Abstract
This study investigated the regulatory function of CD8+ cells in T helper (Th) 17 cell-mediated corneal epithelial barrier disruption that develops in a murine desiccating stress (DS) model that resembles Sjögren syndrome. CD8+ cell depletion promoted generation of IL-17A producing CD4+ T cells via activation of dendritic cells in both the ocular surface and draining cervical lymph nodes in C57BL/6 mice subjected to DS. T cell-deficient nude recipient mice receiving adoptively transferred CD4+ T cells from CD8+ cell-depleted donors exposed to DS displayed increased CD4+ T cell infiltration and elevated IL-17A and CCL20 levels in the ocular surface, which was associated with greater corneal barrier disruption. Enhanced DS-specific corneal barrier disruption in CD8-depleted donor mice correlated with a Th17-mediated expression of matrixmetalloproteinases (MMP-3 and MMP-9) in the recipient corneal epithelium. Co-transfer of CD8+ CD103+ Tregs did not affect the ability of DS-specific pathogenic CD4+ T cells to infiltrate and cause ocular surface disease in the nude recipients, showing that CD8+ cells regulate the afferent arm of DS-induced immune response. In summary, CD8+ regulatory cells suppress generation of a pathogenic Th17 response that plays a pivotal role in DS-induced disruption of corneal barrier function.
Keywords: CD8, IL-17A, IL-13, IFN-γ, dry eye and corneal barrier
Introduction
Inflammation mediated by CD4+ T cells has a prominent role in many immunologic disorders. T helper (Th) 1, Th2, and Th17 populations may each be involved in inflammatory processes, reflecting distinct modes of T cell recruitment and divergent mechanisms of inflammatory tissue damage 1,2. Native immune/inflammatory processes are constrained by active cellular quiescence and immunologic tolerance, which offers potential therapeutic approach for enduring control of inflammatory disease. Several regulatory T cells (Tregs) subtypes have been described within each of the two main subcategories, CD4+ Treg and CD8+ Treg 3,4. Several subsets of inhibitory CD8+ Treg have been identified, some of which may have immunotherapeutic values. Evidence has accumulated that specialized CD8+ Treg have the potential to suppress host-injurious responses that develop in autoimmune disorders such as rheumatoid arthritis, systemic lupus erythematosus and multiple sclerosis 4–7.
The Th17 lineage has been found be distinct from traditional Th1 and Th2 lineages. IL-17A-producing Th17 cells have been identified as a key effector in a variety of human and experimental autoimmune diseases, including Sjögren syndrome, multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus and psoriasis 8,9.
Keratoconjunctivitis sicca (KCS) in Sjögren syndrome (SS) is a severe and potentially sight-threatening ocular surface epithelial disease. The pathogenesis of KCS in our mouse model of SS is a multifactorial process that includes activation of stress pathways in the ocular surface epithelia by the hyperosmolar tear film and cytokines produced by resident intraepithelial lymphocytes and infiltrating Th1 and Th17 cells 10–13. In this model, we previously demonstrated that desiccating stress (DS) -activated CD4+ T cells that when adoptively transferred to naïve T cell-deficient nude mice, were sufficient to elicit autoimmune lacrimal KCS with features resembling human SS, suggesting that CD4+ T cells make a prominent contribution to mucosal and glandular inflammation and tissue damage in SS 14. We have previously demonstrated a suppressive function of CD4+ CD25+ Foxp3+ Treg in CD4+ T cell-mediated KCS using this model 14; however, the contribution of CD8+ Treg in this DS model has not been investigated. CD8+ cells have been found to reside in the epithelium and stroma of normal human and mouse conjunctiva, and a significant decrease in CD8+ cells with concomitant increase in CD4/CD8 ratio in the conjunctiva has been observed in human KCS and in our experimental KCS model 15–18.
Herein, we show for the first time that a subset of CD8+ Tregs can significantly mitigate Th17-mediated disease in our SS model. CD8+ cell-depletion augmented pathogenic Th17 cell generation, and consequently worsened IL-17A-induced disruption of corneal barrier function.
Results
The effect of DS on CD8+ population
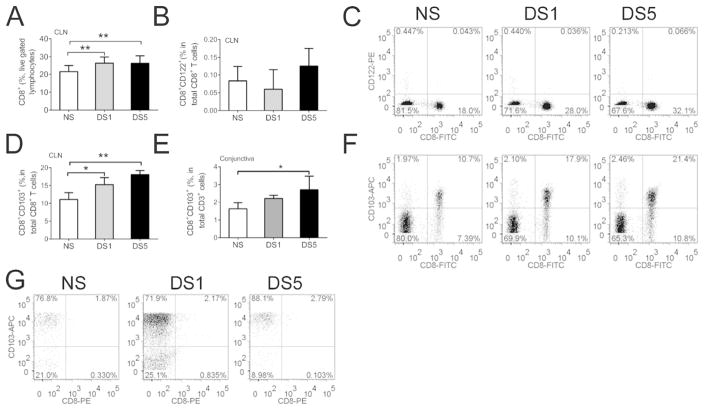
We have previously observed a significant decrease in CD8+ cells with a concomitant increase in CD4/CD8 ratio in the conjunctiva in our DS model of SS 15. A significant increase in the number of CD8+ lymphocytes was noted in the draining cervical lymph nodes (CLN) after DS by flow cytometry (Fig. 1A).
Figure 1. The effects of desiccating stress on CD8+ cell population.
A. Mean ±SD of flow cytometry analysis of CD8+ lymphocytes in draining cervical lymph nodes (CLN) in non-stressed controls (NS) and after desiccating stress for 1 (DS1) or 5 (DS5) days. Experiments were performed two times with at least four mice per group per experiment; ** indicates p<0.01 comparison.
B. Mean ±SD of flow cytometry analysis of CD8+CD122+ lymphocytes in draining cervical lymph nodes (CLN) in NS, DS1 and DS5 groups. Experiments performed two times with at least four mice per group per experiment.
C. Representative dot plots of an experiment showing cells isolated from CLN dual stained for CD8 and CD122. Lymphocytes were gated based on characteristic light-scatter properties, single lymphocytes were gated based on forward scatter height vs. forward scatter area (FSC-A) and live/dead exclusion by propidium iodide. Numbers in the quadrants indicate the percentage of cells.
D. Mean ±SD of flow cytometry analysis of CD8α+CD103+ lymphocytes in draining cervical lymph nodes (CLN) in NS, DS1 and DS5 groups. Experiments were performed two times with at least four mice per group per experiment. *indicates p<0.05 comparison; ** indicates p<0.01 comparison.
E. Mean ±SD of flow cytometry analysis of CD8α+CD103+ T cells in the ocular surface of NS, DS1 and DS5 groups. Experiments were performedtwo times with two/three mice per group per experiment.
F. Representative dot plots of an experiment showing cells isolated from CLN dual stained for CD8 and CD103 in NS, DS1 and DS5 groups. Lymphocytes were gated based on characteristic light-scatter properties, single lymphocytes were gated based on forward scatter height vs. forward scatter area (FSC-A) and live/dead exclusion by propidium iodide. Numbers in the quadrants indicate the percentage of cells.
G. Representative dot plots of an experiment showing cells isolated from the conjunctiva and stained for CD3, CD8 and CD103. Lymphocytes were gated based on characteristic light-scatter properties, single lymphocytes were gated based on forward scatter height vs. forward scatter area and live/dead cell discrimination was obtained by propidium iodide staining. CD8 and CD103 positive cells were gated from CD3 positive cells. Numbers in the quadrants indicate the percentage of cells. Experiments were repeated two times with two/three mice per group per experiment.
CD8+CD122+ and CD8+CD103+ T cell are recently identified Treg populations that can suppress CD4+ T cell proliferation 19–23. Rare CD8+CD122+ Tregs were noted in the ocular surface and CLN, and no obvious change was found in the number of CD8+CD122+ cells in CLN after DS by flow cytometry analysis (Fig. 1B and C). By contrast, 61.3% ± 3.8% of CD8+ lymphocytes in CLN were noted to co-express CD103, and the number of these CD8+CD103+ cells significantly increased after DS (Fig. 1D–G).
The number of CD8+CD103+ cells also increased on the ocular surface after DS (Fig. 1E–G). We have previously surveyed the conjunctiva regarding the presence of intraepithelial lymphocytes 24 and we have found that there is no increase in the percentage of total CD3+ cells during desiccating stress. 24 This evidence suggests that CD8+ CD103+ cells may be one of the cell populations regulating the autoimmune inflammation that develops in this model.
CD8+ cell depletion promoted activation of ocular surface DCs during DS
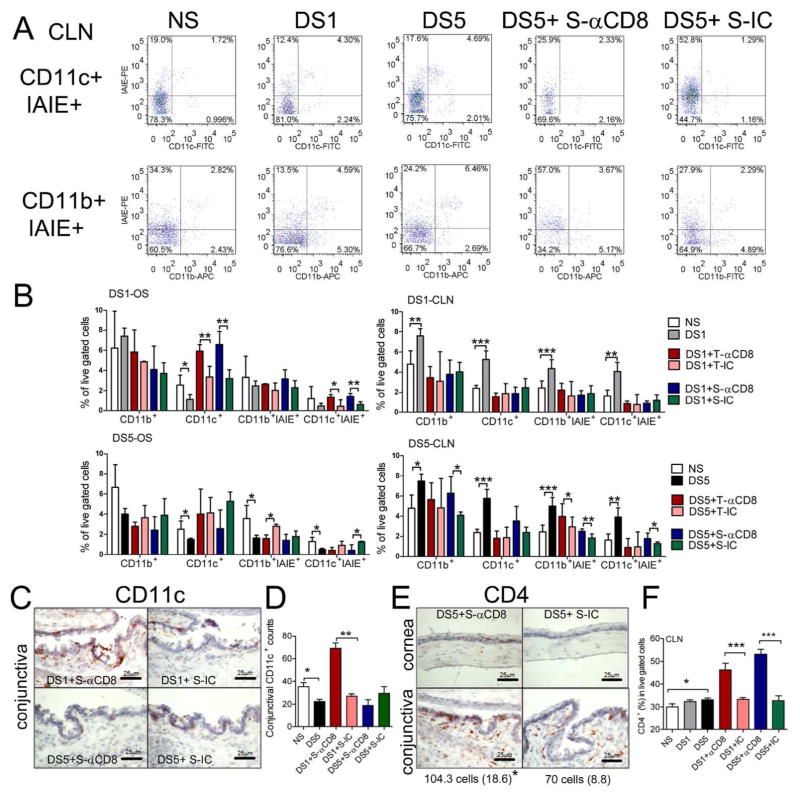
Antigen-captured dendritic cells (DCs) at the ocular surface migrate to the CLN to present antigens to T cells. To evaluate the effect of CD8+ cell depletion on DC activation, we performed flow cytometry analysis of freshly isolated cells from the ocular surface and CLN in mice with and without CD8+ cell depletion during DS. We found a significant or progressive decrease in the number of CD11c+ and CD11b+ cells in the ocular surface, accompanied by a progressive increase in the number of these cells in the CLN after exposure to DS. Dual label of these cells with antibody to the murine HLA-class 2 antigen, IAIE, showed that there was a parallel change in DC activation (Fig. 2A), indicating that DS promoted activation and migration of DCs from the ocular surface.
Figure 2. The effects of CD8+ cell depletion on DC activation.
A. Representative flow cytometry dot plot analysis of dual CD11c+IAIE+ and dual CD11b+IAIE+ cells in the draining cervical lymph node (CLN) in non-stressed controls (NS) and after desiccating stress (DS) for 1 (DS1) or 5 (DS5) days and mice that received systemic (S) injection of anti-CD8 (αCD8) antibody or isotype control (IC) antibody after DS5 treatment. CD11c and CD11b cells were gated based on characteristic light-scatter properties, single cells were gated based on forward scatter height vs. forward scatter area and live/dead cell discrimination was obtained by propidium iodide staining. Numbers in the quadrants indicate the percentage of cells. Experiments were performed two times with at least four mice per group per experiment.
B. Mean ±SD of flow cytometry analysis of single cell suspensions isolated from the ocular surface (OS) or CLN stained for CD11c+, CD11b+ or dual stained with the MHC II marker, IAIE in NS, DS1, DS5 and mice that received either systemic (S) or topical (T) injections of anti-CD8 (αCD8) antibody or isotype control (IC) antibody after DS1 or DS5 treatment. Experiments were performed two times with at least four mice per group per experiment. * p<0.05; ** p<0.01, *** p<0.001.
C. Representative images of immunohistochemical CD11c+ staining in the conjunctiva of C57BL/6 mice that received systemic (S) injection of anti-CD8 (αCD8) antibody or isotype control (IC) antibody after DS1 or DS5 treatment.
D. Bar graph showing mean ±SD the number of CD11c+ cells in the conjunctiva of C57BL/6 mice that received systemic (S) injection of anti-CD8 (αCD8) antibody or isotype control (IC) antibody after DS1or DS5 treatment. * p<0.05; ** p<0.01. Experiments were performed two times with at least two/three samples mice per group per experiment.
E. Representative images of immunohistochemical CD4+ staining in the cornea and conjunctiva of C57BL/6 mice that received systemic injection of αCD8 antibody or IC antibody after DS1or DS5 treatment.
F. Mean±SD of flow cytometry analysis of CD4+ cells in draining cervical lymph nodes in NS, DS1, DS5 and DS1/5 mice that received either systemic injection of anti-CD8 (αCD8) antibody or isotype control (IC) antibody. Lymphocytes were gated based on characteristic light-scatter properties, single cells were gated based on forward scatter height vs. forward scatter area and live/dead cell discrimination was obtained by propidium iodide staining. Experiments were performed two times with at least four mice per group per experiment. * p<0.05; *** p<0.001.
Both local and systemic CD8+ cell depletion increased the number and activation of DCs in the ocular surface, while they had no effect in the number and activation of DCs in the draining CLN after DS1 (Fig. 2A). Immunohistochemical staining of CD11c+ cells in the conjunctiva confirmed the flow cytometry results, showing a significantly increased number of CD11c+ cells in the CD8+ cell depleted group compared to the isotype control group after 1 day of DS (DS1; Fig. 2B and C). These findings indicate that CD8+ cell depletion promoted the accumulation and activation of DCs in the ocular surface after DS1. After DS5, the number of activated DCs decreased in the ocular surface and increased in the draining CLN (Fig. 2A), indicating that CD8+ cell depletion promoted the migration of DCs from the ocular surface to the regional node at this stage.
CD8+ cell depletion promoted Th17 cell generation during DS
In our murine model of SS, we previously demonstrated that DS generated Th1 and Th17 cells pathogenic CD4+T cells that are capable of adoptively transferring dry eye disease to T cell-deficient nude mice 11,12,14,25. Immunohistochemical staining of CD4+ cells in the ocular surface showed that the number of CD4+ cells in the conjunctiva significantly increased in the systemic CD8 depleted group compared to the isotype control group after DS5 (Fig. 2E). No CD4+ T cell infiltration was found in the cornea of NS, DS5 and the isotype antibody treated controls; however, CD4+ T cell infiltration was noted in the corneas of DS5 mice systemically treated with the anti-CD8 antibody (Fig. 2E). We found that systemic CD8+ cell depletion significantly increased the number of CD4+ T cells in the draining CLN after DS5, as determined by flow cytometry (Fig. 2F). Local depletion of CD8+ cells yielded a similar increase in the number of CD4+ T cells in the ocular surface and draining CLN as systemic CD8+ cell depletion (data not shown). Using absolute cell counts (total splenocytes) normalized by the number of spleens, we observed that there was no difference in the total number of starting cells among all groups (NS: 8.95±2.04; DS5: 7.47±1.50; isotype control: 7.53±1.66 and systemic anti-CD8: 8.27±1.52 x107 total cells) but the recovery of CD4+ cells was higher in the CD8 depleted group (NS: 6.08±2.17; DS5: 5.60±0.83; isotype control: 6.07±2.04 and systemic anti-CD8: 10.22±3.8x 106 isolated CD4+ T cells, P=0.03 between IC treated and systemic CD8 treated). Taking together, these findings suggest that CD8 neutralization enhanced the generation of autoreactive CD4+ T cells following DS.
The absence of obvious differences in DC activation and CD4+ T cell generation between the local (subconjunctival injection) and systemic CD8+ cell antibody depletion groups prompted evaluation of the efficiency of CD8+ cell depletion. Flow cytometric analysis of freshly isolated CLN cells from CD8 or isotype antibody treated mice showed that both local and systemic injection of anti-CD8 effectively depleted CD8α+ (isotype control: 23.13% ± 6.23%; local anti-CD8: 0.70% ± 0.26%; systemic anti-CD8: 0.82% ± 0.29%) and CD8β+ cells (isotype control: 26.97% ± 4.06%; local anti-CD8: 1.1% ± 0.14%; systemic anti-CD8: 0.92% ± 0.33%). No significant difference was found in the efficiency of CD8+ cell depletion between the local and systemic antibody treated groups (P>0.05), suggesting that in addition to local effect, subconjunctival injected anti-CD8 antibody drained to CLN and produced an effect similar to systemically injected antibody.
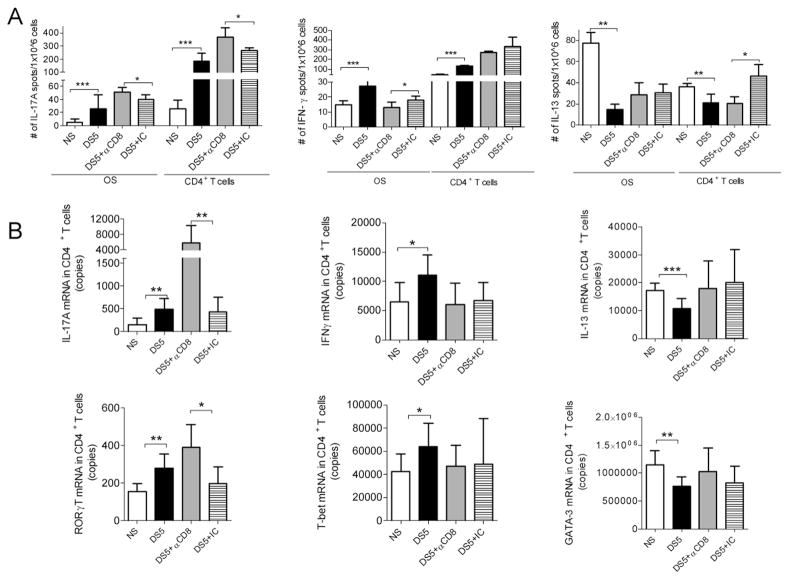
We found increased production of IL-17A in both ocular surface cells and CD4+ T cells, whereas decreased production of IFN-γ was noted in the ocular surface cells and decreased expression of IL-13 was noted in the CD4+ T cell population by ELISPOT (Fig. 3A). Furthermore, real-time PCR performed on isolated CD4+ T cells confirmed expression of the Th signature cytokines and their respective transcription factors (Th17: IL-17A and RORγT; Th1: IFN-γ and T-bet; Th2 cytokine, IL-13 and GATA-3) that was observed by ELISPOT in mice after DS5 with and without systemic CD8+ cell depletion (Fig. 3B). These findings suggest that CD8+ cell depletion exerted the most prominent effect on the generation of pathogenic Th17 cells during DS.
Figure 3. The effects of CD8+ cell depletion on CD4+ T population.
A. Results of ELISPOTs to detect IL-17A, IFN-γ and IL-13 producing cells isolated from the ocular surface (OS) and CD4+ T cells isolated from spleen and cervical lymph nodes (CLN) of C57BL/6 before (NS) or after DS5 in mice that received systemic injection of αCD8 antibody or isotype control (IC) antibody after DS5 treatment. Experiments were performed two times with five mice per group per experiment.
B. Gene expression analysis showing copies of IL-17A, IFN-γ, IL-13 mRNA, RORγT, T-bet and GATA-3 transcripts in the CD4+ T cells isolated from spleen and CLN of C57BL/6 before (NS) or after DS5 in mice mice that received systemic injection of αCD8 antibody or IC.
* p<0.05; ** p<0.01; *** p<0.001. Experiments were performed three times with three to four mice per group per experiment.
CD8+ cell depletion worsened pathogenic Th17 mediated corneal barrier disruption
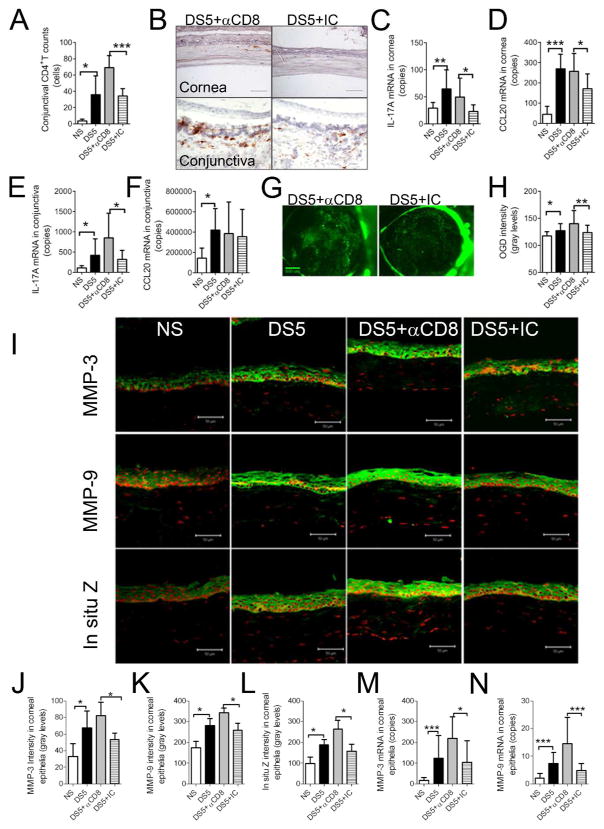
To investigate the effects of CD8+ cell depletion on generation of pathogenic Th-17 cells, we compared corneal and conjunctiva disease severity following CD4+ T cell adoptive transfer into T cell-deficient nude recipient mice. We found that adoptive transfer of CD4+ T cells from CD8+ cell-depleted DS5 donor mice increased CD4+ T cell infiltration in the recipient conjunctiva compared to the isotype control group (Fig. 4A and B). Reduced CD4+ T cell infiltration was noted in corneas of the recipients receiving CD4+ T cells from isotype control antibody treated DS5 donor mice (2/5 mice); however, widespread CD4+ T infiltration was noted in corneas of all recipients of CD4+ T cells from DS5 donor mice with CD8+ cell depletion (5/5 mice) (Fig. 4A).
Figure 4. The effects of pathogenic Th-17 cells stimulated by CD8+ cell depletion on corneal disease.
A. Bar graph showing the number of CD4+ T cells in the conjunctiva of nude mouse adoptive transfer recipients of CD4+ T cells from non-stressed controls (NS), desiccating stress for 5 (DS5) donor days or DS5 donor mice subjected to systemic injection of anti-CD8 (αCD8) antibody or isotype control (IC) antibody. Experiments were performed two times with at least two to three mice per group per experiment.
B. Representative images of CD4+ immunohistochemical staining in the cornea and conjunctiva (CJ) of T cell deficient (nude) recipient mice following adoptive transfer of CD4+ T cells from DS5 donor mice who received anti-CD8 or isotype antibody after DS5 treatment.
C–D. Gene expression analysis showing copies of IL-17A (C) and CCL20 mRNA (D) transcripts in recipient corneal epithelia. Experiments were performed three times with three to four mice per group per experiment.
E–F. Gene expression analysis showing copies of IL-17A (E) and CCL20 mRNA (F) transcripts in recipient conjunctiva. Experiments were performed three times with three to four mice per group per experiment.
G. Representative images of Oregon-Green-Dextran (OGD) corneal staining in the adoptive transfer recipients of CD4+ T cells obtained from DS5 donor mice which received anti-CD8 or isotype antibody. Experiments were performed three times with five mice per group per experiment (total of 30 eyes/group).
H. Mean±SD of OGD staining intensity in corneas of nude mouse adoptive transfer recipients. Experiments were performed three times with at least five mice per group per experiment.
I. Laser scanning immunofluorescent confocal microscopy of cornea stained for matrix metalloproteinases (MMP) -3 and MMP-9 and in situ zymography (in situ Z) in the adoptive transfer recipients of CD4+ T cells isolated from donor mice who received systemic injection of anti-CD8 (αCD8) or isotype control (IC) antibody during 5 days of desiccating stress (DS5). Experiments were performed two times with two to three mice per group per experiment.
J–L. Bar graphs showing mean±SD of fluorescence intensity measured in the corneal epithelium of adoptive transfer recipients for MMP-3 (J), MMP-9 (K) and in situ zymography (L). Experiments were performed two times with two to three mice per group per experiment.
M–N. Gene expression analysis showing copies of MMP-3 (M) and MMP-9 (N) mRNA transcripts in cornea epithelia of adoptive transfer recipients. Experiments were performed two times with two to three mice per group per experiment.
NS, nude mice that received CD4+ T cells from non-stressed donor mice; DS5, nude mice that received CD4+ T cells from 5 days of desiccating stress (DS) donor mice; DS5+αCD8 and DS5+ IC, nude mice that received CD4+ T cells from DS5 donor mice that received systemic injection anti-CD8 (αCD8) antibody or isotype control (IC) antibody * p<0.05; ** p<0.01; *** p<0.001
To further confirm the role of CD8+ cell depletion on generation of Th17 cells, we evaluated IL-17A and CCL20 gene expression in the recipient corneal epithelia and conjunctiva as Th17 committed cells produce IL-17A and CC-chemokine attractant ligand 20 (CCL20), among other mediators 26,27. Our results show that CD8+ cell depletion significantly increased levels of IL-17A (cornea and conjunctiva) and CCL20 mRNA transcripts (cornea) (Fig. 4C–F).
We have previously shown that IL-17A has a pathogenic role in the corneal disease that develops in an experimental murine model of SS 13. To confirm the effect of CD8+ cell depletion on generation of Th17 cells capable of causing corneal epithelia disease, we evaluated corneal barrier function in the CD4+ adoptive transfer recipients. As seen in Figures 4G and H, mice that received CD4+ T cells from CD8 deficient mice showed greater corneal barrier disruption function than recipients of isotype control treated mice.
To determine if CD8+ cell depletion augments the IL-17A-mediated MMP production, we evaluated expression of MMP-3 and MMP-9 in the corneal epithelia of adoptive transfer recipients. We found significantly increased immunoreactivity to MMP-3 and MMP-9 in the recipients of CD4+ T cells from CD8+ cell depleted donors (Fig. 4I, J, K). Both in situ zymography in corneal cryosections and real time PCR further confirmed the results of immunostaining as there was increased gelatinase activity and increased expression of MMP-3 and MMP-9 mRNA transcripts in the corneal epithelia of the CD8+ cell depleted recipient group compared to recipients of the isotype control treated group (Figures 4I–N).
Co-transfer of CD8+ CD103+ Treg did not suppressed the Th17 pathogenic response in the ocular surface
The integrin CD103 (αE/β7) is widely expressed on intraepithelial CD8+ T cells 28. In the conjunctiva, we also found CD8+ cells co-express CD103 (Fig. 1). To investigate the role of CD8+CD103+ Treg in regulating migration of pathogenic Th17 cells generated in mice exposed to DS, CD8+ CD103+ Tregs were co-adoptively transferred with CD4+ T cells from mice subjected to DS10 to nude recipients. Efficiency of isolation was confirmed by flow cytometry (Figure 5A–E). We found that intraperitoneally injected CD8+ CD103+ Tregs homed to the conjunctiva (data not shown) but did not regulate the number of CD4+ T cells infiltrating the ocular surface (Figure 5F) nor conjunctival goblet cell density (data not shown). These findings suggest that CD8+ CD103+ Treg can suppress the generation of pathogenic Th17 cells of this murine SS model before priming by suppressing dendritic cell activation but not by blocking the efferent arm of the immune response.
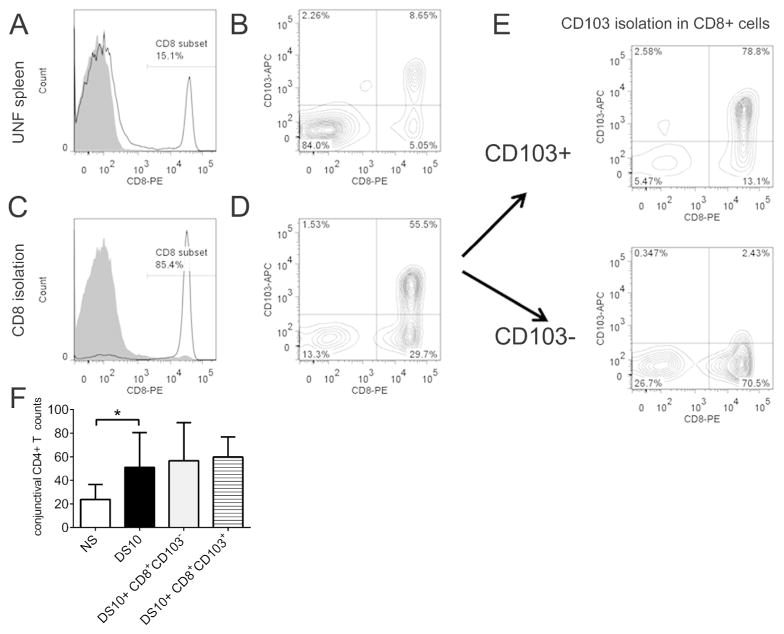
Figure 5. CD8+CD103+ isolation using magnetic beads and co-adoptive transfer results.
A. Unfractionated (UNF) splenocytes were stained for CD8 (white histogram) and showed ~15% CD8+ T cells (isotype control antibody, shaded histogram).
B. About 50% of CD8α+ T cells are also CD103+.
C. Histogram showing successful separation of CD8+ (white histogram) and CD8 negative cells (shaded histogram).
D. 55.5% of isolated CD8+ cells co-express CD103 marker.
E. CD8+ T cells were further isolated based on the expression of CD103: CD103+ expression increased to 78.8 % in the positive population, while the negative population had just 2.43% of double positive cells.
F. Bar graph showing the number of CD4+ T cells in the conjunctiva of nude mouse adoptive transfer recipients of CD4+ T cells. Nude mouse recipients were divided into four groups in this experiment: 1) NS that received CD4+ T cells from NS B6 mice; 2) DS10 which received CD4+ T cells from DS10 B6 mice; 3) DS10+ CD8+ CD103+ Treg mice, which received CD4+ T cells from DS10 B6 mice and CD8+ CD103+ (2 × 106) and 4) DS10+ CD8+ CD103− mice which received CD4+ T cells from DS10 B6 mice and CD8+ CD103− (2 × 106). Experiments were performed twice with at least two to three mice per group per experiment.
Discussion
This study demonstrated the suppressive effects of CD8+ Treg on Th17 cell-mediated corneal epithelial barrier disruption in our DS model resembling SS. A compensatory increase in CD8+ cells consisting primarily of CD8+CD103+ Tregs was noted in the draining nodes over the 5 day exposure to DS. CD8+ cell depletion promoted the generation of IL-17A producing CD4+ T cells via activation of DCs in mice subjected to DS. T cell-deficient nude recipient mice receiving adoptive transfer of CD4+ T cells from CD8+ cell-depleted donors exposed to DS displayed increased CD4+ T cell infiltration and enhanced DS-specific Th17-mediated corneal barrier disruption. Co-transfer of CD8+ CD103+ regulatory T cells had no effect on the ability of DS-specific pathogenic CD4+ T cells to infiltrate and cause ocular surface disease in the nude recipients.
A progressive increase in CD8+ lymphocytes was noted in the draining cervical lymph nodes (CLN) in mice subjected to DS. Our findings suggest that these CD8+ cells function to regulate the initiation of the autoimmune keratoconjunctivitis that develops in this model. In fact, a compensatory Treg response has been noted during the progression of disease in other autoimmune models. For instance, CD4+ Tregs accumulate to a higher frequency within the CNS of mice with experimental autoimmune encephalomyelitis 29. Therefore, based on this finding, we hypothesized that the increase in CD8 cells in the CLN is an immunoregulatory response to the DS-induced ocular surface autoimmunity.
In this study, we found that depletion of CD8+ regulatory cells stimulated DC activation and homing during immunopathogenesis of this DS-induced SS model. Systemic CD8+ cell depletion promoted resident ocular surface DC recruitment and activation at an early stage (day 1) of DS treatment, while it enhanced migration of ocular surface DCs at a late stage (day 5) of DS treatment. It is well known that activated DCs play a fundamental role in coordinating the innate and adaptive immune response by capturing antigen within the peripheral tissues and migrating to the regional lymph nodes via afferent lymphatic vessels where they present antigens to naïve T cells in the context of the necessary costimulatory molecules. This evidence suggests that CD8+ cell depletion promoted generation of CD4+ T cells via activation of DCs. To investigate the definitive role of intraepithelial CD8+ cells in activation of DCs, we also performed subconjunctival injection of anti-CD8 in this study; however, to our surprise, subconjunctivally injected anti-CD8 antibody almost completely depleted the CD8+ cells in the draining CLN, similar to systemic CD8 antibody depleted group. This suggests that subconjunctivally injected anti-CD8 antibody gained access to the draining CLN where it effectively depleted CD8+ Tregs and promoted generation of CD4+ effector T cells.
In this study, we found the CD8+ cell population contains regulatory cells that play a pivotal role in suppressing generation of pathogenic Th17 cells in our DS model. This is a major finding because mechanisms that control CD4+ T cell-mediated tissue damage are a significant factor in averting and resolving chronic inflammatory diseases. Several lines of evidence support the claim that CD8+ Tregs dampen the SS-like disease in our model: 1) CD8+ cell depletion during DS promoted generation of pathogenic CD4+ T cells with increased IL-17A expression in both the ocular surface and draining CLN of donor mice; 2) adoptive transfer of CD4+ T cells from CD8+ cell depleted DS-treated donors increased ocular surface infiltration with CD4+ T cells that manifested increased IL-17A and CCL20 production; 3) abundant CD4+ T cells infiltrated the corneal epithelia and central stroma in both the donor and recipient mice in the CD8+ cell depleted group in our current study. These pathogenic CD4+ T cells appear to have a Th17 phenotype. It has been demonstrated that Th17 differed from Th1 by exhibiting a stronger affinity for the cornea of inflamed eyes, and after adoptive transfer, Th17 polarized CD4 cells were found to abundantly invade the corneal stroma and epithelia in the recipient eyes, while Th1 polarized CD4 cells not 8; and 4) Enhanced pathogenicity of CD4+ T cells derived from CD8+ cell-depleted mice was associated with increased levels of IL-17A in both donor and recipient mice. IL-17A has been shown to stimulate production of IL-1, tumor necrosis factor alpha (TNF-α), IL-6, IL-8 and MMPs by epithelial cells and fibroblasts 30–32. We have previously shown that IL-17A contributes to the development of epithelial corneal in our DS-model 13 via increased MMP-9 production and activation. MMP-9 has been found to have a central role in DS-induced corneal barrier disruption, as MMP-9 deficient mice were resistant to barrier disruption in DS 33,34. We have also reported that IL-17 neutralization during DS decreased the expression of MMP-9 and MMP-3 and improved corneal barrier function 13. In our current study, we found that increased IL-17A production in donor CD4+ T cells resulted in greater corneal barrier disruption and increased MMP-3 and MMP-9 expression in the recipient corneal epithelia, further supporting the link between CD8+ Treg suppression of pathogenic Th17 cells in this SS model.
Multiple CD8+ Treg subtypes are now recognized as essential regulators of the immune system that prevent autoimmunity through secretion of multiple cytokines and subsequent inhibition of effector lymphocyte function 4–7. According to their origin, CD8+ Treg subsets have been grouped into natural and inducible Tregs. Natural Tregs are generated and mature within the thymus and are characterized by the expression of either human leukocyte antigen (HLA)-G or CD122 and CD28 35. The mechanism of suppression is cell contact independent and mediated by soluble factors, such as soluble HLA-G or IL-10 35. In contrast to naturally occurring Tregs, inducible Tregs are generated from naïve cells in the periphery after encounter with antigens, presented by DCs that have an activation status distinct from those DCs that promote Th cell differentiation 36. The mechanism of suppression of T effector cells by inducible Tregs is by cell–cell contact and/or release of soluble factors 36. CD8+CD122+ T cells are a recently identified natural Treg that appear to have a function in immune homeostasis, because activated T cells expand without restraint and cause lethal disorders in mice in their absence 19. In our current study, rare CD8+CD122+ T cells were noted in CLN and ocular surface, and no obvious change was found in the number of CD8+CD122+ Tregs in CLN after DS, suggesting that CD8+CD122+ Treg may not contribute to the regulation of autoimmune inflammation in this model.
Thus far, the effects of CD8+CD103+ Treg on suppressing autoimmune Th17 biased disease have not been established. In our study, CD8+ CD103+ Tregs were noted in the mouse ocular surface, and intraperitoneally injected CD8+ CD103+ Treg homed to the conjunctiva. More than 60% of CD8+ lymphocytes in the CLN were found to co-express CD103, and the number of these CD8+CD103+ Tregs significantly increased after DS. Co-adoptive transfer of CD8+ CD103+ Tregs failed to prevent accumulation of adoptively transfer CD4+ T cells infiltrating the ocular surface suggesting that CD8+ T cells regulates generation of pathogenic CD4+ T cells in the afferent arm but not migration of cells to the ocular surface. CD103 is the integrin αE that forms the heterodimer αEβ7 molecule with β7 integrin and it has been identified as a marker for intraepithelial lymphocytes in a number of mucosal sites 28. Recently, CD8+CD103+ T cells have also been reported to exert regulatory activity on CD4+ T cell proliferation in a cell–cell contact dependent fashion 21–23,35. That alloreactive CD8+CD103+ Tregs are inducible and generated at the effector site of an immune response was suggested by data indicating that CD8+ T cells responding to donor alloantigens presented by DCs within draining lymph nodes rapidly upregulated CD103 expression, and subsequently migrated into the transplanted graft 37. The findings of this study suggest that the activated DCs in ocular surface in response to DS migrate to CLN and consequently promote generation of CD8+CD103+ Treg. To develop novel immunomodulatory therapies for SS, it may be valuable to further investigate the mechanisms by which CD8+ CD103+ Treg suppress generation of Th17 effector cells in response to DS in this murine SS model.
In summary, the CD8+ cell population contains regulatory cell subsets that suppress generation of the pathogenic Th17 response that plays a pivotal role in DS-induced disruption of mucosal barrier function. This study provides new insight into the pathogenesis of Th17 biased autoimmune diseases.
Materials and methods
Mice
This research protocol was approved by the Baylor College of Medicine Center for Comparative Medicine, and it conformed to the standards in the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Female C57BL/6 (C57BL/6NTac, B6) and B6.Cg/NTac-Foxn1nuNE9 (nude) were purchased from Taconic, Inc. (Germantown, NY). Mice were used at 6 to 10 weeks of age.
Murine desiccating stress model
DS was created in B6 mice by subcutaneous injection of scopolamine hydrobromide (0.5 mg/0.2 ml; Sigma-Aldrich, St. Louis, MO), QID (08:00, 12:00, 14:00, and 17:00 h) and exposure to an air draft and <35% ambient humidity for 1, 5 or 10 consecutive days (DS1, DS5 and DS10, respectively) 12–14. A group of female mice that did not receive any treatment to induce dry eye served as a non-stressed (NS) controls.
Antibody-depletion of CD8+ cells in B6 mice
Eight groups of B6 mice were evaluated: DS1/DS5 mice that received systemic (i.p.) injections of anti-CD8α antibody (500 μg/dose, 1mg/mL, clone YTS 169.4; DS1+S-αCD8 and DS5+S-αCD8, respectively) or rat-IgG isotype control (500 μg/dose, 1mg/mL, Sigma-Aldrich; DS1+S-IC and DS5+S-IC, respectively); and DS1/ DS5 mice that received local (subconjunctival) injections of anti-CD8 antibody (50 μg/dose, 2.5mg/mL, clone YTS 169.4; DS1+T-αCD8 and DS5+L-αCD8, respectively) or rat-IgG isotype control (50 μg/dose, 2.5mg/mL, Sigma-Aldrich; DSL+T-IC and DS5+L-IC, respectively). Mice received a total of three injections at days −4,−2 and 0 when subjected to DS for 1 day and a total of four injections at days −4,−2, 0 and +2 when subjected to DS for 5 days.
Isolation of murine CD4+ T cells and adoptive transfer
Superior CLN and spleens from donor mice were collected and meshed gently between two frosted end glass slides, as previously described 14. Untouched CD4+ cells were isolated using magnetic beads according to the manufacturer’s instructions (MACS system; Miltenyi Biotec, Bergisch Gladbach, Germany). The cells were analyzed by flow cytometry and were found to be greater than 90% pure (data not shown). One donor-equivalent of cells were transferred intraperitoneally (i.p.) to T cell deficient nude mice (approximately 5 × 106 CD4+ T cells). The adoptive transfer recipients were sacrificed after 72 hours. In some experiments, eyes were collected for histology, were evaluated for corneal permeability while in others cornea and conjunctiva were processed for RNA analysis. Nude mouse recipients were divided into four groups: 1) NS, that received CD4+ T cells from NS B6 mice; 2) DS5, that received CD4+T cells from DS5 B6 mice; 3) DS5+ αCD8, that received CD4+T cells from DS5 +S- αCD8 B6 mice and 4) DS5+ IC, that received CD4+T cells from DS5+S-IC B6 mice.
Isolation and reconstitution of murine CD8+ CD103+ Treg
To investigate the role of CD8+CD103+ Treg in generation of pathogenic CD4+ T cells during DS, CD8+ CD103+ Treg were simultaneously co-adoptively transferred with CD4+ T cells isolated from B6 mice subjected to DS10.
CD8+ T cells were isolated from splenocytes and superficial CLN using anti-CD8 antibody –conjugated magnetic microbeads and columns according to the manufacturer’s protocol (MACS system). CD8+ cells were then incubated with anti-CD103-APC antibody (clone 2E7, Biolegend, San Diego) followed by an incubation with APC conjugated beads (MACS system). Flow cytometry was used to confirm CD8 isolation and effective separation of cells into CD8+CD103+ and CD8+CD103- populations (Figure 5).
Nude mouse recipients were divided into four groups in this experiment: 1) NS that received CD4+ T cells from NS B6 mice; 2) DS10 which received CD4+ T cells from DS10 B6 mice; 3) DS10+ CD8+ CD103+ Treg mice, which received CD4+ T cells from DS10 B6 mice and CD8+ CD103+ (2 × 106); 4) DS10+ CD8+ CD103− mice which received CD4+ T cells from DS10 B6 mice and CD8+ CD103− (2 × 106). CD4+ T cells were isolated as above described, and 2×106 cells suspended in 100Tl of sterile PBS were injected (i.p.) into nude recipient mice. Eyes were collected for histology 72 hours after the adoptive transfer (n=5 samples).
Histology and Immunohistochemistry
Eyes and anexae from each group (n=5) were excised, embedded in optimal cutting temperature (OCT) compound (VWR, Suwanee, GA), and flash frozen in liquid nitrogen. Sagittal 8 μm sections were cut with a cryostat (HM 500; Micron, Waldorf, Germany) and placed on glass slides that were stored at −80°C.
Immunohistochemistry was performed to detect and count the cells in the conjunctiva that stained positively for CD4 (clone H129.9, 10 ug/mL, BD Pharmingen) and CD11c (clone HL3CN, 10 u/mL, BD Pharmingen). Cryosections were stained with the above mentioned primary antibodies and appropriate biotinylated secondary antibodies (BD Pharmingen; Jackson Immune Laboratories, West Grove, PA) and Vectastain Elite ABC using NovaRed reagents (Vector, Burlingame, CA). Secondary antibody alone and appropriate anti-mouse isotype (BD Pharmingen) controls were also performed. Three sections from each animal were examined and photographed with a microscope equipped with a digital camera (Eclipse E400 with a DS-Fi1; Nikon, NY). Positively stained cells were counted in the conjunctiva using image-analysis software (NIS Elements Software, version 3.0; Nikon) software.
Isolation of murine ocular surface cells
The eyes and lids of mice were excised, pooled and incubated in 10mL of 5mg/mL of Dispase II (Roche Molecular Biochemicals, Indianapolis) as previously described 24. Collected cells were used either for flow cytometry or ELISPOT.
Flow Cytometry analysis of murine cells
Single-cell suspensions of CLN were stained with anti-CD16/32 (BD Pharmingen, Sand Diego, CA) at 4°C for 10 minutes, followed by staining with anti-CD8α-FITC (clone 53–6.7; BD Pharmingen) or anti-CD4-FITC (GK1.5; BD Pharmingen), anti-CD8α-PE (clone 53–6.7; BD Pharmingen), anti-CD122 –PE (clone TM-β1; BD Pharmingen), and anti-CD103-APC (clone 2E7, Biolegend, San Diego), anti-CD11c-FITC (clone HL3, BD Pharmingen), anti-CD11b-APC (clone M1/70, BD Pharmingen) or anti-IAIE-PE (clone 2G9, BD Pharmingen). Single-cell suspensions isolated from cornea and conjunctiva were stained with anti-CD16/32, followed by staining with anti-CD3-pacific blue (Clone 500A2, BD Pharmingen), anti-CD8α–PE (clone 53–6.7) and anti-CD103-APC (clone 2E7, Biolegend).
Negative controls consisted of splenocytes stained with isotype antibodies (BD Pharmingen). Cells were resuspended in propidium iodide and kept on ice until flow cytometry analysis was performed. A BD LSRII Benchtop cytometer was used and data analyzed using FlowJo software (TreeStar Inc.).
Mouse IL-17A, IL13 and IFN-γ ELISPOT
Replicate 50uL cell suspensions containing 1.0×106 freshly ocular surface cells and CD4+ T cells isolated as described above were added to 96-well PVDF plates (Millipore, Billerica, MA), pre-coated with anti-mouse IL-17A, IL-13 or IFN-γ capture antibody (R&D Systems; Minneapolis, MN), as previously described 24. The positive, red spots were counted under a dissecting microscope (SMZ 1500, Melville, NY). Replicate wells were averaged from two individual experiments. Results are presented as number of spots/1 × 106 cells.
Corneal Permeability
Corneal epithelial permeability to Oregon-green-dextran (OGD; 70,000 molecular weight [MW]; Invitrogen, Eugene, OR) was assessed in the nude recipient mice (n=10 eyes/5 mice/group/experiment in three independent experiments) as previously described 13. Briefly, 0.5 μL of 50 mg/mL OGD was instilled onto the ocular surface 1 minute before euthanasia. Corneas were rinsed with PBS and photographed with a high dynamic and resolution digital camera (Coolsnap HQ2, Photometrics, Tucson, AZ) attached to a stereoscopic zoom microscope (SMZ 1500; Nikon, Melville, NY) under fluorescence excitation at 470 nm. The severity of corneal OGD staining was graded in digital images by two masked observers, using NIS Elements (version 3.0, Nikon) within a 2-mm diameter circle placed on the central cornea. The mean fluorescent intensity measured by the software inside this central zone was transferred to a database and the results averaged within each group.
RNA isolation and quantitative PCR
Cornea epithelium from B6.nude recipient mice was scrapped with a scalpel, conjunctiva was surgically excised, and CD4+T cells were isolated as described above. Six to twelve samples were used in each group. Total RNA was extracted using a Pico Pure RNA isolation® Kit (Arcturus, Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions, quantified by a NanoDrop® ND-1000 Spectrophotometer (Thermo scientific, Wilmington, DE) and stored at −80°C. Samples were treated with DNase to eliminate genomic DNA contamination, according to the manufacturer’s instructions (Qiagen, Valencia, CA). First-strand cDNA was synthesized with random hexamers by M-MuLV reverse transcription (Ready-To-Go You-Prime First-Strand Beads; GE Healthcare, Inc., Arlington Heights, NJ), as previously described 10.
Quantitative real-time PCR was performed with specific MGB probes (Taqman; Applied Biosystems, Inc., Foster City, CA) and PCR master mix (Taqman Gene Expression Master Mix), in a commercial thermocycling system (StepOnePlus™ Real-Time PCR System, Applied Biosystems), according to the manufacturer’s recommendations. Quantitative real time PCR was performed using gene expression assay primers and MGB probes specific for murine targets IL-17A (Mm00439618), IFN-γ (Mm00801778), IL13 (Mm99999190), MMP-3 (Mm00440295), MMP-9 (Mm00442991), CCL20 (Mm00444228), GATA-3 (Mm00484683), RORγT (Mm00441139) and T-bet (Mm00450960).
The copy number of the genes of interest was calculated by comparing the samples to the gene-specific standard curves, previously prepared using commercial mouse cDNA (Zyagen, San Diego, CA). Samples and standards were assayed in duplicate. A non-template control and total RNA without retrotranscription were included in all the experiments to evaluate PCR and DNA contamination of the reagents.
MMPs Immunofluorescent Staining and in situ zymography
Immunofluorescent staining was performed with polyclonal goat anti-matrix metalloproteinases (MMP) −3 (2ug/mL; SC6834; Santa Cruz Biotechnology; Santa Cruz, CA) or rabbit anti-MMP-9 (5ug/mL, Chemicon-Billerica, MA, USA) and with appropriate Alexa-Fluor 488 conjugated IgG secondary antibodies as previously described 38. In situ zymography was performed to localize the gelatinase activity in the corneal epithelia, as previously described 38.
The images were captured with a fluorescence microscope (Eclipse E400; Nikon) and a attached digital camera (Eclipse E400 with a DS–F1; Nikon). Fluorescent intensity was measured were analyzed using the NIS Elements Software (Nikon).
Statistical analysis
One way analysis of variance (ANOVA) with Tukey’s post hoc testing was used for statistical comparisons using GraphPad Prism 5.0 software (GraphPad Software Incorporation, San Diego, CA). P ≤ 0.05 was considered statistically significant.
Acknowledgments
Supported by NIH Grant EY018888 (CSDP), EY11915 (SCP), EY018090 (SCP, MES, JYN), NIH Core Grants-EY002520 & EY020799, Research to Prevent Blindness, the Oshman Foundation, William Stamps Farish Fund, the Hamill Foundation and by the Cytometry and Cell Sorting Core at Baylor College of Medicine which is funded by the NIH NIAID P30AI036211, NCI P30CA125123, and NCRR S10RR024574.
Footnotes
Disclosure: C.S. Schaumburg, K. F. Siemasko and M.E. Stern are employees of Allergan, Inc.
References
- 1.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 2.Jabs DA, Lee B, Whittum-Hudson JA, Prendergast RA. Th1 versus Th2 immune responses in autoimmune lacrimal gland disease in MRL/Mp mice. Invest Ophthalmol Vis Sci. 2000;41:826–831. [PubMed] [Google Scholar]
- 3.O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 4.Tang XL, Smith TR, Kumar V. Specific control of immunity by regulatory CD8 T cells. Cell Mol Immunol. 2005;2:11–19. [PubMed] [Google Scholar]
- 5.Dinesh RK, Skaggs BJ, La CA, Hahn BH, Singh RP. CD8+ Tregs in lupus, autoimmunity, and beyond. Autoimmun Rev. 2010;9:560–568. doi: 10.1016/j.autrev.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki M, Konya C, Goronzy JJ, Weyand CM. Inhibitory CD8+ T cells in autoimmune disease. Hum Immunol. 2008;69:781–789. doi: 10.1016/j.humimm.2008.08.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konya C, Goronzy JJ, Weyand CM. Treating autoimmune disease by targeting CD8(+) T suppressor cells. Expert Opin Biol Ther. 2009;9:951–965. doi: 10.1517/14712590903020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox CA, Shi G, Yin H, et al. Both Th1 and Th17 are immunopathogenic but differ in other key biological activities. J Immunol. 2008;180:7414–7422. doi: 10.4049/jimmunol.180.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 11.de Paiva CS, Hwang CS, Pitcher JD, III, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology (Oxford) 2010;49:246–258. doi: 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Paiva CS, Villarreal AL, Corrales RM, et al. Dry Eye-Induced Conjunctival Epithelial Squamous Metaplasia Is Modulated by Interferon-{gamma} Invest Ophthalmol Vis Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 13.de Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunology. 2009 May;2(3):243–53. doi: 10.1038/mi.2009.5. Epub 2009 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating Stress Induces T Cell-Mediated Sjogren’s Syndrome-Like Lacrimal Keratoconjunctivitis. J Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 15.de Paiva CS, Villarreal AL, Corrales RM, et al. IFN- γ Promotes Goblet Cell Loss in Response to Desiccating Ocular Stress. Invest Ophthalmol Vis Sci 2006. 2006;47:E-Abstract 5579. [Google Scholar]
- 16.Sacks EH, Wieczorek R, Jakobiec FA, Knowles DM. Lymphocytic subpopulations in the normal human conjunctiva. A monoclonal antibody study. Ophthalmology. 1986;93:1276–1283. doi: 10.1016/s0161-6420(86)33580-2. [DOI] [PubMed] [Google Scholar]
- 17.Rojas B, Cuhna R, Zafirakis P, et al. Cell populations and adhesion molecules expression in conjunctiva before and after bone marrow transplantation. Exp Eye Res. 2005;81:313–325. doi: 10.1016/j.exer.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Raphael M, Bellefqih S, Piette JC, Le HP, Debre P, Chomette G. Conjunctival biopsy in Sjogren’s syndrome: correlations between histological and immunohistochemical features. Histopathology. 1988;13:191–202. doi: 10.1111/j.1365-2559.1988.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 19.Rifa’i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endharti AT, Okuno Y, Shi Z, et al. CD8+CD122+ regulatory T cells (Tregs) and CD4+ Tregs cooperatively prevent and cure CD4+ cell-induced colitis. J Immunol. 2011;186:41–52. doi: 10.4049/jimmunol.1000800. [DOI] [PubMed] [Google Scholar]
- 21.Lu L, Yu Y, Li G, et al. CD8(+)CD103(+) regulatory T cells in spontaneous tolerance of liver allografts. Int Immunopharmacol. 2009;9:546–548. doi: 10.1016/j.intimp.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. 2008;180:2573–2580. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RA, ten Berge IJ. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006;177:2775–2783. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Volpe EA, Gandhi NB, et al. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One. 2012;7:e36822. doi: 10.1371/journal.pone.0036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Paiva CS, Volpe EA, Gandhi NB, et al. Disruption of TGF-beta Signaling Improves Ocular Surface Epithelial Disease in Experimental Autoimmune Keratoconjunctivitis Sicca. PLoS One. 2011;6:e29017. doi: 10.1371/journal.pone.0029017. Epub 2011 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Homey B, eu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 27.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schon MP, Arya A, Murphy EA, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 29.Subramanian S, Shahaf G, Ozeri E, et al. Sustained expression of circulating human alpha-1 antitrypsin reduces inflammation, increases CD4+FoxP3+ Treg cell population and prevents signs of experimental autoimmune encephalomyelitis in mice. Metab Brain Dis. 2011;26:107–113. doi: 10.1007/s11011-011-9239-9. [DOI] [PubMed] [Google Scholar]
- 30.Cortez DM, Feldman MD, Mummidi S, et al. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38. Am J Physiol Heart Circ Physiol. 2007;293:H3356–H3365. doi: 10.1152/ajpheart.00928.2007. [DOI] [PubMed] [Google Scholar]
- 31.Yagi Y, Andoh A, Inatomi O, Tsujikawa T, Fujiyama Y. Inflammatory responses induced by interleukin-17 family members in human colonic subepithelial myofibroblasts. J Gastroenterol. 2007;42:746–753. doi: 10.1007/s00535-007-2091-3. [DOI] [PubMed] [Google Scholar]
- 32.Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:748–753. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- 33.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Koch SD, Uss E, van Lier RA, ten Berge IJ. Alloantigen-induced regulatory CD8+CD103+ T cells. Hum Immunol. 2008;69:737–744. doi: 10.1016/j.humimm.2008.08.281. [DOI] [PubMed] [Google Scholar]
- 36.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Yuan R, Feng Y, et al. Regulation of CD103 expression by CD8+ T cells responding to renal allografts. J Immunol. 2004;172:214–221. doi: 10.4049/jimmunol.172.1.214. [DOI] [PubMed] [Google Scholar]
- 38.Corrales RM, Stern ME, de Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:3293–3302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]