Abstract
Background
Alcohol abuse increases the risk for acute lung injury (ALI). In both experimental models and in clinical studies, chronic alcohol ingestion causes airway oxidative stress and glutathione depletion and increases the expression of transforming growth factor beta-1 (TGFβ1), a potent inducer of fibrosis, in the lung. Therefore, we hypothesized that alcohol ingestion could promote aberrant fibrosis following experimental ALI and that treatment with the glutathione precursor s-adenosylmethionine (SAMe) could mitigate these effects.
Methods
Three month old C57BL/6 mice were fed standard chow ± alcohol (20% v/v) in their drinking water for 8 wks and ± SAMe (4% w/v) during the last 4 wks. ALI was induced by intratracheal instillation of bleomycin (2.5 units/kg) and lungs were assessed histologically at 7 and 14 days for fibrosis, and at 14 days for the expression of extracellular matrix proteins and TGFβ1.
Results
Alcohol ingestion had no apparent effect on lung inflammation at 7 days, but at 14 days after bleomycin treatment it increased lung tissue collagen deposition, hydroxyproline content, and the release of activated TGFβ1 into the airway. In contrast, SAMe supplementation completely mitigated alcohol-induced priming of these aberrant fibrotic changes through decreased TGFβ1 expression in the lung. In parallel, SAMe decreased alcohol-induced TGFβ1 and Smad3 mRNA expressions by lung fibroblasts in vitro.
Conclusion
These new experimental findings demonstrate that chronic alcohol ingestion renders the experimental mouse lung susceptible to fibrosis following bleomycin-induced ALI, and that these effects are likely driven by alcohol-mediated oxidative stress and its induction and activation of TGFβ1.
Keywords: ARDS, glutathione, s-adenosylmethionine, fibrosis, TGFβ1
INTRODUCTION
Alcohol is the most widely used and abused substance in the world, with a prevalence of alcohol abuse and dependence of ∼ 30% at some time throughout adult life in the USA (Merikangas and McClair, 2012). Its abuse accounts for up to 41% of emergency room visits (Dobson, 2003) as well as approximately 10% of ICU admissions (Moss and Burnham, 2006) and results in billions of dollars in annual health care expenditures (Dobson, 2003). Alcohol abuse is associated with both an increased susceptibility to pneumonia and an increased incidence of the acute respiratory distress syndrome (ARDS) in critically ill individuals (Moss et al., 1996). Further, our group previously identified that chronic alcohol ingestion is associated with depletion of the critical anti-oxidant glutathione within the alveolar airspace in both experimental animals and in otherwise healthy alcoholic humans, and there is compelling experimental evidence implicating oxidative stress and glutathione depletion as a fundamental mechanism underlying alcohol-induced lung dysfunction (Guidot et al., 1999). In addition, we determined that alcohol-mediated oxidative stress induces the expression of transforming growth factor beta-1 (TGFβ1), which impairs alveolar epithelial barrier function (Bechara et al., 2004) and exaggerates tissue remodeling through activation of lung fibroblasts (Roman et al., 2005), and that alcohol-induced induction of TGFβ1 can be abolished by supplementing the diets of alcohol-fed rats with glutathione precursors such as N-acetylcysteine (NAC), SAMe or procysteine (Brown et al., 2007, Velasquez et al., 2002, Holguin et al., 1998). Although there have been no studies examining a potential link between alcohol consumption and the development of lung fibrosis following ARDS, both elevated levels of TGFβ1 and glutathione depletion have been implicated in lung fibrogenesis (Liu et al., 2012, Cantin et al., 1989). Importantly, as many as 53% of patients with ARDS develop fibrosis and up to 55% of non-survivors have lung fibrosis at autopsy (Rocco et al., 2009). Further, it has been well established that activation of TGFβ1 is associated with extracellular matrix production and the development of fibrosis in a variety of tissues including the lung (Coward et al., 2010). In the liver, the development of fibrosis (i.e. ‘cirrhosis’) following a variety of insults including chronic alcohol abuse has been linked to activation of TGFβ1 (Dooley and ten Dijke, 2012). However, although chronic alcohol abuse is clearly linked to an increased risk of ALI and is a potent inducer of TGFβ1 in the lung, there is essentially nothing known regarding the effects of chronic alcohol exposure on the fibrotic phase of lung repair following acute injury.
We hypothesized that alcohol-induced TGFβ1 expression and activation during acute lung injury primes the lung for aberrant fibroproliferation and even fibrosis following ALI. We used a well-characterized mouse model of bleomycin-induced ALI to evaluate the effects of chronic alcohol ingestion on the lung following an acute inflammatory lung injury. We further hypothesized that treatment with SAMe would attenuate the alcohol-induced oxidative stress and consequent induction and activation of TGFβ1, and would thereby mitigate lung fibrosis following bleomycin-induced ALI.
MATERIALS AND METHODS
Animals and dietary treatments
3 month old C57BL/6 mice were obtained from Jackson Laboratories. After acclimatization to our animal facility including feeding with standard chow and water ad lib, mice were started on alcohol with an initial concentration of 5% (v/v) in their drinking water that was increased by 5% every 3 days until a final concentration of 20% was reached. Mice were then continued on 20% alcohol in drinking water for 8 weeks; in some mice, SAMe (4% w/v) was added to their water/alcohol mixture for the last 4 weeks of the feeding protocol. At the end of this 8-week period, mice were subjected to the bleomycin injury protocol (see below) and then maintained on their chronic feeding protocol for 1 or 2 weeks at which time they were sacrificed. All studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Emory University and conformed to institutional standards for the humane treatment of laboratory animals.
Bleomycin-induced acute lung injury
Bleomycin-induced acute lung injury was chosen in our study for its reproducibility, including its predictable resolution phase that enabled us to assess lung tissue remodeling following acute injury. Mice were anesthetized by intraperitoneal injection with Ketamine/Xylazine (100 mg/kg and 10 mg/kg) and the trachea was exposed using sterile technique. Bleomycin (2.5 units/kg) (Sigma-Aldrich, St Louis, MO) in phosphate-buffered saline (PBS) or vehicle (PBS) alone was injected into the tracheal lumen. After instillation the incision was closed and the animals were allowed to recover. At 7 or 14 days after bleomycin injection the mice were euthanized by isofluorane inhalation. Bronchoalveolar lavage (BAL) fluid was collected by instilling and withdrawing 500 µl of PBS in and out of the mouse lung 3 times. Samples were immediately centrifuged and the supernatants collected and stored at −80°C until analyzed. The lungs were harvested and prepared for histological examination as well as quantification of hydroxyproline content.
Hydroxyproline Assay
The frozen right lower lobes of the lungs were analyzed for hydroxyproline content using a commercially available assay kit using the manufacturer’s protocol (BioVision, San Francisco, CA). A standard curve was generated for each assay using a hydroxyproline standard and the hydroxyproline content in each sample was calculated using this standard curve. Results were expressed as micrograms of hydroxyproline per 10 milligrams of lung tissue.
Histology
Masson’s trichrome staining (for collagen deposition) was performed on non-adjacent 5 µm paraffin-embedded lung sections. Morphometric analyses for collagen deposition quantification were performed on 3 random lung sections (3 high power fields (40x) per section) using ImageJ 1.42 (http://rsbweb.nih.gov/ij/). The relative amount of collagen deposition was averaged and normalized to that of lungs from untreated mice.
Isolation of primary lung fibroblasts
Primary lung fibroblasts (PLF) were harvested from young and old C57BL/6 mice as previously described (Roman et al., 2005). Cells were cultured in fibroblast culture medium (Dulbecco’s Modified Eagle’s Medium (DMEM) with 4.5 g/L glucose supplemented with 20% fetal bovine serum (FBS), 100 U/ml penicillin G sodium, 100 U/ml streptomycin, 0.25 µg/ml amphotericin B). Primary lung fibroblasts between passages 3–5 were used for treatment of cells.
Treatment of the lung fibroblasts
Passage 3–5 primary lung fibroblasts isolated were plated in 6 well plates at 5,000 cells/cm2 for 24 hours prior to treatment. Cells were treated with or without SAMe (100, 250 and 500 µM) in the presence or absence of alcohol (60 mM). Cells were harvested at 7 hours for to examine for TGF-β1 and α-Smooth muscle actin-1 mRNA expressions.
Messenger RNA expression analysis
Messenger RNA mRNA was isolated from primary lung fibroblasts (PLF) as previously described using RNeasy kit (Qiagen)(Sueblinvong et al., 2008). First-strand cDNA was synthesized. Quantitative PCR was performed with primers set for 18s, TGF-β1, and α-Smooth muscle actin-1 using iQ SYBR Green Supermix (Bio-Rad); the real-time iCycler sequence detection system (Bio-Rad) was used for the real-time PCR analysis. The level of target mRNA expression was normalized to 18s housekeeping gene levels and relative target mRNA levels were determined according to the comparative cycle threshold method (Applied Biosystems 7900HT Sequence Detection System, User Bulletin No. 2; Applied Biosystems) and relative expression values were calculated as previously described (Sueblinvong et al., 2008).
Enzyme linked immunosorbent assay (ELISA) for TGFβ1
We previously determined that all of the TGFβ1 protein released into the alveolar space of alcohol-fed animals during acute inflammation was already in the activated form (i.e. dissociated from the latency activating peptide or LAP) (Bechara et al., 2004); therefore, in this study the BAL fluid supernatants were not acidified prior to performing the ELISA as acidification was unnecessary to separate the active TGFβ1 protein from the LAP. Levels of active TGFβ1 in the BAL fluid were determined with a commercially available ELISA kit (R&D Systems, Minneapolis, MN) using the manufacturer’s protocol. Absorbance was read at 450 nm and quantified using a standard curve.
Statistical Analyses
One-way ANOVA (using GraphPad Prism and GraphPad InStat version 4) was used for comparisons among groups, and post-test analysis using Bonferroni’s method performed if statistical significance was reached. Significant differences were accepted at a P level of < 0.05.
RESULTS
Chronic alcohol ingestion increased lung fibrosis 14 days following bleomycin-induced lung injury
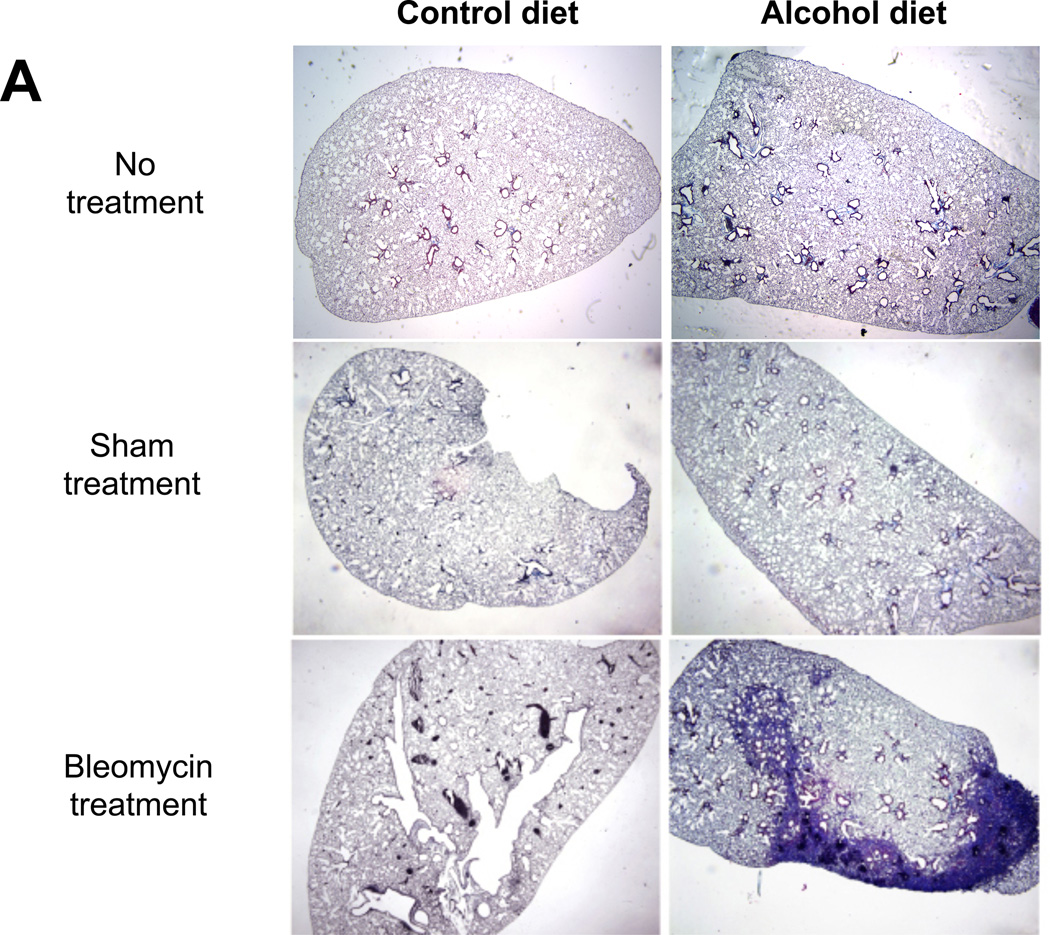
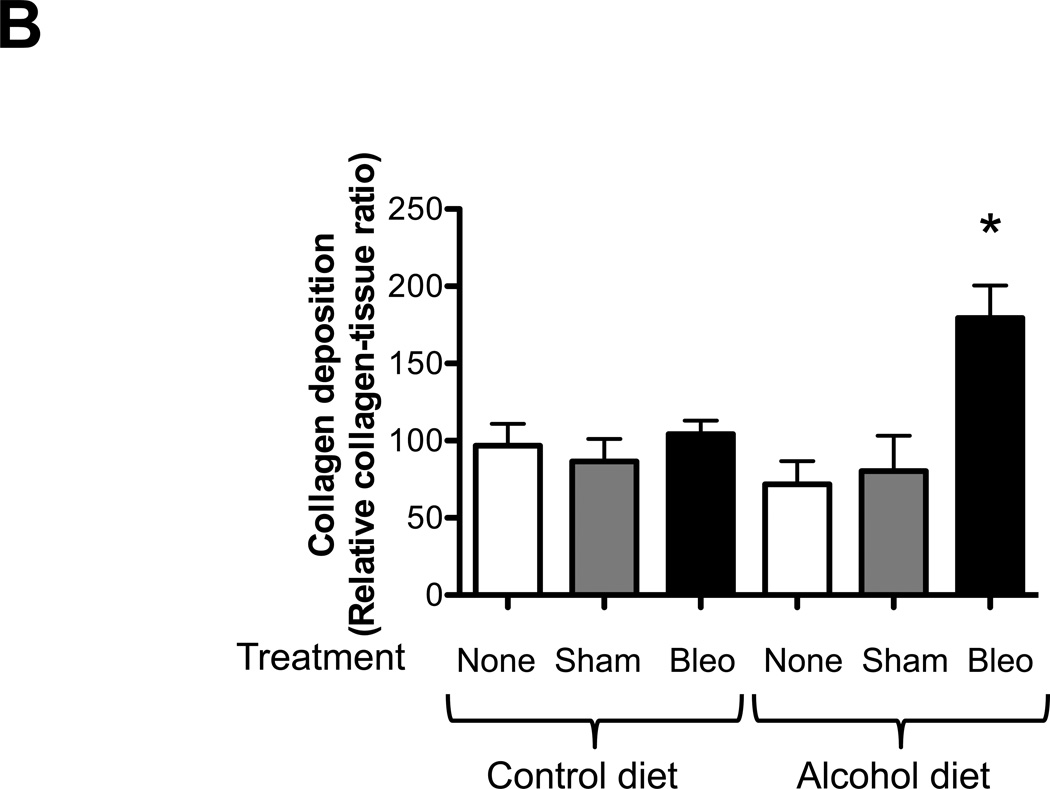
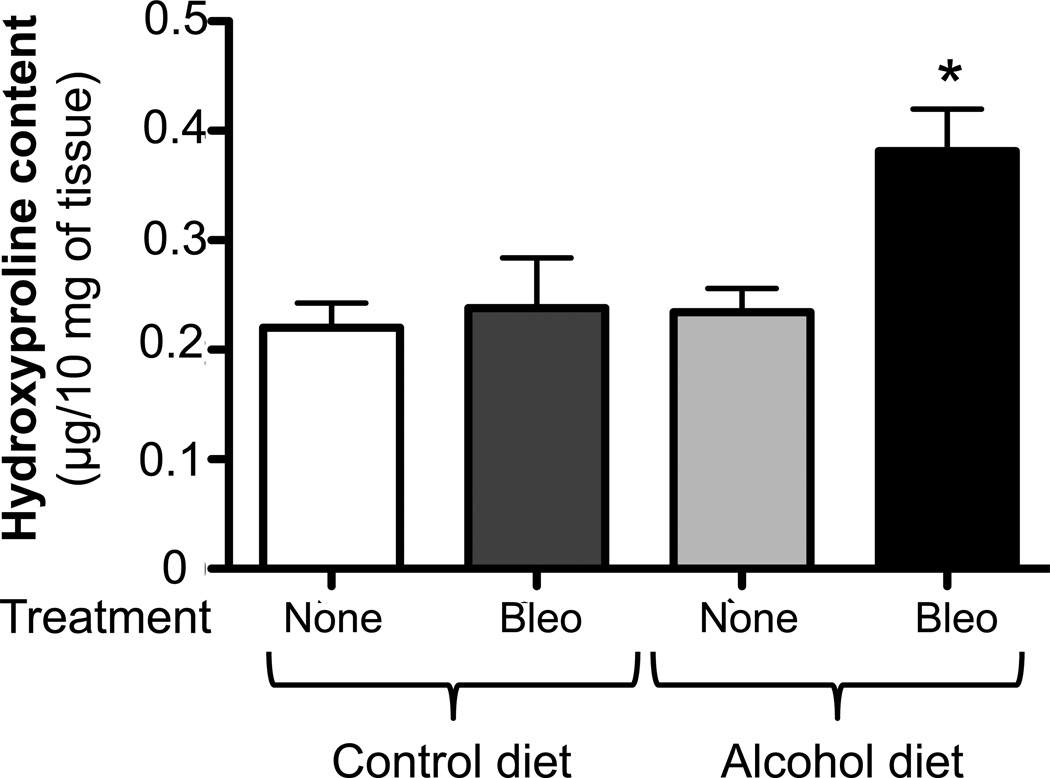
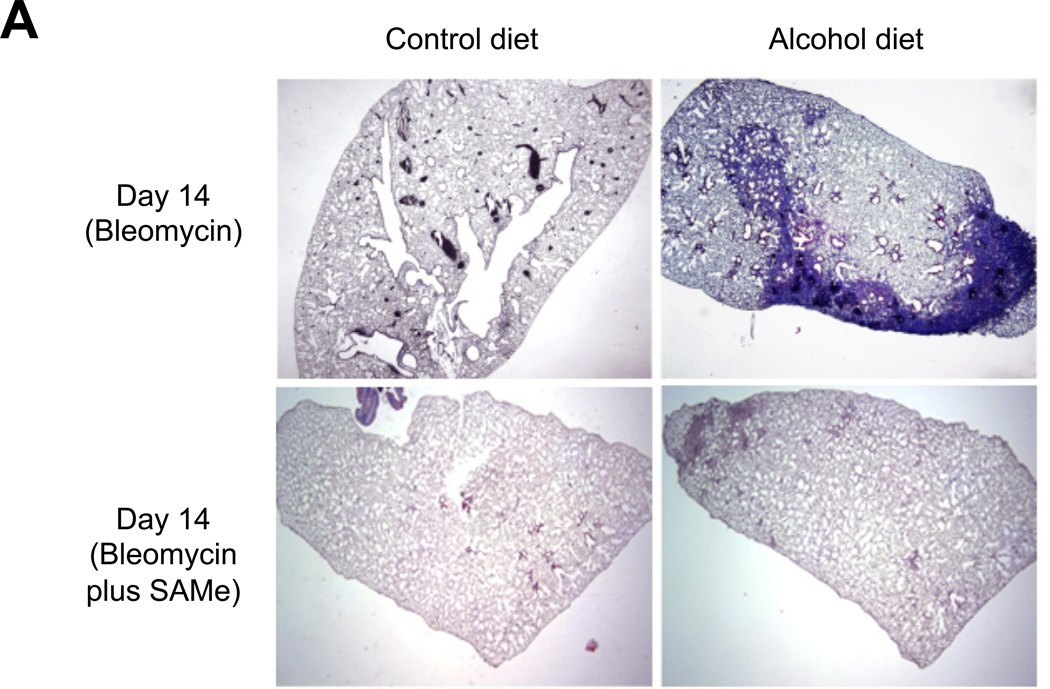
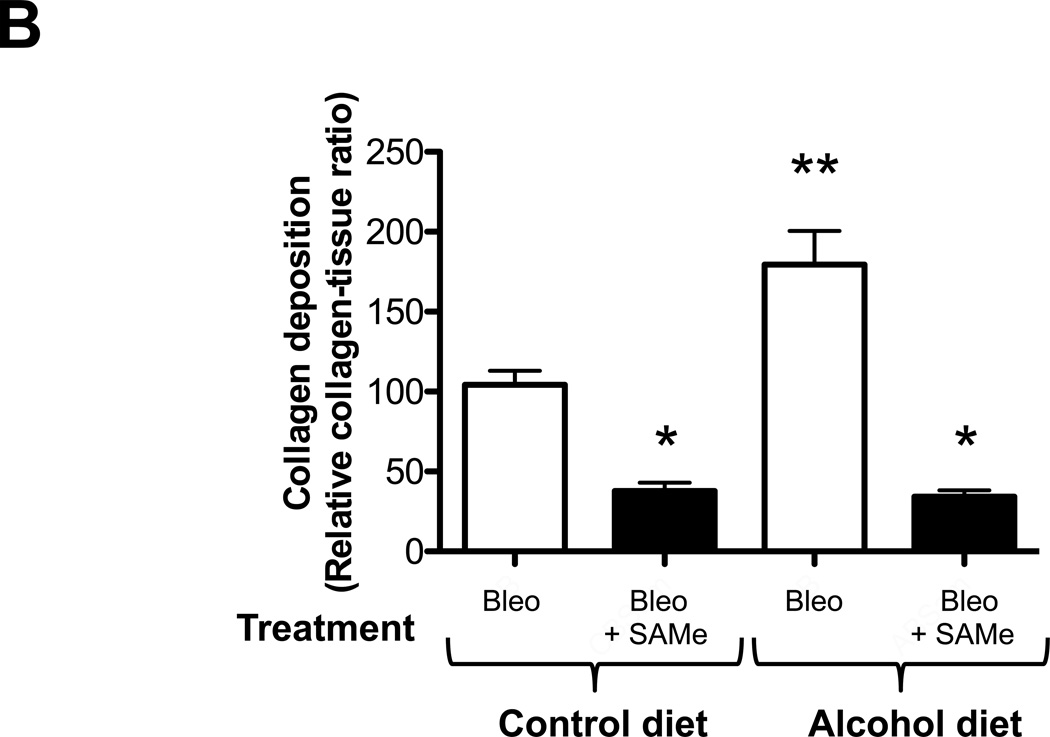
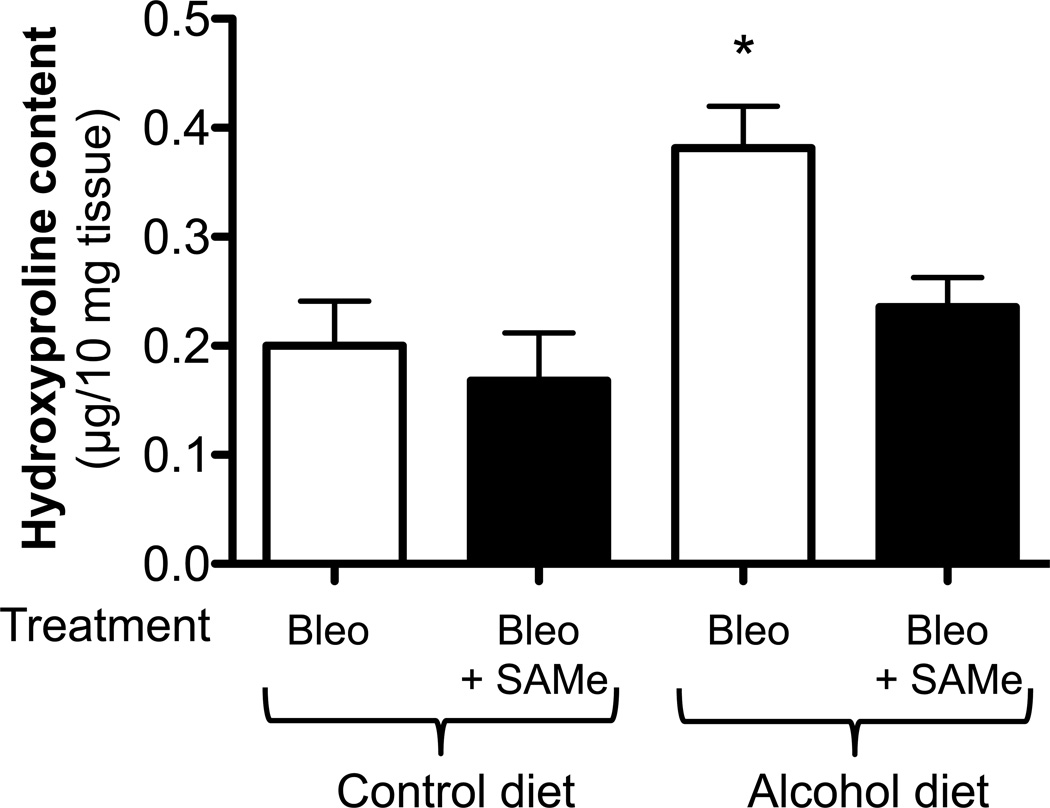
We first examined histological changes and collagen deposition (using Masson’s trichrome staining) in the lungs of control-fed and alcohol-fed mice 7 and 14 days following bleomycin-induced acute lung injury. At 7 days following intratracheal bleomycin instillation, the lungs of alcohol-fed and control-fed mice showed similar mild inflammatory responses (not shown). However, at day 14 when remodeling response is expected to be most evident, the lungs from bleomycin-treated alcohol-fed mice showed evidence of aberrant repair as reflected by fibrosis and increased collagen deposition when compared to either control-fed mice treated with bleomycin, or to control-fed or alcohol-fed mice that were either untreated or sham treated (representative images are shown in Figure 1, panel A). These qualitative findings were verified by quantitative morphometric analyses showing an ∼ 75% increase (P<0.05) in lung collagen deposition in bleomycin-treated alcohol-fed mice when compared to untreated control-fed or alcohol-fed mice, sham-treated control-fed or alcohol-fed mice, or bleomycin-treated control-fed mice, at day 14 (Figure 1, panel B). In parallel, and as shown in Figure 2, there was a significant increase (∼70%; P<0.05) in hydroxyproline content (Figure 2) in bleomycin-treated alcohol-fed mice, consistent with the histological findings in Figure 1.
Figure 1. Chronic alcohol ingestion increased fibroproliferation and collagen deposition following bleomycin-induced acute lung injury.
Control-fed and alcohol-fed mice were treated intratracheally with bleomycin (see Methods) and then sacrificed 14 days later. The lungs were stained with Masson’s trichrome to assess tissue collagen deposition. Panel A shows representative low-power (2X) images and Panel B shows the summary data for the relative intensity of collagen staining as analyzed by ImageJ 1.42 software; values were normalized to those of lungs from uninjured control-fed mice. N = 3–5 per group.
* P<0.05 increased compared to lungs from uninjured control-fed mice.
Figure 2. Chronic alcohol ingestion increased lung hydroxyproline content following bleomycin-induced lung injury.
Control-fed and alcohol-fed mice were treated intratracheally with bleomycin (see Methods) and then sacrificed 14 days later. Lungs were analyzed for hydroxyproline content. Graph depicts hydroxyproline content (µg per 10 mg of lung tissue) in these same lungs. N = 3–5.
* P<0.05 increased compared to lungs from untreated control-fed mice.
Chronic alcohol ingestion caused aberrant tissue remodeling as reflected by persistently increased TGFβ1 expression following bleomycin-induced lung injury
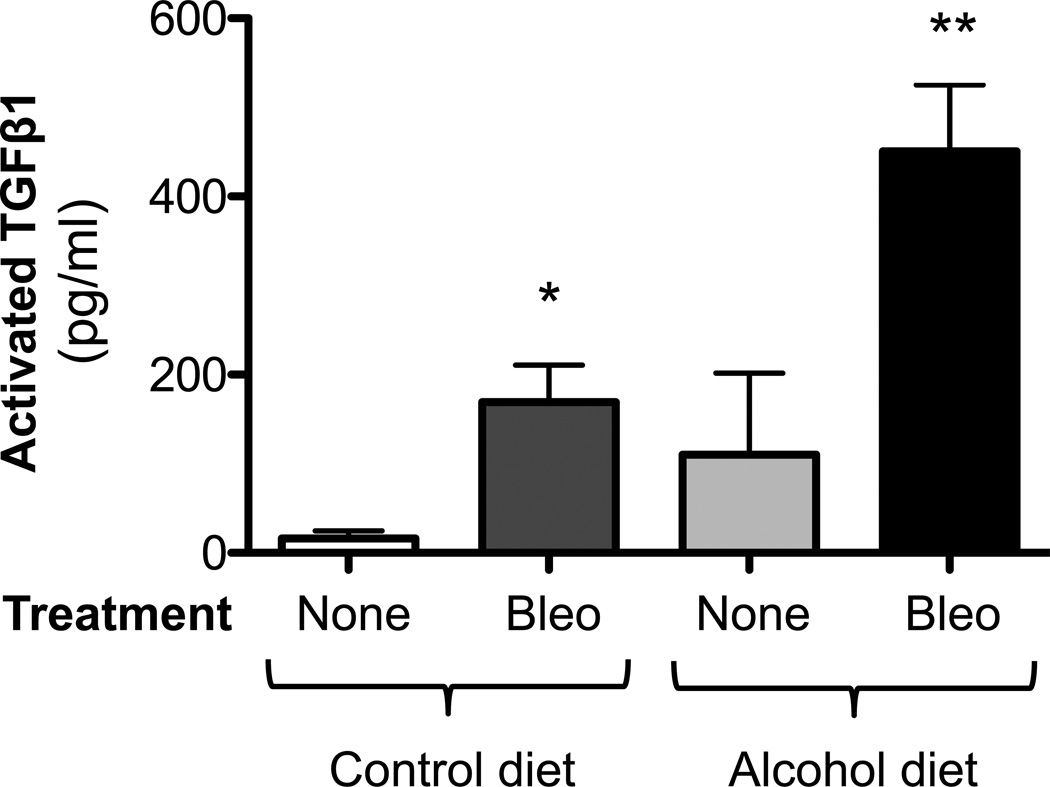
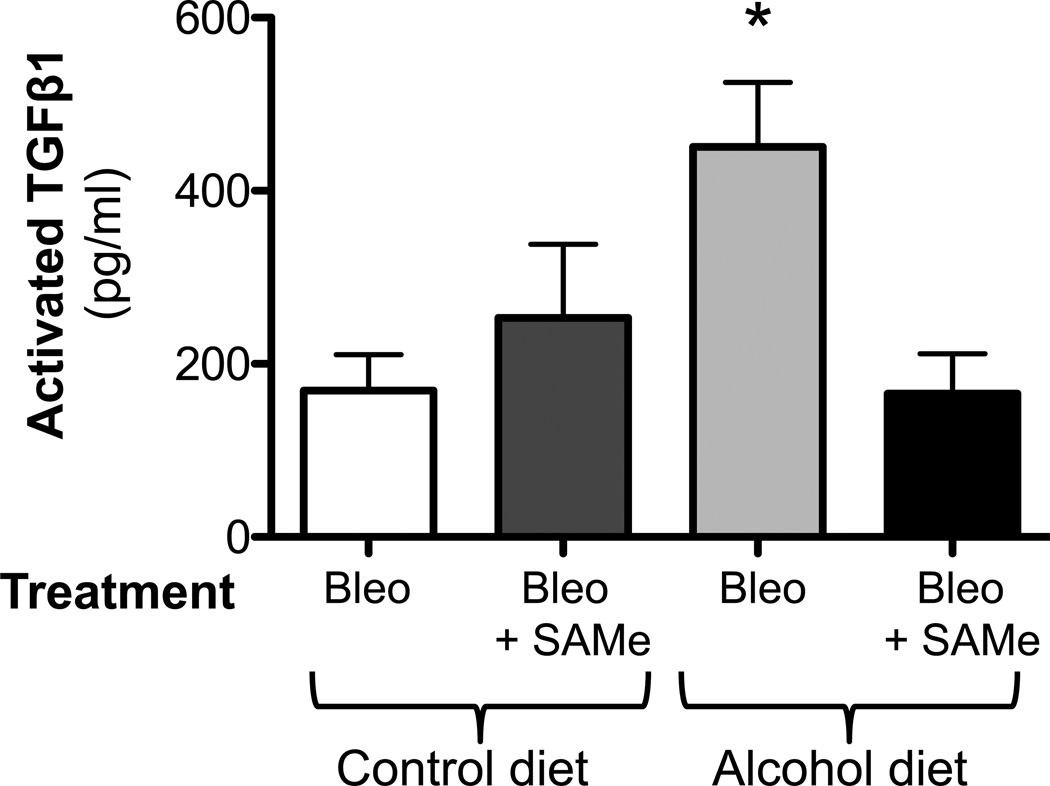
It has been well established that TGFβ1 is involved in fibroblast activation and fibrogenesis (Coward et al., 2010). Therefore, we examined TGFβ1 expression in our experimental model. At 14 days after bleomycin treatment, we quantified the release of activated TGFβ1 into the BAL fluid by ELISA and determined that bleomycin-induced lung injury was associated with a significant (P<0.05) release of activated TGFβ1 into the BAL fluid of both control-fed and alcohol-fed mice; as expected, however, the alcohol ingestion accentuated this effect (∼120% increase; P<0.05) compared to the injured control-fed mice (Figure 3).
Figure 3. Chronic alcohol ingestion increased TGFβ1 expression following bleomycin-induced lung injury.
Control-fed and alcohol-fed mice were treated intratracheally with bleomycin (see Methods) and then sacrificed 14 days later. Bronchoalveolar lavage (BAL) fluid were analyzed for TGFβ1 expression by ELISA. Graph shows the levels of activated TGFβ1 in the BAL fluids. N = 5–6.
* P<0.05 increased compared to lungs from untreated control-fed mice
** P<0.05 increased compared to lungs from bleomycin-treated, control-fed mice
Treatment with SAMe attenuated the fibrotic response following bleomycin-induced acute lung injury
As previously discussed, chronic alcohol ingestion profoundly decreases the levels of the anti-oxidant glutathione in the lungs of experimental animals and of humans. In addition, it is associated with a decrease in endogenous levels of SAMe (Beier et al., 2011), which is a precursor to glutathione in its biosynthetic pathway. To examine the role of supplemental SAMe on lung repair following injury, alcohol-fed mice received supplemental SAMe in the drinking water for 4 weeks prior to the induction of lung injury and this treatment was continued until they were sacrificed. At 14 days after induction of lung injury with bleomycin we examined the lungs for collagen deposition using Masson’s trichrome staining. The lungs of alcohol-fed mice that were treated with SAMe had less collagen deposition than the lungs of alcohol-fed mice not treated with SAMe (representative images are shown in Figure 4, panel A. These qualitative findings were supported by quantitative analyses of fibrotic areas showing that treatment with SAMe reduced (P<0.05) bleomycin-induced collagen deposition in both the lungs of control-fed and alcohol-fed mice; however, the relative reduction was much greater in the alcohol-fed mice (Figure 4, panel B). In parallel, SAMe treatment significantly (P<0.05) decreased the hydroxyproline content (Figure 5) in the lungs of bleomycin-treated, alcohol-fed mice.
Figure 4. S-adenosylmethionine (SAMe) supplementation attenuated collagen deposition following bleomycin-induced lung injury.
Control-fed and alcohol-fed mice had their water supplemented with SAMe starting 4 weeks prior to bleomycin treatment and then continued for 2 weeks following (see Methods), at which time the mice were sacrificed. Masson’s trichrome staining was performed identically to lungs shown in Figure 1 to assess for collagen deposition. Panel A shows representative low-power (2X) images. Panel B shows the summary data for the relative intensity of collagen staining as analyzed by ImageJ 1.42 software; data were normalized to lungs from bleomycin-treated, control-fed mice and expressed as percentage of bleomycin-treated, control-fed group (N = 4 – 6).
* P<0.05 decreased compared to lungs from bleomycin-treated, control-fed mice. ** P<0.05 increased compared to lungs from bleomycin-treated, control-fed mice.
Figure 5. S-adenosylmethionine (SAMe) supplement reduced lung hydroxyproline content following bleomycin-induced lung injury.
Control-fed and alcohol-fed mice had their water supplemented with SAMe starting 4 weeks prior to bleomycin treatment and then continued for 2 weeks following bleomycin-induced lung injury (see Methods), at which time the mice were sacrificed. Lungs were harvested for hydroxyproline content analyses. Graph depicts hydroxyproline content (µg per 10 mg of lung tissue) in these same lungs. N = 3–5.
* P<0.05 compared to lungs from bleomycin-treated, control-fed mice.
SAMe treatment decreased TGFβ1 expression in the lungs of bleomycin-treated, alcohol-fed mice
To examine the potential mechanisms by which SAMe attenuates collagen deposition following bleomycin-induced lung injury, we examined the expression of TGFβ1 in both lung tissue and BAL fluid. Although, SAMe treatment did not have an effect on TGFβ1 expression in the lung lavage of control-fed mice with and without injury, it significantly (P<0.05) attenuated the release of activated TGFβ1 into the alveolar space as reflected by decreased levels of TGFβ1 in the BAL fluid in the injured alcohol-fed mice (Figure 6).
Figure 6. S-adenosylmethionine (SAMe) supplementation attenuated TGFβ1 expression in mice lung following bleomycin-induced lung injury.
Control-fed and alcohol-fed mice had their water supplemented with SAMe starting 4 weeks prior to bleomycin treatment and then continued for 2 weeks following (see Methods), at which time the mice were sacrificed and bronchoalveolar lavage (BAL) fluid were analyzed for TGFβ1 expression by ELISA. Graph shows the levels of activated TGFβ1 in the BAL fluids. N = 4–6.
* P<0.05 increased compared to lungs from bleomycin-treated, control-fed mice.
SAMe treatment decreased TGFβ1 and attenuated alcohol-induced fibroblast to myofibroblast transdifferentiation in the lung fibroblasts in vitro
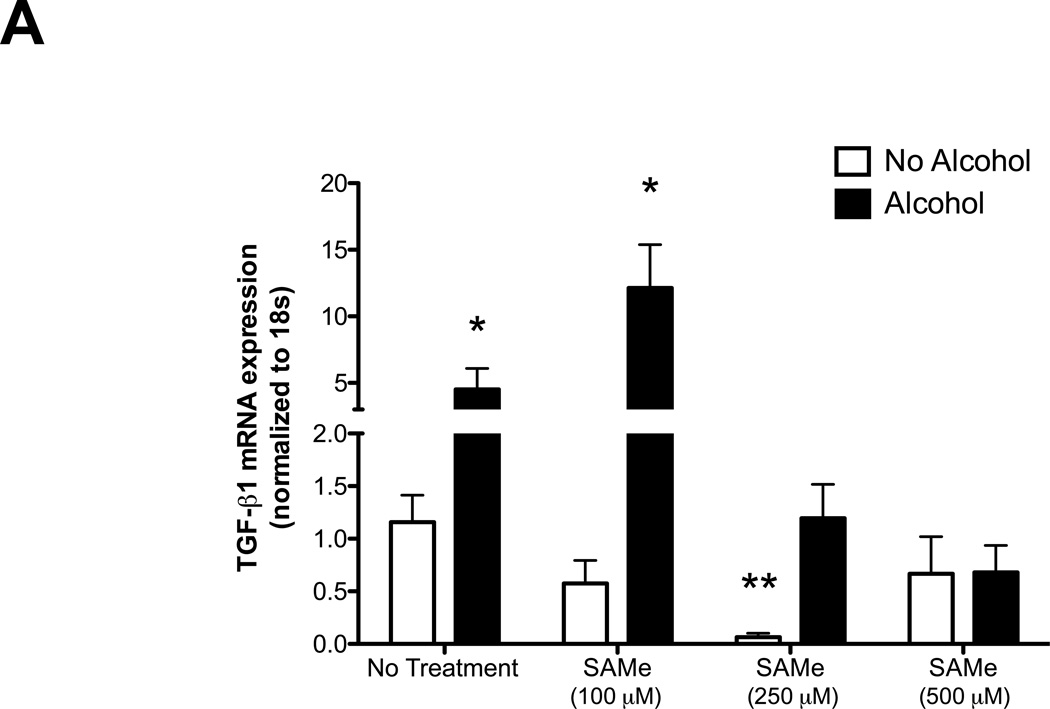
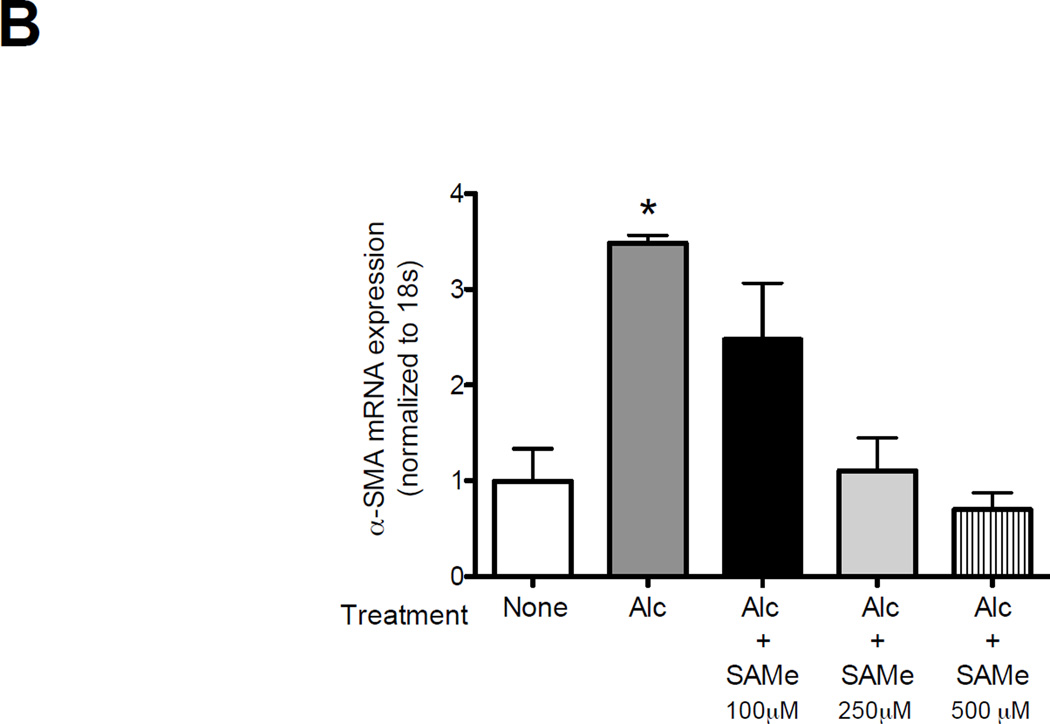
To examine the potential mechanisms by which SAMe attenuates collagen deposition and TGFβ1 activation following bleomycin-induced lung injury, we examined the effect of SAMe on the lung fibroblasts. As previously shown by our group, alcohol treatment stimulated TGFβ1 mRNA expression and lung fibroblast to myofibroblast transdifferentiation as seen by increase in α-SMA-1 mRNA expression (Figure 7, panel A and B, respectively). Here, we further demonstrated that SAMe treatment suppressed TGFβ1 and α-SMA-1 mRNA expressions in the dose dependent manner (Figure 7, panel A and B). Interestingly, at 100 µM of SAMe in the presence of alcohol, there was an induction of TGFβ1 but not α-SMA-1 mRNA expression. This suggested that at higher doses, SAMe might suppress fibroblast to myofibroblast transdifferentiation, possibly via its properties as a methyl donor and/or as a precursor to cysteine and GSH biosynthesis. In contrast, at lower doses SAMe may dampen cellular responses to TGFβ1 including the induction of α-SMA-1 expression. At present this is only speculative but these results are consistent with the conclusion that fibroblasts are influenced by their ambient redox potential, and that these effects likely depend on whether the redox potential is more positive (oxidized) or more negative (reduced).
Figure 7. SAMe treatment suppressed the alcohol-induced TGFβ1 and α-smooth muscle actin (α-SMA-1) mRNA expression in the lung fibroblasts.
The lung fibroblasts were isolated from uninjured control-fed mice (C57Bl/6). Cells were treated ± alcohol (60 mM) ± SAMe (100, 250 and 500 µM) for 7 hours. Cells lysate were analyzed for TGFβ1 (Panel A) and α-SMA-1 (Panel B) mRNA expressions using quantitative PCR. N = 6.
* P<0.05 increased compared to no alcohol and no SAMe treatment group.
** P < 0.05 decreased compared to no alcohol and no SAMe treatment group.
DISCUSSION
In this study we determined that chronic alcohol ingestion promoted an aberrant fibrotic response to bleomycin-induced experimental acute lung injury. Specifically, the lungs of alcohol-fed mice developed significant fibrosis at two weeks after bleomycin treatment while the lungs of control-fed mice displayed typical recovery from the acute injury with little evidence of fibrosis. Consistent with these gross histological changes, chronic alcohol ingestion increased the expression and activation of TGFβ1 following bleomycin-induced lung injury and, in parallel (or perhaps as a direct consequence), increased the deposition of mature collagen in the injured lung tissue. In contradistinction, treatment with the glutathione precursor SAMe attenuated the aberrant fibrotic responses induced by chronic alcohol ingestion through direct effect on the lung fibroblasts. To our knowledge, these results provide the first experimental evidence that chronic alcohol ingestion promotes the development of fibrosis following bleomycin-induced acute lung injury. As alcohol abuse dramatically increases the risk of ARDS, a devastating form of acute lung injury for which there are no effective pharmacological therapies, our experimental findings suggest that these vulnerable individuals may also have dysfunctional repair mechanisms that could be amenable to treatment with thiol anti-oxidants such as SAMe to prevent long-term consequences such as the development of a prolonged fibroproliferative phase or even irreversible pulmonary fibrosis.
Our group had previously identified that chronic alcohol abuse is associated with a 2–4-fold increased incidence of ARDS (Moss et al., 1996, Moss et al., 2003). Although the pathological effects of chronic alcohol ingestion on the development of liver fibrosis had been well established (Beier et al., 2011), to our knowledge there have been no studies examining whether chronic alcohol ingestion promotes disrepair and fibrosis following acute inflammatory lung injury. However, there were many reasons to predict that alcohol could interfere with normal reparative processes in the lung. Specifically, we identified that chronic alcohol ingestion produces a previously unrecognized state of severe oxidative stress and cellular dysfunction within the lung as reflected by decreased glutathione levels (Guidot et al., 1999), increased fibronectin expression (Brown et al., 2007), and increased expression and activation of TGFβ1 (Bechara et al., 2004); each of these factors has been implicated in the development of pulmonary fibrosis (Coward et al., 2010). Therefore, we hypothesized that chronic alcohol ingestion alters lung repair processes and promotes fibrosis formation following acute inflammatory injury. In our current study, we determined that alcohol-fed mice developed overt lung fibrosis 14 days after bleomycin-induced lung injury, as evidenced by histological analyses (Masson’s trichrome staining) and increased protein content of hydroxyproline, whereas the lungs of mice that did not ingest alcohol (control-fed) recovered back to baseline with little to no evidence of tissue fibrosis. In light of our previous studies showing that alcohol ingestion induces the expression of TGFβ1 expression in the lung (Bechara et al., 2004), we suspected that TGFβ1 was a mediator of alcohol-induced susceptibility to fibrosis following acute lung injury and this study provides circumstantial evidence in support of such a role.
TGFβ1 is known to promote extracellular matrix (ECM) production and accumulation along with mesenchymal cell (i.e. fibroblast) proliferation and differentiation, all of which appear to contribute to the development of fibrosis during the repair phase of tissue injury (Biernacka et al., 2011). Under healthy conditions, there is very little TGFβ1 protein expressed in the lung and most of it is in a latent form that is bound to the ECM. During acute inflammation, the latent TGFβ1 is released and activated by both proteolytic and oxidative mechanisms (Biernacka et al., 2011, Beier et al., 2011). Excessive alcohol ingestion increases the expression of TGFβ1 in various tissues including the liver and the lung, and this TGFβ1 expression is associated with the development of liver fibrosis (cirrhosis) (Beier et al., 2011). In parallel, TGFβ1 is increased in the lung lavage fluid and the lung tissue of rats and humans following chronic alcohol ingestion (Bechara et al., 2004, Brown et al., 2007, Brown and Brown, 2012). Therefore, we hypothesized that the alcohol-mediated susceptibility to fibrosis following lung injury would be associated with, if not caused by, increased TGFβ1 expression in the lung as well as its activation and release into the airspace. The new experimental findings in this study support that hypothesis.
A previous study in a similar mouse model of bleomycin-induced lung injury showed that TGFβ1 mRNA expression was induced and peaked within 5–7 days after treatment, and that TGFβ1protein expression persisted up to 10–14 days after initial treatment but declined back to baseline thereafter. However, these changes varied among strains of mice (Phan and Kunkel, 1992, Zhang et al., 1995). In the current study we used a relatively lower dose of bleomycin (a single dose of 2.5 units/kg) than has been typically used in this model. We intentionally induced a milder lung injury in control-fed animals so that we could identify any exacerbating effects of chronic alcohol ingestion. At 14 days following initiation of acute lung injury, we found modest elevation of activated TGFβ1 expression in the lung lavage fluid of control-fed mice compared to uninjured control-fed groups. In this study, alcohol feeding alone was associated with trend toward increased levels of activated TGFβ1 in the lung lavage fluid as compared to uninjured control-fed mice; however, these changes were not statistically significant. This is consistent with our previous studies in which we determined that TGFβ1 levels were increased in the lung lavage fluid of alcohol-fed rats compared to control-fed rats during acute endotoxemia (Bechara et al., 2004). However, there was no detectable TGFβ1 in the lavage fluid of either alcohol-fed or control-fed rats in the absence of endotoxemia. In fact, the primary finding in that study was that chronic alcohol ingestion increased the expression of the latent (inactive) form of TGFβ1 in the lung tissue, and we speculated that this has few if any detectable effects in the otherwise healthy alcoholic lung but sets the stage for TGFβ1 activation and epithelial barrier disruption during acute inflammatory stress. Consistent with those earlier findings, in this study we identified an increase in active TGFβ1 in the alveolar space (lung lavage fluid) of alcohol-fed mice 14 days following initiation of acute lung injury and when aberrant fibroproliferation is evident. Altogether, these experimental findings complement our previous studies and provide novel circumstantial evidence that the induction and activation of TGFβ1 may be causally implicated in alcohol-mediated priming of a ‘pro-fibrotic’ response to acute inflammatory lung injury.
Although the mechanisms by which alcohol induces TGFβ1 expression and thereby augments fibrosis following acute lung injury are still being investigated, oxidative stress and glutathione depletion appear to play a central role (Guidot et al., 1999, Moss et al., 2000). Therefore, we predicted that dietary supplementation with a glutathione precursor would mitigate the fibrosis in alcohol-fed mice in response to bleomycin-induced acute lung injury. SAMe is an intermediate step in the endogenous synthesis of glutathione but also has its own effects as a thiol anti-oxidant as well as being a key methyl donor. We have previously shown that dietary supplementation with glutathione precursors such as SAMe, N-acetylcysteine (NAC), or procysteine prevents alcohol-induced TGFβ1 expression and the associated alveolar epithelial and macrophage dysfunction that increases the susceptibility to acute lung injury in experimental models (Holguin et al., 1998, Velasquez et al., 2002, Bechara et al., 2004, Gauthier et al., 2009).
Consistent with these salutary effects in those previous studies, we determined that SAMe treatment decreased the activation of TGFβ1 and the associated (if not consequent) aberrant fibrosis in the alcohol-fed mice following bleomycin-induced acute lung injury. We further demonstrated that SAMe directly suppressed the lung fibroblasts activation as shown by decreased TGFβ1 mRNA expression and attenuated fibroblast to myofibroblasts transdifferentiation as shown by decreased α-SMA-1 mRNA expression in the dose dependent manner. Clearly the findings in an experimental model must be interpreted with caution, as clinical studies of NAC treatment did not show any benefit in patients with idiopathic pulmonary fibrosis (Homma et al., 2012, Meyer et al., 1994, Behr et al., 2009) despite its benefit as a preventative measure in an animal model (Sugiura et al., 2009). However, the potential efficacy of NAC for IPF is still being evaluated in clinical trials. Further, there may be an important window of opportunity between the onset of lung injury and the development of disrepair and fibroproliferative abnormalities that likely precedes the formation of ‘fixed’ lung fibrosis. In addition, fibroproliferation and disrepair following acute lung injury prolongs hospitalization and delays functional recovery in many acute illnesses that have no relationship to IPF. In that regard, strategies such as SAMe (or NAC) might be effective in more acute settings such as ARDS when fibroproliferation, but not yet end-stage and ‘fixed’ fibrosis, is present. Importantly, individuals with underlying alcohol use disorders may well benefit the most from this therapeutic strategy. Specifically, SAMe and/or other glutathione precursors may be able to delimit the formation of lung fibrosis but may have little or no effect on resolving established fibrosis. Therefore, the salutary effects of SAMe in this experimental model raise the possibility that such strategies could promote tissue repair and limit fibroproliferative changes following acute lung injury, particularly in individuals with alcohol abuse who are at such high risk for ARDS and its associated morbidity and mortality.
In summary, we determined that chronic alcohol ingestion renders the experimental mouse lung susceptible to the development of pulmonary fibrosis following bleomycin-induced acute lung injury. Alcohol-mediated susceptibility to lung fibrosis was associated with increased expression and activation of the pro-fibrotic cytokine TGFβ1 and increased collagen deposition as seen by increased in hydroxyproline content in the lungs. Consistent with our previous studies showing that alcohol-induced expression of TGFβ1 is mediated by oxidative stress and glutathione depletion, the aberrant fibrotic response was completely inhibited by dietary supplementation with SAMe, a thiol-containing anti-oxidant and glutathione precursor. To our knowledge, this is the first report that demonstrates not only a delayed pathological effect of chronic alcohol ingestion on lung repair following acute inflammatory injury, but also that alcohol-mediated susceptibility to disrepair and fibrosis can be modified with a relatively simple dietary intervention. Taken together, these findings suggest that such a strategy could have salutary effects in individuals at high risk for acute lung injury and delayed resolution of tissue injury because of underlying chronic alcohol abuse.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Michael Koval and Dr. Pratibha Joshi for their helpful reviews of this manuscript.
SUPPORT
Cystic Fibrosis Foundation Program for Adult Care Excellence for VS, Emory Center for Respiratory Health for VS, NIH Career Development Award (NIAAA 1K08AA021404–01) for VS, Emory Alcohol and Lung Biology Center (NIAAA P50 AA013757) for VS and DMG, and a VA Merit Review for DMG.
Footnotes
DISCLOSURES
No conflicts interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
VS and DMG designed the research project; VS, VEK, RS and STM performed experiments and analyzed data; VS, XF and DMG interpreted the results of the experiments, VS and DMG drafted, edited and revised the manuscript.
REFERENCES
- Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM. Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. American journal of respiratory and critical care medicine. 2004;170:188–194. doi: 10.1164/rccm.200304-478OC. [DOI] [PubMed] [Google Scholar]
- Behr J, Demedts M, Buhl R, Costabel U, Dekhuijzen RP, Jansen HM, Macnee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Petruzzelli S, De Vuyst P, Van Den Bosch JM, Rodriguez-Becerra E, Lankhorst I, Sardina M, Boissard G. Lung function in idiopathic pulmonary fibrosis--extended analyses of the IFIGENIA trial. Respiratory research. 2009;10:101. doi: 10.1186/1465-9921-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JI, Arteel GE, Mcclain CJ. Advances in alcoholic liver disease. Current gastroenterology reports. 2011;13:56–64. doi: 10.1007/s11894-010-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Ritzenthaler JD, Guidot DM, Roman J. Alveolar type II cells from ethanol-fed rats produce a fibronectin-enriched extracellular matrix that promotes monocyte activation. Alcohol. 2007;41:317–324. doi: 10.1016/j.alcohol.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Brown LA. Ethanol (EtOH)-Induced TGF-beta(1) and Reactive Oxygen Species Production Are Necessary for EtOH-Induced Alveolar Macrophage Dysfunction and Induction of Alternative Activation. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- Coward WR, Saini G, JenkinS G. The pathogenesis of idiopathic pulmonary fibrosis. Therapeutic advances in respiratory disease. 2010;4:367–388. doi: 10.1177/1753465810379801. [DOI] [PubMed] [Google Scholar]
- Dobson R. Heavy drinking costs the NHS 1.7bn pounds sterling a year, says report. BMJ. 2003;327:701. doi: 10.1136/bmj.327.7417.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley S, Ten Dijke P. TGF-beta in progression of liver disease. Cell and tissue research. 2012;347:245–256. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TW, YOUNG PA, Gabelaia L, Tang SM, Ping XD, Harris FL, Brown LA. In utero ethanol exposure impairs defenses against experimental group B streptococcus in the term Guinea pig lung. Alcoholism, clinical and experimental research. 2009;33:300–306. doi: 10.1111/j.1530-0277.2008.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidot D, Moss M, Holguin F, Lois M, Brown L. Ethanol ingestion impairs alveolar epithelial glutathione homeostasis and function, and predisposes to endotoxin-mediated acute lung injury. Chest. 1999;116:82S. doi: 10.1378/chest.116.suppl_1.82s. [DOI] [PubMed] [Google Scholar]
- Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest. 1998;101:761–768. doi: 10.1172/JCI1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma S, Azuma A, Taniguchi H, Ogura T, Mochiduki Y, Sugiyama Y, Nakata K, Yoshimura K, Takeuchi M, Kudoh S. Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology. 2012;17:467–477. doi: 10.1111/j.1440-1843.2012.02132.x. [DOI] [PubMed] [Google Scholar]
- Liu RM, Vayalil PK, Ballinger C, Dickinson DA, Huang WT, Wang S, Kavanagh TJ, Matthews QL, Postlethwait EM. Transforming growth factor beta suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Radic Biol Med. 2012;53:554–563. doi: 10.1016/j.freeradbiomed.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Mcclair VL. Epidemiology of substance use disorders. Hum Genet. 2012;131:779–789. doi: 10.1007/s00439-012-1168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Buhl R, Magnussen H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 1994;7:431–436. doi: 10.1183/09031936.94.07030431. [DOI] [PubMed] [Google Scholar]
- Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. 1996. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA : the journal of the American Medical Association. 275:50–54. [PubMed] [Google Scholar]
- Moss M, Burnham EL. Alcohol abuse in the critically ill patient. Lancet. 2006;368:2231–42. doi: 10.1016/S0140-6736(06)69490-7. [DOI] [PubMed] [Google Scholar]
- Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. American journal of respiratory and critical care medicine. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Experimental lung research. 1992;18:29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- Rocco PR, Dos Santos C, Pelosi P. Lung parenchyma remodeling in acute respiratory distress syndrome. Minerva anestesiologica. 2009;75:730–740. [PubMed] [Google Scholar]
- Roman J, Ritzenthaler JD, Bechara R, Brown LA, Guidot D. Ethanol stimulates the expression of fibronectin in lung fibroblasts via kinase-dependent signals that activate CREB. Am J Physiol Lung Cell Mol Physiol. 2005;288:L975–L987. doi: 10.1152/ajplung.00003.2004. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura H, Ichikawa T, Liu X, Kobayashi T, Wang XQ, Kawasaki S, Togo S, Kamio K, Mao L, Ann Y, Ichinose M, Rennard SI. N-acetyl-L-cysteine inhibits TGF-beta1-induced profibrotic responses in fibroblasts. Pulmonary pharmacology & therapeutics. 2009;22:487–91. doi: 10.1016/j.pupt.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Velasquez A, Bechara RI, Lewis JF, Malloy J, Mccaig L, Brown La, Guidot DM. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcoholism, clinical and experimental research. 2002;26:1245–1251. doi: 10.1097/01.ALC.0000024269.05402.97. [DOI] [PubMed] [Google Scholar]
- Zhang K, Flanders KC, Phan SH. Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. The American journal of pathology. 1995;147:352–361. [PMC free article] [PubMed] [Google Scholar]