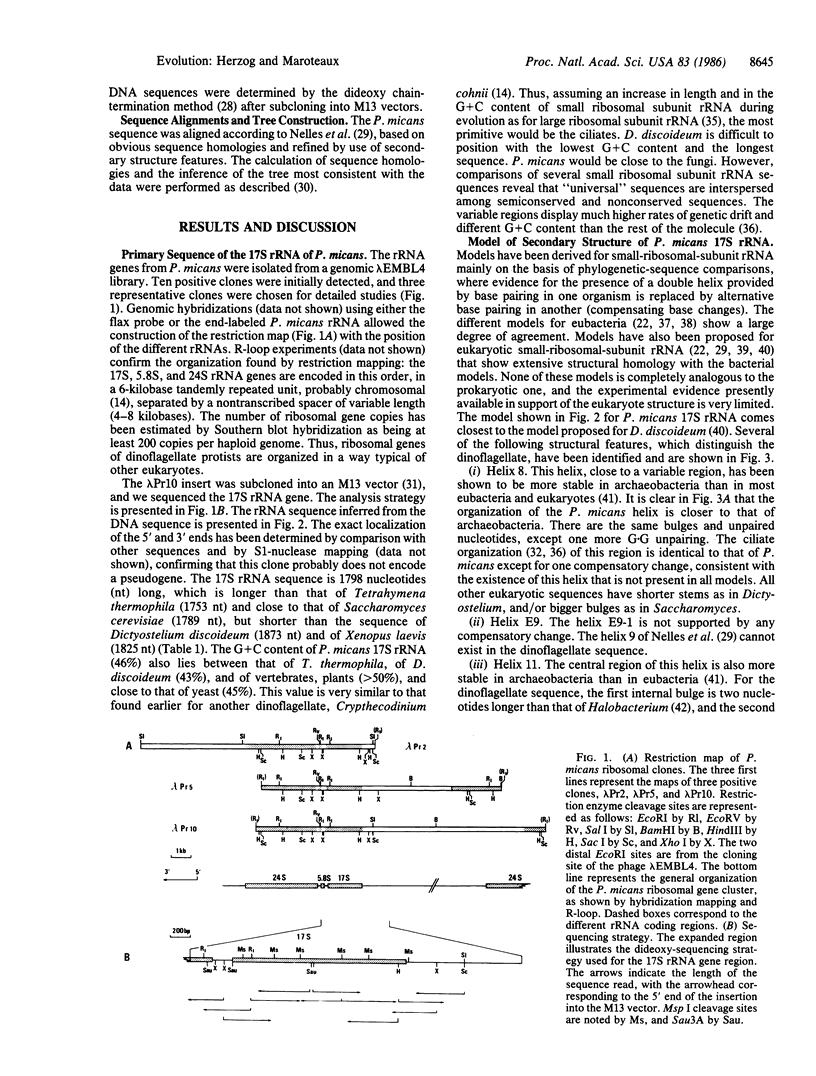
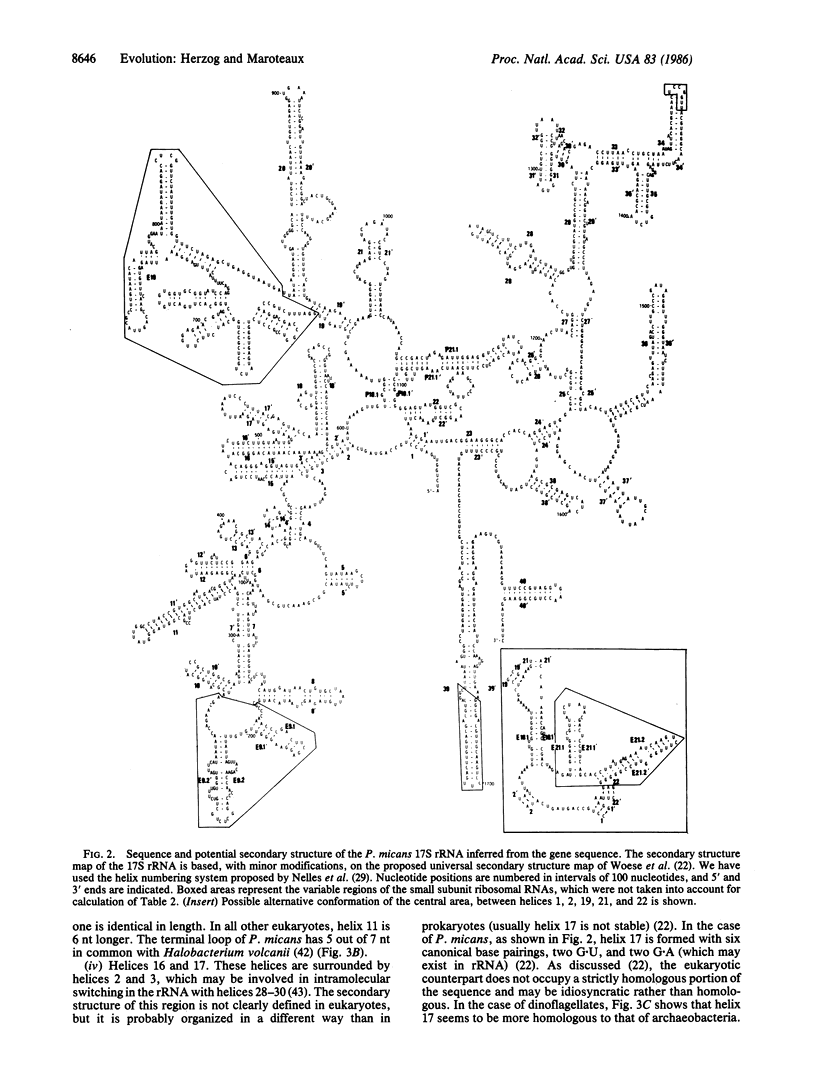
Abstract
We present the complete sequence of the nuclear-encoded small-ribosomal-subunit RNA inferred from the cloned gene sequence of the dinoflagellate Prorocentrum micans. The dinoflagellate 17S rRNA sequence of 1798 nucleotides is contained in a family of 200 tandemly repeated genes per haploid genome. A tentative model of the secondary structure of P. micans 17S rRNA is presented. This sequence is compared with the small-ribosomal-subunit rRNA of Xenopus laevis (Animalia), Saccharomyces cerevisiae (Fungi), Zea mays (Planta), Dictyostelium discoideum (Protoctista), and Halobacterium volcanii (Monera). Although the secondary structure of the dinoflagellate 17S rRNA presents most of the eukaryotic characteristics, it contains sufficient archaeobacterial-like structural features to reinforce the view that dinoflagellates branch off very early from the eukaryotic lineage.
Keywords: ribosomal gene, rRNA secondary structure, Prorocentrum micans, phylogenetic tree, primitive eukaryote
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. R., Roberts M., Loeblich A. R., 3rd, Klotz L. C. Characterization of the DNA from the dinoflagellate Crypthecodinium cohnii and implications for nuclear organization. Cell. 1975 Oct;6(2):161–169. doi: 10.1016/0092-8674(75)90006-9. [DOI] [PubMed] [Google Scholar]
- Atmadja J., Brimacombe R., Blöcker H., Frank R. Investigation of the tertiary folding of Escherichia coli 16S RNA by in situ intra-RNA cross-linking within 30S ribosomal subunits. Nucleic Acids Res. 1985 Oct 11;13(19):6919–6936. doi: 10.1093/nar/13.19.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmadja J., Brimacombe R., Maden B. E. Xenopus laevis 18S ribosomal RNA: experimental determination of secondary structural elements, and locations of methyl groups in the secondary structure model. Nucleic Acids Res. 1984 Mar 26;12(6):2649–2667. doi: 10.1093/nar/12.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodansky S., Mintz L. B., Holmes D. S. The mesokaryote Gyrodinium cohnii lacks nucleosomes. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1329–1336. doi: 10.1016/0006-291x(79)91126-4. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Lazar E., Haendler B., Jacob M., Galego-Dias L., Pousada C. High evolutionary conservation of the secondary structure and of certain nucleotide sequences of U5 RNA. Nucleic Acids Res. 1983 Dec 10;11(23):8359–8367. doi: 10.1093/nar/11.23.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Stiege W. Structure and function of ribosomal RNA. Biochem J. 1985 Jul 1;229(1):1–17. doi: 10.1042/bj2290001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. L., Gutell R., Noller H. F., Wool I. G. The nucleotide sequence of a rat 18 S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18 S ribosomal ribonucleic acid. J Biol Chem. 1984 Jan 10;259(1):224–230. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Eckenrode V. K., Arnold J., Meagher R. B. Comparison of the nucleotide sequence of soybean 18S rRNA with the sequences of other small-subunit rRNAs. J Mol Evol. 1984;21(3):259–269. doi: 10.1007/BF02102358. [DOI] [PubMed] [Google Scholar]
- Elwood H. J., Olsen G. J., Sogin M. L. The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol Biol Evol. 1985 Sep;2(5):399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- Erdmann V. A., Wolters J., Huysmans E., De Wachter R. Collection of published 5S, 5.8S and 4.5S ribosomal RNA sequences. Nucleic Acids Res. 1985;13 (Suppl):r105–r153. doi: 10.1093/nar/13.suppl.r105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Goldsbrough P. B., Cullis C. A. Characterisation of the genes for ribosomal RNA in flax. Nucleic Acids Res. 1981 Mar 25;9(6):1301–1309. doi: 10.1093/nar/9.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Lanter J. M., Woese C. R. Sequence of the 16S Ribosomal RNA from Halobacterium volcanii, an Archaebacterium. Science. 1983 Aug 12;221(4611):656–659. doi: 10.1126/science.221.4611.656. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Iida Y., Yano T., Takaiwa F., Iwabuchi M. Phylogenetic relationships among eukaryotic kingdoms inferred from ribosomal RNA sequences. J Mol Evol. 1985;22(1):32–38. doi: 10.1007/BF02105802. [DOI] [PubMed] [Google Scholar]
- Herzog M., Soyer M. O. Distinctive features of dinoflagellate chromatin. Absence of nucleosomes in a primitive species Prorocentrum micans E. Eur J Cell Biol. 1981 Feb;23(2):295–302. [PubMed] [Google Scholar]
- Herzog M., Soyer M. O. The native structure of dinoflagellate chromosomes and their stabilization by Ca2+ and Mg2+ cations. Eur J Cell Biol. 1983 Mar;30(1):33–41. [PubMed] [Google Scholar]
- Hinnebusch A. G., Klotz L. C., Blanken R. L., Loeblich A. R., 3rd An evaluation of the phylogenetic position of the dinoflagellate Crypthecodinium cohnii based on 5S rRNA characterization. J Mol Evol. 1981;17(6):334–337. doi: 10.1007/BF01734355. [DOI] [PubMed] [Google Scholar]
- Hogan J. J., Gutell R. R., Noller H. F. Probing the conformation of 18S rRNA in yeast 40S ribosomal subunits with kethoxal. Biochemistry. 1984 Jul 3;23(14):3322–3330. doi: 10.1021/bi00309a032. [DOI] [PubMed] [Google Scholar]
- Leffers H., Garrett R. A. The nucleotide sequence of the 16S ribosomal RNA gene of the archaebacterium Halococcus morrhua. EMBO J. 1984 Jul;3(7):1613–1619. doi: 10.1002/j.1460-2075.1984.tb02019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. H., Reddy R., Henning D., Spector D., Busch H. Primary and secondary structure of dinoflagellate U5 small nuclear RNA. Nucleic Acids Res. 1984 Feb 10;12(3):1529–1542. doi: 10.1093/nar/12.3.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L., Herzog M., Soyer-Gobillard M. O. Molecular organization of dinoflagellate ribosomal DNA: evolutionary implications of the deduced 5.8 S rRNA secondary structure. Biosystems. 1985;18(3-4):307–319. doi: 10.1016/0303-2647(85)90031-0. [DOI] [PubMed] [Google Scholar]
- Messing J., Carlson J., Hagen G., Rubenstein I., Oleson A. Cloning and sequencing of the ribosomal RNA genes in maize: the 17S region. DNA. 1984;3(1):31–40. doi: 10.1089/dna.1.1984.3.31. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nelles L., Fang B. L., Volckaert G., Vandenberghe A., De Wachter R. Nucleotide sequence of a crustacean 18S ribosomal RNA gene and secondary structure of eukaryotic small subunit ribosomal RNAs. Nucleic Acids Res. 1984 Dec 11;12(23):8749–8768. doi: 10.1093/nar/12.23.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen G. J., McCarroll R., Sogin M. L. Secondary structure of the Dictyostelium discoideum small subunit ribosomal RNA. Nucleic Acids Res. 1983 Nov 25;11(22):8037–8049. doi: 10.1093/nar/11.22.8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae P. M. The nature and processing of ribosomal ribonucleic acid in a dinoflagellate. J Cell Biol. 1970 Jul;46(1):106–113. doi: 10.1083/jcb.46.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Spector D., Henning D., Liu M. H., Busch H. Isolation and partial characterization of dinoflagellate U1-U6 small RNAs homologous to rat U small nuclear RNAs. J Biol Chem. 1983 Nov 25;258(22):13965–13969. [PubMed] [Google Scholar]
- Rizzo P. J. Comparative aspects of basic chromatin proteins in dinoflagellates. Biosystems. 1981;14(3-4):433–443. doi: 10.1016/0303-2647(81)90048-4. [DOI] [PubMed] [Google Scholar]
- Rizzo P. J., Morris R. L. Some properties of the histone-like protein from Crypthecodinium cohnii (HCc). Biosystems. 1983;16(3-4):211–216. doi: 10.1016/0303-2647(83)90005-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler E. A., Blackburn E. H. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J Biol Chem. 1985 May 25;260(10):6334–6340. [PubMed] [Google Scholar]
- Stiegler P., Carbon P., Zuker M., Ebel J. P., Ehresmann C. Structural organization of the 16S ribosomal RNA from E. coli. Topography and secondary structure. Nucleic Acids Res. 1981 May 11;9(9):2153–2172. doi: 10.1093/nar/9.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. F., Hearst J. E. Structure-function relations in E. coli 16S RNA. Cell. 1983 May;33(1):19–24. doi: 10.1016/0092-8674(83)90330-6. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Werner E., Sohst S., Gropp F., Simon D., Wagner H., Kröger H. Presence of poly (ADP-ribose) polymerase and poly (ADP-ribose) glycohydrolase in the dinoflagellate Crypthecodinium cohnii. Eur J Biochem. 1984 Feb 15;139(1):81–86. doi: 10.1111/j.1432-1033.1984.tb07979.x. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Gutell R., Gupta R., Noller H. F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Schnabel R., Stetter K. O. Archaebacteria and the origin of the eukaryotic cytoplasm. Curr Top Microbiol Immunol. 1985;114:1–18. doi: 10.1007/978-3-642-70227-3_1. [DOI] [PubMed] [Google Scholar]



