replacements, a reduced energy density eating program, or both to behavioral treatment. The relatively high attrition rate (44%) during the weight loss phase was presumably due to the fact that any eligible patient referred by his or her primary care doctor could enroll. Notably, the elevated attrition rate did not affect the internal validity of our study because our hypotheses were based solely on the maintenance of lost weight.
The weight loss phase produced smaller weight losses (a mean reduction of 6 kg or 3.3%) than those reported in most previous lifestyle change programs, which could be due to the relatively short weight loss phase of 12 weeks or to the fact that treatment was administered through individual phone calls instead of a group format. The significant three-way interaction between experimental conditions (MR and ED status) and time indicated that there were significant differences in the extent to which the four groups kept off their lost weight. The only significant between-group difference indicated that the MR−/ED+ group maintained their weight losses better than the control group at 24 and 36 months. In a previous study, individuals in a reduced energy density diet program maintained their weight loss for 6 months but then started regaining weight (3).
The weight loss maintenance shown by the MR−/ED+ group is encouraging because in most past studies of lifestyle change for weight loss, weight regain began shortly after treatment ended. On the other hand, if MRs have the potential to also facilitate weight loss maintenance as some past research suggests (11), then it is unclear why the MR+/ED+ group did so poorly during maintenance. One plausible explanation is that the convenience and simplicity of the MRs, and the fact that they were given free of charge during the 9-month maintenance phase of the intervention, led participants to rely on them to continue losing weight or avoid weight regain, thereby undermining these participants’ motivation to learn and consistently implement the strategies that were being taught simultaneously in the ED program from months 4–9. If accurate, this would suggest that the potential benefits of MRs for weight loss (10) may be counteracted during maintenance if interventions taught simultaneously with MR use are not well learned because participants are relying too heavily on MRs to manage their weight.
To better understand the poor results of the MR+ condition, we examined participants’ reports of the use of MRs (which were collected at the 24 and 36 month assessments). At 24 months, 69.5% of those in the MR+ condition and 50% of those in the MR− condition reported using MRs for weight control; the comparable figures at 36 months were 43.1% and 40.5%. There was no difference in weight change between those who did and did not make further use of MRs from 12 to 24 or from 24 to 36 months. Therefore, because the post-intervention compliance with meal replacement usage was incomplete in the MR+ condition, and because a surprisingly large percentage of participants in the MR− condition also reported use of meal replacements, a meaningful difference in MR usage during the 24 maintenenace phase was not achieved. It is possible that many MR− participants, who used MRs during the weight loss phase but then discontinued their use from months 4 through 9, returned to MRs during maintenance in an effort to prevent or reverse weight gain. This possibility suggests that in future studies of MRs for maintenance, investigators should test whether participants in a MR− condition are not in fact using MRs during maintenance and that most of those in a MR+ condition are in fact continuing to use them during maintenance. Most past studies have not assessed MR usage following the intervention period.
In terms of blood lipids, the two ED groups experienced little change in total and LDL cholesterol during maintenance whereas the two groups that did not receive the ED intervention experienced a significant increase in these lipids. The absence of change in total and LDL cholesterol suggests that something about the two ED+ conditions prevented the rise in these outcomes experienced by the two ED− conditions. Finally, the interaction effect on HDL cholesterol presumably reflects the superior weight loss maintenance of the MR−/ED+ condition and the fact that HDL (a protective lipid) is a fraction of total cholesterol (which also declined in this condition).
Given the weight loss maintenance achieved by the MR−/ED+ group, it was surprising that none of the possible mediators of this outcome changed differentially in this group. A possible explanation for the lack of differences in the total energy and energy density of the participants’ diets is that the ED group, who must have shown enduring improvements in their energy intake to maintain their weight losses for two years, devoted more attention and effort to maintaining changes in their diets and therefore demonstrated less underreporting of energy intake than the other three groups when dietary intake was assessed at follow-up. That is, it is possible that the success demonstrated by the MR−/ED+ group reflected reduced energy intake but also less underreporting of intake, which could account for why energy intake reports of this group were actually the highest of the four groups.
A novel feature of this study was the use of weight loss during the first 12 weeks as a covariate when examining the effects of the interventions on weight loss maintenance. This procedure accounted for some of the individual differences in success at weight control that otherwise would have been treated as error variance, increasing the ability of the study to detect group differences during maintenance.
This is one of the few studies that have evaluated interventions for weight loss maintenance as distinct from weight loss. Other strengths of the study include its use of primary care patients, its inclusion of a high percentage of African-Americans, the comprehensiveness of its outcome measures, and its measurement of follow-up one and two years after the end of treatment. One weakness of the study was its relatively high attrition rate during the weight loss phase, which raises questions about the generalization of our findings to those who join a weight loss program. Also, the relatively modest weight losses achieved (an average of 3.3% of starting weight) may have reduced the potential to identify mediators of the treatment effect that was found.
In conclusion, the present findings can be seen from two opposing perspectives. On one hand any intervention that offers the hope of improving the typically disappointing long-term outcomes of previous weight loss studies is noteworthy. On the other hand, this implication is balanced by several others, including the poor outcome found for the MR+/ED+ group and the fact that several nutrition-related variables that were expected to partially account for the superior performance of the ED group failed to do so. More research is warranted on the use of reduced energy density diets and a focus on changing participants’ personal food environments for weight loss maintenance. Such research might also benefit from incorporating additional nutrition-focused strategies (2) to extend the durability of weight losses.
TABLE 4.
Secondary outcomes at 3, 12, 24, and 36 months by condition
| MR−/ED− (Control) | MR−/ED+ | MR+/ED− | MR+/ED+ | |
|---|---|---|---|---|
| 3 Months | ||||
| Blood Pressure (sys) | 122.4 ± 2.7 | 123.5 ± 2.8 | 122.8 ± 2.7 | 122.5 ± 2.3 |
| Blood Pressure (dia) | 78.9 ± 1.8 | 78.6 ± 1.8 | 81.1 ± 1.8 | 81.3 ± 1.5 |
| Cholesterol (total) | 168.0 ± 6.8 a | 195.0 ± 7.0 b | 179.4 ± 6.8 ab | 188.4 ± 5.7 ab |
| Cholesterol (HDL) | 49.5 ± 2.8 | 52.6 ± 2.8 | 52.1 ± 2.8 | 54.6 ± 2.3 |
| Cholesterol (LDL) | 97.5 ± 6.0 a | 119.7 ± 6.1 b | 106.3 ± 6.0 ab | 112.0 ± 5.0ab |
| Cholesterol (ratio) | 3.59 ± .17 | 3.85 ± .18 | 3.56 ± .17 | 3.58 ± .14 |
| Triglycerides | 104.9 ± 8.5 | 113.2 ± 8.7 | 105.1 ± 8.5 | 109.0 ± 7.2 |
| HbA1c | 6.01 ± .16 | 5.90 ± .16 | 6.02 ± .16 | 6.05 ± .13 |
| 12 Months | ||||
| Blood Pressure (sys) | 123.7 ± 1.0 | 123.0 ± 1.1 | 125.3 ± 1.1 | 124.7 ± 0.9 |
| Blood Pressure (dia) | 81.2 ± 0.7 | 79.9 ± 0.7 | 81.2 ± 0.7 | 79.9 ± 0.6 |
| Cholesterol (total) | 187.2 ± 3.0a | 179.5 ± 3.4b | 192.5 ± 2.9 a | 184.7 ± 2.6 b |
| Cholesterol (HDL) | 55.7 ± 1.0 a | 51.7 ± 1.3 b | 54.8 ± 1.0 a | 56.8 ± 0.9 a |
| Cholesterol (LDL) | 109.5 ± 2.6 | 103.7 ± 2.9 | 113.3 ± 2.5 | 107.6 ± 2.3 |
| Cholesterol (ratio) | 3.62 ± .07 | 3.48 ± .08 | 3.63 ± .07 | 3.49 ± .06 |
| Triglycerides | 111.2 ± 6.1 | 116.6 ± 7.0 | 108.8 ± 6.3 | 107.7 ± 5.5 |
| HbA1c | 6.09 ± .07 | 6.04 ± .08 | 6.08 ± .07 | 6.03 ± .06 |
| 24 Months | ||||
| Blood Pressure (sys) | 126.0 ± 1.5 | 123.0 ± 1.7 | 129.9 ± 1.6 | 125.9 ± 1.4 |
| Blood Pressure (dia) | 82.9 ± 1.1 | 80.1 ± 1.2 | 82.4 ± 1.1 | 79.6 ± 1.0 |
| Cholesterol (total) | 196.6 ± 6.7 a | 178.3 ± 7.6 b | 205.7 ± 6.5 a | 187.4 ± 5.9 b |
| Cholesterol (HDL) | 58.8 ± 2.4 a | 50.7 ± 3.0 b | 57.0 ± 2.3 a | 62.0 ± 2.0 a |
| Cholesterol (LDL) | 114.5 ± 5.7 a | 100.3 ± 6.5b | 121.1 ± 5.6a | 106.9 ± 5.0 b |
| Cholesterol (ratio) | 3.68 ± .16 | 3.37 ± .161 | 3.67 ± .16 | 3.34 ± 1.4 |
| Triglycerides | 124.8 ± 11.9 | 120.3 ± 13.4 | 120.8 ± 12.1 | 109.7 ± 11.1 |
| HbA1c | 6.25 ± .17 | 6.12 ± .19 | 6.22 ± .17 | 6.09 ± .15 |
| 36 Months | ||||
| Blood Pressure (sys) | 128.2 ± 2.4 | 122.9 ± 2.9 | 132.4 ± 2.5 | 127.1 ± 2.2 |
| Blood Pressure (dia) | 84.5 ± 1.7 | 80.4 ± 1.8 | 83.5 ± 1.7 | 79.4 ± 1.5 |
Note: 3-month values are raw means ± SEM. The 12-, 24, and 36-month values are estimated means ± SEM obtained from linear mixed models analysis. Means with the same superscript do not differ at p< .05.
What is already known?
Weight regain almost inevitably follows weight loss in lifestyle change programs
Meal replacements and interventions to reduce the energy density of the diet hold promise to improve weight loss maintenance
What does this study add?
Randomized controlled trial of weight loss maintenance in mostly African-American primary care patients
meal replacements did not facilitate weight loss maintenance
the reduced energy density diet without meal replacements produced long-term maintenance
Acknowledgments
This research was supported by grant R01-DK066759 from the National Institute of Diabetes and Digestive and Kidney Diseases. We wish to thank the Slim Fast Company for their provision of MRs at cost. The authors’ responsibilities were as follows – MRL and MLB project conception; MRL and MLB development of overall research plan; MRL, MLB, JGT and MC conducted research; JGT performed statistical analyses; MRL, MLB, JGT and MC wrote the first draft of the manuscript; and all authors had primary responsibility for final content.
Footnotes
Conflicts of Interest
Michael R. Lowe is a member of the Scientific Advisory Committee for Weight Watchers International®.
References
- 1.Perri MG, Corsica JA, Wadden TA. Improving the maintenance of weight lost in behavioral treatment of obesity. In: Stunkard AJ, editor. Handbook of obesity treatment. xv. New York, NY, US: Guilford Press; 2002. pp. 357–379. [Google Scholar]
- 2.Lowe MR. Self-regulation of energy intake in the prevention and treatment of obesity: is it feasible? Obes Res. 2003;11 (Suppl):44S–59S. doi: 10.1038/oby.2003.223. [DOI] [PubMed] [Google Scholar]
- 3.Lowe MR, Tappe KA, Annunziato RA, Riddell LJ, Coletta MC, Crerand CE, Didie ER, Ochner CN, McKinney S. The effect of training in reduced energy density eating and food self-m(onitoring accuracy on weight loss maintenance. Obesity. 2008;16:2016–2023. doi: 10.1038/oby.2008.270. [DOI] [PubMed] [Google Scholar]
- 4.Rolls B, Hermann M. The Ultimate Volumetrics Diet: Smart, Simple, Science-Based Strategies for Losing Weight and Keeping it Off. New York, NY: Harper Collins Publishers, Inc; 2012. [Google Scholar]
- 5.Jansen A, Vanreyten A, van Balveren T, Roefs A, Nederkoorn C, Havermans R. Negative affect and cue-induced overeating in non-eating disordered obesity. Appetite. 2008;51:556–562. doi: 10.1016/j.appet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Stroebe W. Dieting, Overweight, and Obesity: Self-Regulation in a Food-Rich Environment. Washington, D.C: American Psychological Association; 2008. [Google Scholar]
- 7.Vartanian LR, Herman CP, Wansink B. Are we aware of the external factors that influence our food intake? Health Psychology. 2008;27:533–538. doi: 10.1037/0278-6133.27.5.533. [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. JAMA. 2007;297:1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- 9.Nederkoorn C, Houben K, Hofmann W, Jansen A, Jansen A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010;29(4):389–393. doi: 10.1037/a0019921. [DOI] [PubMed] [Google Scholar]
- 10.Heymsfield SB, van Mierlo C, van dK, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27:537–549. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- 11.Ditschuneit HH, Fletchner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69:198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- 12.Rolls BJ, Roe LS, Beach AM, Kris-Etherton PM. Provision of foods differing in energy density affects long-term weight loss. Obes Res. 2005;13:1052–1060. doi: 10.1038/oby.2005.123. [DOI] [PubMed] [Google Scholar]
- 13.Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing two weight-loss diets. Am J Clin Nutr. 2007;85:1465–77. doi: 10.1093/ajcn/85.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brownell KD. The LEARN Program for Weight Control. Dallas, TX: American Health Publishing; 1994. [Google Scholar]
- 15.Rolls BJ, Barnett R. Volumetrics: Feel Full on Fewer Calories. New York: HarperCollins; 2000. [Google Scholar]
- 16.DeMaio TJ, Ciochetto S, Davis WL. Proceedings of the Section on Survey Research Methods. Vol. 2. Alexandria, VA: American Statistical Association; 1993. Research on the continuing survey of food intakes by individuals; pp. 1021–1025. [Google Scholar]
- 17.Derr JA, Mitchell DC, Brannon D, Smiciklas-Wright H, Dixon LB, Shannon BM. Time and cost analysis of a computer-assisted telephone interview system to collect dietary recalls. Am J Epidemiol. 1992;136:1386–1392. doi: 10.1093/oxfordjournals.aje.a116451. [DOI] [PubMed] [Google Scholar]
- 18.Freskanich D, Buzzard IM, Welch BT, Asp EH, Dieleman LS, Chong KR, Bartsch GE. Comparison of a computerized and a manual method of food coding for nutrient intake studies. J Am Med Assoc. 1988;88:1263–1267. [PubMed] [Google Scholar]
- 19.Wadden TA. Relaxation therapy for essential hypertension: specific or nonspecific effects. J Psychosom Res. 1984;28:53–61. doi: 10.1016/0022-3999(84)90040-0. [DOI] [PubMed] [Google Scholar]
- 20.d’Amore A, Massignan C, Montera P, Moles A, DeLorenzo A, Scucchi S. Relationship between dietary restraint, binge eating, and leptin in obese women. Int J Obes. 2001;25:373–377. doi: 10.1038/sj.ijo.0801565. [DOI] [PubMed] [Google Scholar]
- 21.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 22.Westenhoefer J, Stunkard AJ, Pudel V. Validation of the flexible and rigid control dimensions of dietary restraint. Int J Eat Disord. 1999;26:53–64. doi: 10.1002/(sici)1098-108x(199907)26:1<53::aid-eat7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Clark MM, Abrams DB, Niaura RS, Easton CA, Rossi JS. Self-efficacy in weight management. J Consult Clin Psychol. 1991;59:739–744. doi: 10.1037//0022-006x.59.5.739. [DOI] [PubMed] [Google Scholar]
- 24.Prochaska JO, Norcross JC, Fowler JL, Follick MJ, Abrams DB. Attendance and outcome in a work site weight control program: processes and stages of change as process and predictor variables. Addict Behav. 1992;17:35–45. doi: 10.1016/0306-4603(92)90051-v. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs DRJ, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: Cardia and the Minnesota Heart Health Program. Journal of Cardiopulmonary Rehabilitation and Prevention. 1989:9. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorin AA, Wing RR, Fava JL, Jakicic JM, Jeffery R, West DS, Brelje K, DiLillo VG. Weight loss treatment influences untreated spouses and the home environment: Evidence of a ripple effect. Int J Obes. 2008;32:1678–1684. doi: 10.1038/ijo.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorin AA. Randomized controlled trial of a comprehensive home environment-focused weight-loss program for adults. Health psychology. 2012 doi: 10.1037/a0026959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sackett DL, Richardson WS, Rosenberg W, et al. Evidence-based medicine: How to practice and teach EBM. New York, NY: Churchill Livingstone; 1997. [Google Scholar]