Abstract
Lay Abstract
Parents, clinicians, teachers, and researchers seem to agree that individuals with autism spectrum disorders often have sharper hearing, including abilities like perfect pitch, better memory for specific sounds, better abilities to tell one sound apart from even a very similar sound, and so on. We asked whether this sharper hearing ability is related to some of the difficulties in autism, including later development of language: Is better hearing part of having ASD? One important part of this study is that it includes a group of people from all over the U.S. and Canada who had ASD when they were younger (diagnosed before age five years), but who do not have any symptoms now that they are teenagers. We call this an “optimal outcome”. They do not have any symptoms, they do not need any special education services, and their IQ scores are in the normal or high-normal range. We measured the ability to hear the difference between two tones, or pitches, in 26 teenagers with optimal outcomes, 29 teenagers with high-functioning autism, and 20 teenagers with typical development. The teenagers who have ASD were better at hearing pitch differences, suggesting that this special ability is part of ASD; the teenagers with optimal outcomes did not differ from the typically-developing group. Also, the teenagers with the best pitch abilities tended to be the latest to start talking when they were toddlers. The paper discusses what these results mean for communication in ASD, and for the process of learning language more generally.
Scientific Abstract
The autism spectrum disorders (ASD) are neurodevelopmental disorders, diagnosed behaviorally but associated with differences in brain development. Individuals with ASD exhibit superior auditory perceptual skills, which may correlate with ASD symptomatology, particularly language skills. We describe findings from individuals diagnosed with ASD before age five, who now have no symptoms (e.g., having optimal outcomes). Unlike an ASD group, which shows heightened pitch discrimination, the Optimal Outcome group’s abilities do not differ from those of typically developing controls. Furthermore, pitch discrimination is associated with both current autism symptomatology and early language milestones. Findings illuminate processes associated with resolution of autism. We also discuss a specific mechanism by which heightened auditory discrimination leads to language delays in ASD.
Keywords: Language, Language Delays, Auditory perception, Autism, Long-Term Outcomes
Introduction
The autism spectrum disorders (ASD) are diagnosed via deficits in social and communication skills and the presence of repetitive behaviors and stereotyped interests, but the presence of exceptional skills may also be part of the ASD phenotype. A significant proportion of individuals with ASD demonstrates exceptional pitch perception, as measured by perfect or absolute pitch, heightened pitch discrimination, and enhanced memory for pitches and musical passages (Heaton, 2003, 2005; Heaton et al., 1998; Jarvinen-Pasley et al., 2008; Miller, 1999; reviewed in Mottron et al., 2006; Mottron et al., 2000; O’Riordan & Passetti, 2006). Furthermore, findings of enhanced discrimination of tones (Heaton et al., 2008) and heightened pitch perception (Heaton, 2003) in children with ASD suggest that expertise arises early in development. Some researchers have proposed that there are fundamental differences in the processing of auditory information in ASD; certainly, studies consistently demonstrate language-relevant differences in early perceptual tuning in ASD, with atypical responses to child-directed speech (Kuhl et al., 2005) and speech more generally (e.g., Lepisto et al., 2005).
Perceptual hypersensitivity could serve as a useful “biomarker” in ASD; but there may be an even stronger causal relationship between pitch perception and ASD symptomatology, specifically, language deficits. For example, one study, designed to link auditory perceptual abilities to hypersensitivities reported that of individuals with an ASD who displayed enhanced auditory discrimination, a significantly greater proportion also displayed early language delays (Jones et al., 2009). The present study examines the link between current pitch sensitivity, language delays in ASD, and symptom persistence in ASD. Specifically, we hypothesize that hyper-acuity for pitch could contribute to overly detailed representations of phonological information, thereby delaying the development of phonological categories and subsequent word learning.
Differences in perceptual tuning, particularly pitch, are likely to be critically implicated in early language acquisition (Seidl & Johnson, 2008), particularly the segmentation of words from fluent speech; pitch accents and phrasal boundaries appear to play an important role in word segmentation (Nazzi et al., 2005). Nine-month-old infants are able to make use of pitch and durational cues to segment speech (Goodsitt et al., 1993), and these cues may be more influential than frequency information for word segmentation (Jusczyk et al., 1999). For example, infant word recognition can be disrupted by changes in pitch (Singh et al., 2008); 7.5-month-old infants familiarized with words produced with raised pitch failed to recognize those words in sentences when produced with lowered pitch, and vice versa. Prosodic cues of lengthening, pausing, and large changes in fundamental frequency are not relevant only for word segmentation, but rather may signal the presence and arrangement of syntactic constituents for language learners (Gerken et al., 1994).
Sensitivity to phonologically meaningful information undergoes an interesting developmental shift. Infants initially show sensitivities to close phonological contrasts that are not meaningfully different in their native language, conveyed through a variety of auditory cues. For example, pitch contour and segment duration are lexically contrastive in Latvian and Mandarin, respectively, but non-contrastive in English. Although all infants are initially sensitive to all these allophonic and phonotactic properties during the first year of life (Bortfeld & Morgan, 2010), by age 12 months, infants can distinguish only those contrasts occurring in their language (Werker & Yeung, 2005). Word learners suppress their perception of distinctions between slightly dissimilar sounds that fall within a phonological category (Kuhl et al., 2003), because phonological categorization -- that is, clustering -- is critical to distinguishing words in the speech stream. Infants must discover which dimensions should be dismissed and which require attention (Bortfeld & Morgan, 2010); infants who experience their input as containing a larger set of contrasts may be slower to learn the phonotactic regularities that are informative. In this way, infants with heightened discriminatory capacity for dimensions such as pitch may exhibit delays in language acquisition. The ability to build abstract categories rather than continually focusing on immediate perceptual qualities may be critical in early language development (e.g., Son et al., 2008).
Heightened discrimination of auditory stimuli could thus impede phonological development and subsequent word learning. Some data are consistent with a relationship between perceptual tuning and language delays in ASD; known and unknown words (Coffey-Corina et al., 2008) and syllable changes (Kuhl et al., 2005) were found to elicit distinctive electrophysiological response in children with ASD, and response patterns correlated with language abilities. Some studies have reported group differences in pitch perception between individuals with an ASD diagnosis with versus without a history of early language delay (Bonnel et al., 2010). However, most studies find no association between pitch perception and current language skills in ASD at age 10 years (Heaton et al., 2008), and no special sensitivity to non-native phonological contrasts in ASD at 12 years (Constantino et al., 2007). Perhaps developmental processes such as joint attention and social engagement intervene, such that pitch perception shapes early language development, but has less measurable influence on later language skills. Certainly, these developmental processes are significantly impaired in ASD.
The present study examined pitch discrimination in (i) children with current ASD diagnoses (e.g., the ASD group), (ii) children with a history of ASD who have optimal outcomes (e.g., the Optimal Outcome group), and (iii) children with typical development; and its relationship with current symptomatology, early language milestones, and current language skills. The goal was to probe for a link between auditory sensitivity and the ASD phenotype.
Methods
Participants included individuals ages 8–21 years with a current diagnosis of Autism Spectrum Disorders (ASD; N=29), functioning in the average range of cognitive ability; individuals diagnosed with ASD prior to age five years, but no longer having symptoms of ASD (Optimal Outcome, OO; N=26); and a typically developing comparison group (TD, N=20). Groups were matched on full-scale IQ and chronological age. Demographic data are shown in Table 1. All procedures used in this study were approved by the University of Connecticut Institutional Review Board.
Table 1.
Demographic information for participants with ASD, Optimal Outcome (OO), and typically development (TD).
| ASD M (SD) | OO M (SD) | TD M (SD) | χ2 or F | p | η2p | |
|---|---|---|---|---|---|---|
| N (M:F) | 25:4 | 20:6 | 17:3 | 0.93 | 0.63 | |
| Chron. age (yrs) | 12.3 (2.3) 8 – 17 |
12.5 (3.6) 8 – 21 |
13.7 (2.9) 9 – 21 |
1.37 | 0.26 | 0.002 |
| Nonverbal IQa | 111 (14) 78 – 147 |
112 (14) 92 – 142 |
115 (12) 89 – 139 |
0.60 | 0.55 | 0.002 |
| Verbal IQa | 104 (13)a* 81 – 133) |
113 (13) 91 – 137 |
113 (12) 99 – 136 |
4.91 | 0.01 | 0.16 |
| ADOS Com + Socb | 10.3 (3.0)a 7 – 19 |
1.7 (2.1) 0 – 5 |
0.8 (1.1) 0 – 4 |
155.48 | < .001 | 0.82 |
| ADI-R Com (Lifetime)c | 16.4 (5.0) 5 – 24 |
13.2 (5.1) 0 – 20 |
4.84 | 0.03 | 0.11 | |
| ADI-R Social (Lifetime)c | 20.54 (5.4) 8 – 29 |
14.1 (6.2) 2 – 23 |
15.60 | < .001 | 0.24 | |
| ADI-R Rep (Lifetime)c | 6.5 (1.7) 3 – 10 |
5.6 (2.7) 2 – 9 |
0.89 | 0.35 | 0.00 | |
| SCQ Total (Lifetime)d | 23.0 (5.9)a 10 – 33 |
16.5 (6.6)b 5 – 28 |
1.4 (1.3)c 0 – 4 |
91.57 | < .001 | 0.24 |
| Age of first words (months) | 21.0 (11.2) 6 – 54 |
26.9 (11.6) 8 – 48 |
3.32 | 0.08 | 0.10 | |
| Age of first phrases (months) | 35.2 (11.5) 15 – 54 |
35.4 (10.1) 10 – 48 |
0.004 | 0.95 | 0.002 | |
| CELF Core Languagee | 100.4 (13.1)a 70 – 124 |
110.8 (9.8)b 91 – 126 |
119.2 (7.9)c 109 – 132 |
17.52 | < .001 | 0.15 |
Note: Superscripts identify means that differ significantly from other means in the comparison not sharing that superscript. Group means (SD) are followed by the data range.
Wechsler Abbreviated Scale of Intelligence (WASI’, Wechsler, 1999), Nonverbal and Verbal subscales.
Autism Diagnostic Observation Schedule (ADOS, Lord, Rutter, DiLavore, & Risi, 2002). Communication plus social domain summed score. Cutoff is 7 for ASD and 10 for autistic disorder.
Autism Diagnostic Interview-Revised (ADI-R, Rutter, Le Couteur, & Lord, 2003). Scores refer to presence of symptoms “ever” (Lifetime). Cutoff scores are 8, 10, and 3 for Communication, Social Reciprocity and Repetitive behaviors, respectively.
On the Social Communication Questionnaire (Rutter, Bailey, & Lord, 2003), Lifetime, 15 is the cutoff for an ASD.
Clinical Evaluation of Language Fundamentals-4 (Semel, Wiig, & Secord, 2003).
OO participants
All participants were part of a larger study of Optimal Outcomes in ASD, in which children from across the U.S. and Canada were recruited in order to better understand the phenomenology of possible resolution of ASD symptoms following early intervention. Participants had to have received a diagnosis early in life (prior to age five years) from a specialist in the field of autism, focusing directly on the ASD diagnosis, and verified in a written report covering the period prior to age five years. Participants in the OO group could not exhibit current ASD symptomatology on the basis of the ADOS or by clinical judgment. Inclusion criteria for OO included full-scale, verbal, and performance IQ scores in the normal range (e.g., of 78 or above - within 1.5 SD of average), and participation in a regular education class. This study, including the full methodology to verify early diagnoses in the OO group, is described in detail in Fein et al. (2013).
Multiple studies have confirmed, by use of the ADI-R, the presence of significant behavioral, cognitive, or communicative improvements in between 9 and 43% of individuals with childhood ASD (e.g., Eaves & Ho, 1996), but often with ongoing symptoms in at least one domain and an ongoing need for developmentally appropriate services (e.g., Seltzer et al., 2003). The current study extends these data in suggesting that for some proportion of children with childhood ASD, the developmental trajectory may include a full remission of symptoms. In contrast to prior studies demonstrating “significant reductions” in difficulties but with ongoing pragmatic oddness, participants in the OO group exhibited no current ASD symptomatology according to gold-standard diagnostic evaluation. In addition to ADOS and SCQ data, other measures were used to verify the presence of age-appropriate social and communicative skills (Clinical Evaluation of Language Fundamentals, Test of Language Competence, Vineland Adaptive Behavior Scales). Data from these measures is described in full elsewhere (Fein et al., 2013).
To confirm the presence of childhood ASD in the OO and ASD groups, early (prior to age 5) diagnostic reports were solicited (see below). In addition to confirming childhood ASD in the ASD and OO groups, it was important to establish current ASD status. Diagnosis, based on expert clinician judgment using DSM-IV criteria (APA, 2000), was confirmed in ASD and OO groups using gold-standard clinical tools: the Autism Diagnostic Observation Schedule (Lord et al., 2002), the Autism Diagnostic Interview-Revised (Rutter et al., 2003b), the Social Communication Questionnaire, Lifetime version (Rutter et al., 2003a), as reviewed below, and clinical judgment. SCQ scores were used to rule out ASD in the TD group. In addition, participants with TD were excluded if they had any first-degree relatives with ASD or any history of neurological problems.
ADI-R lifetime scores focus on symptomatology at ages four to five; by this time-period, many children have already had several years of intensive therapy and experienced symptom remission. Thus, lifetime-ASD scores were inappropriate for determining the presence of childhood ASD in the OO group.
There is further evidence that our OO group is characterized by a history of childhood ASD rather than early speech language impairment or general developmental delay. Specifically, in a group of 15 OO and 24 ASD children described by our group, there was evidence of early macrocephaly in the OO group, with no difference between OO and ASD groups (Mraz et al., 2009). Macrocephaly (head circumference > 97th percentile) during the first year of life is one of the most consistent biological findings in ASD (Aylward et al., 2002). While this finding alone cannot confirm the ASD diagnosis, because it could be a familial trait (e.g., Lainhart et al., 2006), it is consistent with the suggestion that members of the OO group were similar to the HFA group at least in early childhood.
Measures
Autism Diagnostic Observation Schedule (ADOS)
The ADOS (Lord et al., 2002) is a semi-structured assessment for the diagnosis of pervasive developmental disorders. Participants completed either Module 3 or Module 4, depending on their maturity level.
Autism Diagnostic Interview-Revised (ADI-R)
The ADI-R (Rutter et al., 2003b) is a structured caregiver interview that, in conjunction with the ADOS, is the “gold standard” instrument for the diagnosis of pervasive developmental disorders. Trained graduate-level clinicians administered and scored the ADOS and ADI-R.
Social Communication Questionnaire, Lifetime version (SCQ)
The SCQ (Rutter et al., 2003a) is a 40-item parent questionnaire for the screening of ASD symptoms in children. It measures symptoms across the lifespan rather than current symptomatology. This measure was chosen because it provides the most continuous measure of autism symptomatology. Autism was ruled out in the TD group using the SCQ-Lifetime version.
Record Review
An expert clinician reviewed reports written before age five years for all OO participants, along with foils (reports of children with other developmental disorders). Diagnostic formulation, referral questions, and treatment recommendations were redacted. Based on behavioral descriptions, the clinician judged whether participants met criteria for PDD/NOS, autistic disorder, or Asperger’s syndrome, according to DSM-IV criteria. Only children with reports consistent with ASD were retained in the OO group; ultimately, four potential OO participants were rejected because of unclear early symptomatology. All 18 foils were correctly rejected.
Tone discrimination task
To assess pitch discrimination, participants listened to 120 pairs of tones presented diotically via Sony MDR-V150 Dynamic Stereo headphones, making a two-alternative forced-choice same/different judgment. Steady-state pure tones were 100 ms in length, with a 1000 ms inter-stimulus interval. The inter-trial interval was controlled by the subject’s response without upper limit; subsequent trials were presented 500 ms following a response. Tones were generated in Praat (Boersma & Weenink, 2011) using the formula 1/2 * sin(2*pi*Y*x), with Y replaced by 500, 750, 1000, or 1500; sampling rate was 11,025 Hz. Thus, in a tone pair differing by 3%, the tones were at s 750 and 772.5 Hz. Across trials, the order of lower and higher tones was counterbalanced. Forty tone pairs (50% identical) were presented at each of three levels, differing by 3% (easy), 2% (medium), or 1% (hard) of total frequency; pitch stimuli were modeled on prior research on pitch discrimination in adults with ASD (Bonnel et al., 2003) and were designed to be challenging for nonmusicians to avoid ceiling effects. Furthermore, to assess pitch discrimination generally, rather than as a language-specific capacity, the range was distinct from that typically observed in voiced human speech (80–250 Hz). The three tone conditions were presented in a fixed order of increasing difficulty, consistent with prior research (Bonnel et al., 2003). Participants completed 16 training trials (at 4% and 1% difference levels), with feedback, to ensure task comprehension. Practice on training trials continued until a 75% accuracy threshold was reached; no participants required more than 16 practice trials.
Procedures
Participants completed a large battery of measures; diagnostic and standardized tasks were completed during an initial interview, and tone discrimination was completed at a subsequent session, an average of five weeks after the initial session (maximum interval, three months).
Data were checked and all anticipatory responses (RT < 70 ms) or trials with a response slower than 7000 ms were removed (<1%). The d′ statistic, used as a measure of response sensitivity, measures separation between the means of the signal and the noise distributions in units of the standard deviation of the noise distribution. D′ describes accuracy without an influence of response bias and was computed as: d′ = [standard normal cumulative distribution function*(0.5*[(inverse of cumulative distribution function for hits) − (inverse of cumulative distribution function for false alarms)]), where hits = correct response of “same” given two tones of identical frequency, and false alarms = incorrect response of “different” for two tones of identical frequency. Effect sizes were calculated with partial eta squared (η2p), which refers to the proportion of variance attributable to a given effect after partialling out non-error sources of variance.
Results
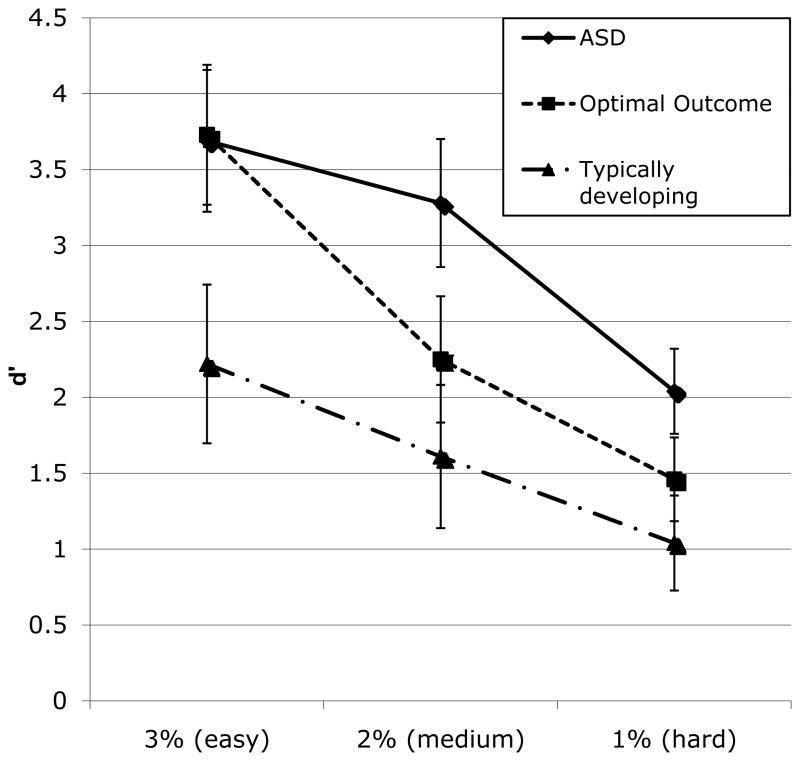
A pitch discrimination task probed for sensitivity to subtle acoustic differences. For the 120 tone pairs, findings were evaluated using the d′ statistic, controlling for age, FSIQ, and RT to avoid speed-response trade-offs. There was a significant group difference in d′, F (2, 65) = 3.25, p = .04, η2p = .09, with a main effect of condition, F(2, 130) = 22.90, p < .001, η2p = .252, and no group X condition interaction, F(4, 130) = 1.22, p = .31, η2p = .04; data are shown in Figure 1. Across conditions, the TD had lower scores than the ASD group, F(1,38)= 1.38, p = .02, whereas group differences for OO versus ASD, F(1, 45) = .08, p = .77, and OO versus TD, F(1, 44) = .003, p = .99, did not reach significance. The ASD group showed the best pitch discrimination, followed by the OO group, followed by the TD group. There was no group difference in RT as a function of tone-condition, indicating that group differences in accuracy did not reflect a speed-accuracy tradeoff, F(2,67) = 1.97, p = .15, η2p = .04.
Figure 1.
Pitch discrimination performance as a function of group status and condition.
Note: Means are presented with FSIQ and chronological age as covariates.
Symptom severity accounted for unique and significant variance in individual differences in pitch perception abilities, even controlling for age and IQ, in linear regression analyses; see Table 2. Each predictor contributed significant independent variance to pitch discrimination, and better discrimination was associated with greater symptom severity.
Table 2.
Summary of hierarchical regression analysis for predictors of pitch perception.
| Pitch discrimination (average of 3 conditions) | B | SE B | β | Model R2 |
|---|---|---|---|---|
| Age (mos) | 0.015 | 0.006 | 0.300** | |
| FSIQa | 0.033 | 0.016 | 0.233* | |
| SCQb | 0.052 | 0.020 | 0.292** | |
| .173** |
F(3, 69) = 4.40, p = .007
FSIQ = Full scale IQ, Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
SCQ = Social Communication Questionnaire, Lifetime form (Rutter, Bailey, & Lord, 2003).
Parents of participants in the ASD (N=16) and OO (N=20) groups reported the age at which children produced single words and multi-word phrases, a process used effectively in prior research (Eigsti & Bennetto, 2009)1; Table 1 presents milestone data as a function of group. Not all parents were able to reliably recall these data; those participants were excluded from the analysis. The OO group showed a tendency to produce first words later, on average, than the ASD group, F(1,45) = 3.32, p = .08, at 27.2 (11.6) months versus 21.0 (11.6) months, respectively, though first phrases were produced at a similar time, at 35.2 (11.5) months versus 35.4 (10.1) months, F(1,45) = 0.004, p = .95. This further strengthens the argument that participants in the OO group had similar symptom severity early in development.
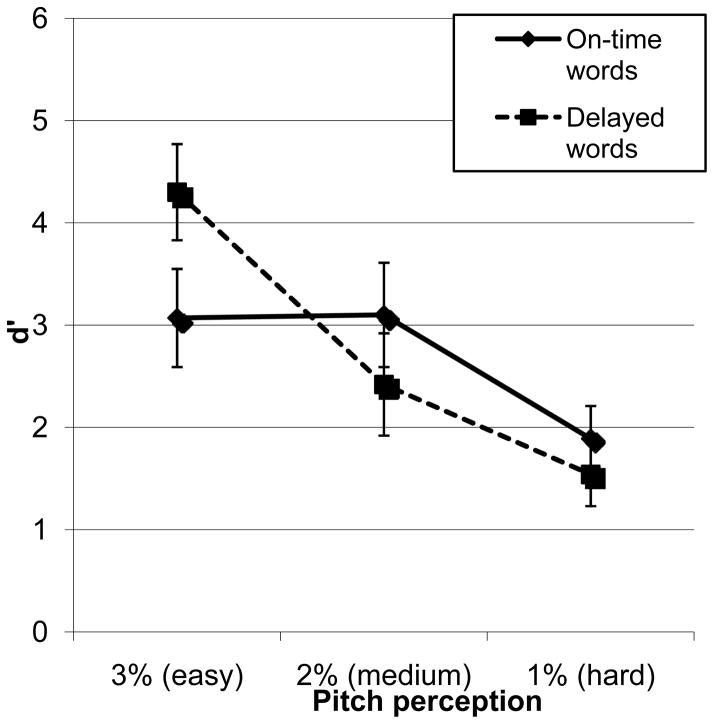
The ASD and OO participants were split into “typical” (n = 25) and “delayed” (no words at age 24 months; n = 25) language milestone groups; pitch discrimination as a function of language milestone was examined in a repeated-measures MANCOVA, controlling for age and FSIQ. The main effects of language milestone, F(1,46) = .11, p = .75, η2p = .002, and pitch condition, F(2,92) = 0.36, p = .70, η2p = 0.01, were not significant, but milestone and pitch condition interacted, F(2,92) = 4.92, p = .009, η2p = .10; see Figure 2. That is, children with delayed first words differed more in performance per condition, with significant differences for each condition (3%, 2%, 1%) relative to the others; performance in the on-time group was similar for the 3% and 2% conditions. Group differences for the 3% pitch conditions just missed significance, with the delayed group displaying better performance, F(1, 46) = 3.79, p = .06, η2p = .08. This pattern of findings confirms the relevance of heightened pitch discrimination in language delays.
Figure 2.
Pitch discrimination ability as a function of language milestone (delayed versus on-time first words).
Note: Means are presented with FSIQ and chronological age as covariates; data are from participants in ASD and OO groups only.
Unlike early language milestones, there was no association between pitch discrimination abilities and current language skills (receptive vocabulary via PPVT scores, and structural language knowledge via CELF scores), all p’s > .20, as reported in the regression analysis shown in Table 3.
Table 3.
Summary of hierarchical regression analysis for pitch perception (d′ averaged across conditions) as a function of concurrent language abilities
| B | SE B | β | Model R2 | |
|---|---|---|---|---|
| Age (mos) | 0.018 | 0.006 | 0.337** | |
| FSIQa | 0.070 | 0.023 | 0.485** | |
| CELFb | −0.039 | 0.026 | −0.277 | |
| PPVTc | −0.018 | 0.023 | −0.132 | .111* |
p < .10,
p < .05,
p < .01
FSIQ = Full scale IQ, Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
CELF = Clinical Evaluation of Language Fundamentals, 4th Edition (Semel, Wiig, & Secord, 2003).
PPVT = Peabody Picture Vocabulary Test (Dunn & Dunn, 2007).
Heightened pitch discrimination might also be associated with a fascination for music. Eight of the participants’ parents (4 ASD, 4 OO) reported intense musical interest or expertise (e.g., “has perfect pitch and a genius for music”). Comparing individuals with and without special musical interests, there was a weak trend for those with music interests to have better pitch overall, F(1, 47) = 3.01, p = .09, η2p = .06. There was no relationship between pitch perception and measures of repetitive behaviors and special interests, all p’s > .13.
Discussion
The current study demonstrates a relationship between low-level perceptual differences and specific aspects of the ASD phenotype. We describe low-level perceptual abilities in individuals with an early history of ASD who experience a current optimal outcome. This OO group showed no enhancement in pitch discrimination – their performance did not differ from the TD group (nor did it differ from the ASD group’s performance). Results were consistent with both significant early ASD symptomatology (including delays in language milestones) and intact current abilities in this OO group. Consistent with the group effect, symptom severity accounted for unique and significant variance in individual differences in pitch perception abilities.
Findings document a correlation between an early language milestone (first words), and auditory perceptual abilities assessed a decade later. That is, difficulty in early word learning was associated with the ability to better distinguish between fine acoustic differences. Interestingly, this heightened skill appears unrelated to current language skills assessed via gold-standard assessments, a finding consistent with several explanations: 1) standardized language assessments may be limited in sensitivity, especially given significant fluency and language experience (Constantino et al., 2007), or 2) pitch discrimination may be salient in the course of early acquisition, but not in later fluent language use.
The relationship between early language milestones and pitch discrimination was most salient in the easiest pitch condition, potentially reflecting the great difficulty of discrimination in the 2% and 1% conditions and thus the more limited variability for those conditions. These relationships did not appear to simply reflect the moderating influence of a distinct attentional style (which would be reflected in a relationship between pitch discrimination and special interests), as the only link with special interests was a marginal trend for individuals with better pitch perception to display a strong expertise and interest in music. One prediction arising from the current findings is that, if initial difficulties with the phototactics of the language are due to heightened sensitivity to acoustic differences in pitch, then individuals with ASD should be relatively later to learn vowel-initial words, whose segmentation is particularly driven by pitch; this is a question for future research.
This study may be one of the first to counter a “Matthew” (rich get richer) effect, in which better early abilities (IQ, language) predict better outcomes. In this study, the optimal outcome group tended to be more delayed in the first-words milestone, compared to children with ongoing ASD, suggesting that being “early” in language is not an important limiting factor for optimal long-term outcomes. Early delays can be overcome, in some children. It should be noted, however, that first words were acquired in the OO group by an average of 27 months; it is likely that more significant delays, with words not acquired until some later time (for example, after the age of 3 or 4 years) may preclude an optimal outcome (see, e.g., Mayo, Chlebowski, Fein & Eigsti, 2013).
The present data document a significant relationship between severity of ASD symptoms, including early language milestones, and a heightened low-level perceptual skill, and add the novel finding that recovery from ASD may be associated with a relative decrease in perceptual sensitivities. Findings add to conceptions of resolution of ASD symptoms. Predictors of optimal outcomes in ASD include greater intelligence, receptive language, verbal and motor imitation, and motor development, as well as initial diagnosis and earlier age at diagnosis and initiation of treatment, but not symptom severity (Helt et al., 2008).
Because of the tremendous phenotypic variation within the autism spectrum, markers of low-level sensory processes would be particularly useful in understanding individual differences, such as who is likely to develop fluent speech (Oram Cardy et al., 2008). Sensory differences have been linked to specific aspects of the behavioral symptoms (phenotype) of autism (Bonnel et al., 2010; Boyd et al., 2010; Chen et al., 2009). It is particularly relevant for the present study that a relationship was demonstrated for auditory stimuli that occupy a distinct range from that occupied by human speech. That is, it cannot be argued that the current pitch discrimination abilities reflect some epiphenomenon of attention to human speech, because such attention (whether typical or atypical) would not likely impact on pitch discrimination for tones at 500–1500 Hz. Perceptual processes are particularly good candidate markers of disorder, because they map onto exquisitely sensitive psychophysical tests and models of neural functioning. Furthermore, the mechanisms underlying pitch representation seem to be shaped by features of the auditory stream rather than speech per se (Swaminathan et al., 2008), allowing for a decoupling of auditory processing and communication (though it should be noted that a meta-analysis of fMRI studies suggests that tone discrimination and lexical decision tasks both activate Broca’s area, regions 44/45; Muller & Basho, 2004). Research has identified relationships between auditory processing and behavioral phenotypes in other disorders; for example, tone discrimination is linked to infant temperamental differences that presage vulnerability to anxiety disorders (e.g., Marshall et al., 2009). Potentially of even greater relevance are findings that individuals with specific language impairment (SLI), dyslexia or other language impairments have significantly poorer frequency discrimination abilities (Loui et al., 2011; McArthur & Bishop, 2004). The present data are distinguished by a novel characteristic in comparison to other disorders, in that enhanced rather than impaired pitch discrimination is linked to language delays and current symptomatology.
As is true for most studies of the association between language skill and pitch discrimination, pitch is assessed long after first language acquisition is complete. It is possible that earlier in development, auditory discrimination abilities play a unique role. For example, enhanced auditory discrimination skills seem to facilitate vowel learning for adults acquiring a second language; adults have presumably already developed a robust understanding of the role of pitch in language (Lengeris & Hazan, 2010). We propose that exceptionally strong pitch discrimination skills may be harmful, rather than helpful, for language acquisition during the first two years of life. Certainly, it is true that heightened or enhanced abilities in a given domain are not necessarily the functional opposite of weak abilities; it may be that both very strong and very weak abilities are associated with learning difficulties, depending on developmental timing.
Some neurophysiological abnormalities that could lead to such alterations in early sensory representation have been documented in ASD (Casanova et al., 2002). As such, superior pitch processing in ASD might reflect the overdevelopment of low-level perceptual processes in general (Bonnel et al., 2003), consistent with local neural overconnectivity (Belmonte et al., 2004). It is possible that attention to non-social auditory stimuli both enhances perceptual discrimination and decreases experience with language-related stimuli, a possibility discussed in detail in other reports (Jarvinen-Pasley et al., 2008). Findings may reflect some feature of brain architecture which preferentially supports auditory perceptual processes, and incidentally predisposes an individual to greater symptom severity. Because the current data are cross-sectional, it is not possible to tease apart these explanations. These findings suggest that heightened sensitivity to acoustic discriminations impedes the extraction of communicative meaning (word learning) from auditory signals, possibly by impairing the formation of phonological categories, and contributes to language impairments in ASD.
Acknowledgments
Research supported by funding from NIMH R01 MH076189-01A1 to DF. A number of research assistants were instrumental in data collection, including Eva Troyb, Alyssa Orinstein, Katherine Tyson, Molly Helt, and Michael Rosenthal, and we acknowledge their contributions. We gratefully acknowledge the time and effort of participating families and their children.
Footnotes
It should be noted that parents of children with ASD might have less reliable reporting of language milestones as children age, reporting greater delays (Hus et al., 2011). The current study used “best practice” strategies for collecting the milestone data.
None of the authors declare a conflict of interest.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–83. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. Journal of Neuroscience. 2004;24:9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat: doing phonetics by computer. 2011 (Version 5.1.37, retrieved 01 September 2009 from http://www.praat.org/)
- Bonnel A, McAdams S, Smith B, Berthiaume C, Bertone A, Ciocca V, et al. Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia. 2010;48:2465–75. doi: 10.1016/j.neuropsychologia.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E, Bonnel AM. Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. Journal of Cognitive Neuroscience. 2003;15:226–35. doi: 10.1162/089892903321208169. [DOI] [PubMed] [Google Scholar]
- Bortfeld H, Morgan JL. Is early word-form processing stress-full? How natural variability supports recognition. Cognitive Psychology. 2010;60:241–66. doi: 10.1016/j.cogpsych.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd BA, Baranek GT, Sideris J, Poe MD, Watson LR, Patten E, et al. Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Research. 2010;3:78–87. doi: 10.1002/aur.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–32. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Chen YH, Rodgers J, McConachie H. Restricted and repetitive behaviours, sensory processing and cognitive style in children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:635–42. doi: 10.1007/s10803-008-0663-6. [DOI] [PubMed] [Google Scholar]
- Coffey-Corina S, Padden D, Kuhl PK, Dawson G. ERPs to words correlate with behavioral measures in children with Autism Spectrum Disorder. Journal of the Acoustical Society of America. 2008;123:3742. [Google Scholar]
- Constantino JN, Yang D, Gray TL, Gross MM, Abbacchi AM, Smith SC, et al. Clarifying the associations between language and social development in autism: a study of non-native phoneme recognition. Journal of Autism and Developmental Disorders. 2007;37:1256–63. doi: 10.1007/s10803-006-0269-9. [DOI] [PubMed] [Google Scholar]
- Eaves LC, Ho HH. Brief report: stability and change in cognitive and behavioral characteristics of autism through childhood. Journal of Autism and Developmental Disorders. 1996;26:557–69. doi: 10.1007/BF02172276. [DOI] [PubMed] [Google Scholar]
- Eigsti IM, Bennetto L. Grammaticality judgments in autism spectrum disorders: Deviance or delay. Journal of Child Language. 2009;19:1–23. doi: 10.1017/S0305000909009362. [DOI] [PubMed] [Google Scholar]
- Fein DA, Barton M, Eigsti IM, Kelley E, Naigles LR, Schultz RT, et al. Optimal outcome in individuals with a history of autism. Journal of Child Psychiatry and Psychology. 2013;52:195–205. doi: 10.1111/jcpp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken L, Jusczyk PW, Mandel DR. When prosody fails to cue syntactic structure: 9-month-olds’ sensitivity to phonological versus syntactic phrases. Cognition. 1994;51:237–65. doi: 10.1016/0010-0277(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Goodsitt JV, Morgan JL, Kuhl PK. Perceptual strategies in prelingual speech segmentation. Journal of Child Language. 1993;20:229–52. doi: 10.1017/s0305000900008266. [DOI] [PubMed] [Google Scholar]
- Heaton P. Pitch memory, labelling and disembedding in autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2003;44:543–51. doi: 10.1111/1469-7610.00143. [DOI] [PubMed] [Google Scholar]
- Heaton P. Interval and contour processing in autism. Journal of Autism and Developmental Disorders. 2005;35:787–93. doi: 10.1007/s10803-005-0024-7. [DOI] [PubMed] [Google Scholar]
- Heaton P, Hermelin B, Pring L. Autism and pitch processing: A precurson for savant musical ability? Music Perception. 1998;154:291–305. [Google Scholar]
- Heaton P, Hudry K, Ludlow A, Hill E. Superior discrimination of speech pitch and its relationship to verbal ability in autism spectrum disorders. Cognitive Neuropsychology. 2008;25:771–82. doi: 10.1080/02643290802336277. [DOI] [PubMed] [Google Scholar]
- Helt M, Kelley E, Kinsbourne M, Pandey J, Boorstein H, Herbert M, et al. Can children with autism recover? If so, how? Neuropsychology Reviews. 2008;18:339–66. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- Hus V, Taylor A, Lord C. Telescoping of caregiver report on the Autism Diagnostic Interview--Revised. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52:753–60. doi: 10.1111/j.1469-7610.2011.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen-Pasley A, Wallace GL, Ramus F, Happe F, Heaton P. Enhanced perceptual processing of speech in autism. Developmental Science. 2008;11:109–21. doi: 10.1111/j.1467-7687.2007.00644.x. [DOI] [PubMed] [Google Scholar]
- Jones CR, Happe F, Baird G, Simonoff E, Marsden AJ, Tregay J, et al. Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia. 2009;47:2850–8. doi: 10.1016/j.neuropsychologia.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW, Houston DM, Newsome M. The beginning of word segmentation in English-learning infants. Cognitive Psychology. 1999;39:159–207. doi: 10.1006/cogp.1999.0716. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Dev Sci. 2005;8:F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM. Foreign-language experience in infancy: effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9096–101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. Am J Med Genet A. 2006;140:2257–74. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeris A, Hazan V. The effect of native vowel processing ability and frequency discrimination acuity on the phonetic training of English vowels for native speakers of Greek. Journal of the Acoustical Society of America. 2010;128:3757–68. doi: 10.1121/1.3506351. [DOI] [PubMed] [Google Scholar]
- Lepisto T, Kujala T, Vanhala R, Alku P, Huotilainen M, Naatanen R. The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Research. 2005;1066:147–57. doi: 10.1016/j.brainres.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- Loui P, Kroog K, Zuk J, Winner E, Schlaug G. Relating pitch awareness to phonemic awareness in children: implications for tone-deafness and dyslexia. Frontiers in psychology. 2011;2:111. doi: 10.3389/fpsyg.2011.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Reeb BC, Fox NA. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Dev Sci. 2009;12:568–82. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur GM, Bishop DV. Frequency discrimination deficits in people with specific language impairment: reliability, validity, and linguistic correlates. Journal of Speech, Language and Hearing Research. 2004;47:527–41. doi: 10.1044/1092-4388(2004/041). [DOI] [PubMed] [Google Scholar]
- Miller LK. The savant syndrome: Intellectual impairment and exceptional skill. Psychological Bulletin. 1999;125:31–46. doi: 10.1037/0033-2909.125.1.31. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mottron L, Peretz I, Menard E. Local and global processing of music in high-functioning persons with autism: beyond central coherence? Journal of Child Psychology and Psychiatry. 2000;41:1057–65. [PubMed] [Google Scholar]
- Mraz KD, Dixon J, Dumont-Mathieu T, Fein D. Accelerated head and body growth in infants later diagnosed with autism spectrum disorders: A comparative study of optimal outcome children. Journal of Child Neurology. 2009;24:833–45. doi: 10.1177/0883073808331345. [DOI] [PubMed] [Google Scholar]
- Muller RA, Basho S. Are nonlinguistic functions in “Broca’s area” prerequisites for language acquisition? FMRI findings from an ontogenetic viewpoint. Brain and Language. 2004;89:329–36. doi: 10.1016/S0093-934X(03)00346-8. [DOI] [PubMed] [Google Scholar]
- Nazzi T, Dilley LC, Jusczyk AM, Shattuck-Hufnagel S, Jusczyk PW. English-learning infants’ segmentation of verbs from fluent speech. Language and Speech. 2005;48:279–98. doi: 10.1177/00238309050480030201. [DOI] [PubMed] [Google Scholar]
- O’Riordan M, Passetti F. Discrimination in Autism Within Different Sensory Modalities. Journal of Autism and Developmental Disorders. 2006;36:665. doi: 10.1007/s10803-006-0106-1. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Roberts TP. Auditory evoked fields predict language ability and impairment in children. International Journal of Psychophysiology. 2008;68:170–5. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire (SCQ) Los Angeles: Western Psychological Services; 2003a. [Google Scholar]
- Rutter M, Le Couteur A, Lord C. ADI-R: The Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003b. [Google Scholar]
- Seidl A, Johnson E. Boundary alignment enables 11-month-olds to segment vowel initial words from speech. Journal of Child Language. 2008;35:1–24. doi: 10.1017/s0305000907008215. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Krauss MW, Shattuck PT, Orsmond G, Swe A, Lord C. The symptoms of autism spectrum disorders in adolescence and adulthood. Journal of Autism and Developmental Disorders. 2003;33:565–81. doi: 10.1023/b:jadd.0000005995.02453.0b. [DOI] [PubMed] [Google Scholar]
- Singh L, White K, Morgan JL. Building a lexicon in the face of variable input: Effects of pitch and amplitude variation on early word recognition. Language Learning and Development. 2008;4:157–78. [Google Scholar]
- Son JY, Smith LB, Goldstone RL. Simplicity and generalization: Short-cutting abstraction in children’s object categorizations. Cognition. 2008;108:626–38. doi: 10.1016/j.cognition.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan J, Krishnan A, Gandour JT. Pitch encoding in speech and nonspeech contexts in the human auditory brainstem. Neuroreport. 2008;19:1163–7. doi: 10.1097/WNR.0b013e3283088d31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker JF, Yeung HH. Infant speech perception bootstraps word learning. Trends in Cognitive Sciences. 2005;9:519–27. doi: 10.1016/j.tics.2005.09.003. [DOI] [PubMed] [Google Scholar]