Abstract
Sézary syndrome (SS) cells express cell surface molecules also found on normal activated CD4 T cells. In an effort to find a more specific surface marker for malignant SS cells, a microarray analysis of gene expression was performed. Results showed significantly increased levels of mRNA for CD164, a sialomucin found on human CD34+ hematopoietic stem cells, and FCRL3, a molecule present on a subset of human natural T regulatory cells. Both markers were increased in CD4 T cells from SS patients compared to healthy donors. Flow cytometry studies confirmed the increased expression of CD164 and FCRL3 primarily on CD4+CD26− T cells of SS patients. Importantly, a statistically significant correlation was found between an elevated percentage of CD4+CD164+ T cells and an elevated percentage of CD4+CD26− T cells in all tested SS patients but not in patients with Mycosis Fungoides and atopic dermatitis or healthy donors. FCRL3 expression was significantly increased only in high tumor burden patients. CD4+CD164+ cells displayed cerebriform morphology and their loss correlated with clinical improvement in treated patients. Our results suggest that CD164 can serve as a marker for diagnosis and for monitoring progression of CTCL/SS and that FCRL3 expression correlates with a high circulating tumor burden.
Introduction
Sézary syndrome (SS), the leukemic variant of cutaneous T-cell lymphoma (CTCL), is a malignancy of skin-trafficking CD4 T cells. The diagnosis is based predominantly on tissue biopsy showing atypical, epidermotropic CD4 T cells in the epidermis, and by microscopic examination of peripheral blood buffy coats for presence of lymphocytes with atypical ceribriform appearing nuclei, known as Sézary cells. In addition to examination of tissue biopsies and blood, flow cytometry is now a widely accepted diagnostic tool. However, since SS cells express molecules also present on normal activated CD4 T cells, diagnosis based on the phenotype of circulating malignant cells can be difficult. (Kim et al., 2005 )
The malignant SS cells have been phenotyped as central memory cells expressing CD4+CD26-CD45RO+. The ability of the malignant cells to localize to the skin is facilitated by skin addressins CLA and CCR4, while the presence of CCR7 facilitates entry into lymph nodes. (Campbell et al., 2010; Ferenczi et al., 2002; Sokolowska-Wojdylo et al., 2005a; Sokolowska-Wojdylo et al., 2005b) Advancing disease in SS patients correlates with the gradual decline in the TCR repertoire, eventually resulting in the presence of malignant CD4 T cells expressing a single TCR Vβ. (Yawalkar et al., 2003) Furthermore, molecules such as NKp46 and CD158k/KIR3DL2, receptors originally identified on NK cells, ganglioside GD3 (CD60) and syndecan 4 (SD-4), present on activated normal T cells, were also found to be expressed at elevated levels in mainly high tumor burden SS patients. (Bensussan et al., 2011; Campbell et al., 2010; Chung et al., 2011; Poszepczynska-Guigne et al., 2004; Scala et al., 2010). Interestingly, T-plastin, an intracellular protein, has been found exclusively in the malignant circulating CD4 T cells in SS patients, but its intracellular expression and lack of specific antibodies applicable for flow cytometry diminish its usefulness as a diagnostic marker. (Kari et al., 2003; Su et al., 2003)
The identification of a clonal malignant TCR Vβ population of CD4 T cells in patients facilitates diagnosis and monitoring of the Sézary cells. However, SS patients without an identifiable circulating clone can pose a diagnostic and therapeutic monitoring challenge particularly since loss of CD26 is indicative of the malignant cells, but it does not distinguish them from normal populations of CD4+CD26− cells present in the circulation. (Bernengo et al., 2001; Jones et al., 2001; Sokolowska-Wojdylo et al., 2005b)
In our attempt to find a specific surface marker for malignant cells, CD4 T cells isolated from SS patients and healthy donors were subjected to microarray analysis of global gene expression. These studies revealed that CD164 and FCRL3 were expressed at significantly higher levels in patients’ CD4 T-cells compared to healthy donors. CD164, a sialomucin adhesion receptor demonstrated on a population of CD34+ hematopoietic progenitor cells, has been reported to be expressed on less than 3% of peripheral CD3 T cells in healthy volunteers. (Watt et al., 1998; Zannettino et al., 1998) FC-receptor-like 3 (FCRL3) is a member of the FCRL gene family encoding proteins, FCRL 1-6, that are homologous to the classical Fc receptors. FCRL3 expression is found on 40% of naturally occurring human CD4+CD25+Foxp3+ T regulatory cells (Tregs), and functional studies showed that CD4+CD25+FCRL3+ cells are non-responsive to anti-CD3/CD28, IL-2, PHA or ConA stimulation. (Nagata et al., 2009) Subsequent studies published by Swainson et al. demonstrated that FCRL3+ cells exhibit a CD4+ memory T cell phenotype and that expression of FCRL3 correlates with high levels of programmed cell death-1 receptor (PD-1). (Swainson et al., 2010)
In this study we provide evidence that CD164 may serve as an early detection marker for Sézary syndrome in low-to-high tumor burden patients, and that FCRL3 expression correlates with disease progression.
Results
Increased mRNA expression of CD164 and FCRL3 genes in CD4 T cells from Sézary syndrome patients as assessed by microarray analysis and QRT-PCR
We performed a microarray analysis of global gene expression on CD4 T cells isolated from SS patients to identify surface markers specific for the malignant cells. We compared gene expression in CD4 T cells from high tumor burden (HT, n=2, ≥50% malignant cells in circulation), medium tumor burden (MT, n=2, 50-20% malignant cells), low tumor burden (LT, n=2, ≤20% malignant cells) patients and healthy donors (HD, n=3).
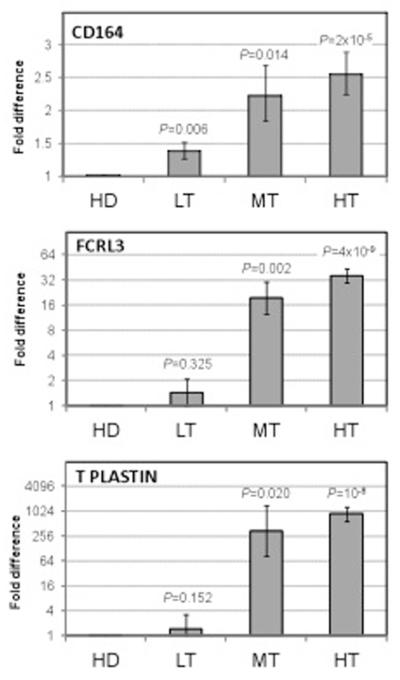
A total of 1,219 genes were identified as significantly differentially expressed (P<0.05, FDR<10%) and differed by at least 80% of the levels between high, medium and low tumor burden patients and healthy donors. We chose two genes coding for cell surface molecules previously not associated with CTCL. CD164 exhibited a significant difference between all 6 SS patient samples and 3 healthy donors (p=0.004, FDR=7%). FCRL3 was the most differentially expressed between high and medium tumor burden versus normal samples (p=0.006, FDR=10%) but was less informative for the low tumor burden patients. (Figure 1)
Figure 1. Increased mRNA expression of CD164 and FCLR3 genes in CD4 T cells from Sézary syndrome patients as assessed by microarray analysis.
The expression of CD164 and FCRL3 in CD4 T cells isolated from PBMC of high (HT), medium (MT), low (LT) tumor burden Sézary patients and healthy donors (HD). Normalized signals with the indicated genes are shown.
To validate the expression of CD164 and FCRL3 on the SS malignant cells, we assessed mRNA levels for these genes in CD4 T cells of 11 high tumor burden patients, 16 medium to low tumor burden patients (6 medium tumor burden and 10 low tumor burden patients) and 9 healthy donors by QRT-PCR. In the same cohort of patients we also assessed expression of T-Plastin. As shown in Figure 2, CD164 was significantly expressed in high, medium and, importantly, in low tumor burden patients’ cells compared to healthy controls. FCRL3, as well as T-plastin, were both expressed by CD4 T cells from patients with high-to medium but not low tumor burdens. These data strongly suggest that malignant cells, particularly in patients with advanced disease, express all three molecules.
Figure 2. CD164, FCRL3 and T-plastin mRNA expression in CD4 T cells from Sézary syndrome patients as assessed by quantitative real-time RT-PCR.
CD4 T cells were isolated from PBMC of 11 high tumor burden patients (≥ 50% malignant cells), 6 medium tumor burden patients (50-20% malignant cells), 10 low tumor burden patients (≤20% malignant cells) and 9 healthy donors. Total RNA was extracted from CD4 T cells followed by QRT-PCR to assess mRNA levels. Fold difference for CD164, FCRL3 and T Plastin is calculated versus expression levels in cells from healthy volunteers. Error bars represent standard error of mean.
Of note, in addition to CD164, FCRL3 and T-plastin, we also assessed mRNA expression of syndecan 4 (SD-4) and NKp46. (Bensussan et al., 2011; Chung et al., 2011) SD-4 mRNA expression was significantly increased in patients compared to healthy donors (p=0.01), whereas NKp46 mRNA was inconsistently expressed and not significantly increased among SS patients (data not shown).
The acquisition of CD164 correlates with the loss of CD26 expression in SS patients; FCRL3 is present mainly on CD4 T cells from high tumor burden patients
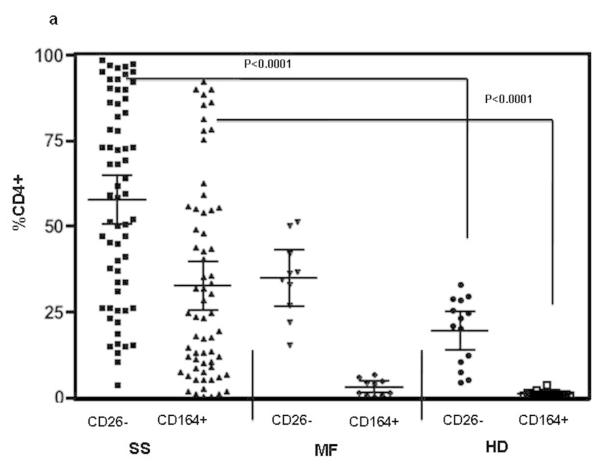
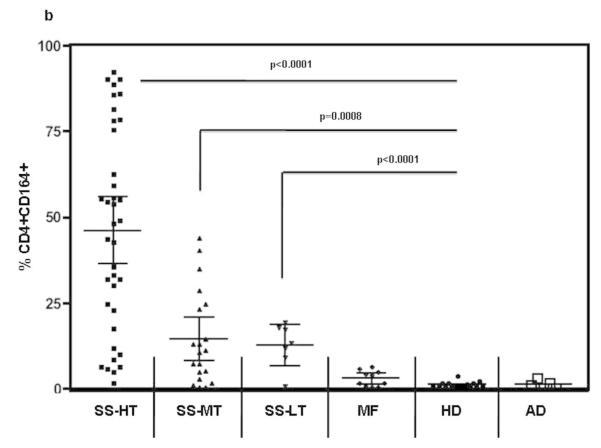
Peripheral blood mononuclear cells (PBMC) from SS patients (high/medium/low tumor burden), MF patients without known blood involvement, healthy donors and atopic dermatitis patients (AD) were analyzed for the presence of CD164 and CD26 on their CD4 T cells by flow cytometry. The percentages of CD4+CD164+ as well as CD4+CD26− T cells were significantly increased in SS patients compared to MF and AD patients or healthy donors (p values for both markers were <0.0001). Importantly, we found a statistically significant correlation between an elevated percentage of CD4+CD164+ T cells and an elevated percentage of CD4+CD26− T cells in all fifty-nine SS patients (Pearson correlations: rho=0.674, p<0.0001) but not in MF patients (n=10, rho= −0.372, p=0.33) or healthy donors (n=14, rho= −0.39, p=0.21). Figure 3a shows percentages of CD4+CD26− and CD4+CD164+ T cells detected in SS patients (mean CD26−: 59.2%, SEM: 3.7, mean CD164+: 34.7%, SEM: 3.7), MF patients (mean CD26−: 36.6%, SEM: 4.6, mean CD164+: 3.0%, SEM: 0.7) and healthy donors (mean CD26−: 19.6%, SEM: 2.6, mean CD164+: 1.2%, SEM: 0.2). Compared to MF and HD, the percentage of CD4+CD26-T cells in SS patients was also significantly increased, p<0.02. Consistent with the data shown in Figure 3a, CD164 protein was detected on the surface of high (n=37) medium (n=14), and importantly, low (n=8) tumor burden patients and was significantly increased in all three groups compared to healthy donors or MF patients without peripheral blood involvement and AD patients (Figure 3b). Interestingly, CD164 expression was not detected on CD4 T cells from six AD patients (mean CD164+: 1.2%, SEM: 0.50). It should be mentioned that only four out of fifty-nine SS patients (6.8%) were low expressers of CD164 with less than 5% of CD4 T cells expressing CD164 (mean: 1.8%, SEM: 0.84).
Figure 3. The acquisition of CD164 correlates with the loss of CD26 on patients’ CD4 T cells; FCRL3 is predominantly expressed in CD4 T cells of high tumor burden patients.
The cell surface expression of CD26, CD164 and FCRL3 was assessed by flow cytometry. (a) SS patients (n=59) demonstrate significantly higher percentages of CD4+CD164+ and CD4+CD26− T cells compared to MF patients (n=10), or HD (n=14). (b) Percentage of CD4+CD164+ T cells is significantly higher in high (HT; n=37), medium (MT; n=14) and low (LT; n=8) tumor burden patients compared to MF, HD or AD patients (n=6). (c) Percentage of CD4+FCRL3+ T cells is significantly higher only in CD4 T cells of HT patients (n=26) but not in MT (n=15) or LT (n=7) compared to HD. Results are expressed as mean with 95% CL (d) CD164 and FCRL3 are predominately expressed on CD4+CD26− and CD4+CD26-Vβ+ T cells. Shown results are from one representative patient (out of seven) with a TCRVβ defined by antibody (upper panel) and from one representative patient (out of fourteen) whose TCRVβ was not defined by antibody (lower panel). Patients’ PBMC were collected prior to initiation of systemic therapy at the University of Pennsylvania.
A statistically significant increase in FCRL3 protein expression was found only in patients with a high tumor burden in the circulation (Figure 3c). On average, 46.6% of CD4 T cells from high tumor burden patients expressed FCRL3 (SEM: 6.35) on their surface compared to 5.1% of CD4+FCRL3+ cells in medium tumor patients (SEM: 1.58) and 2.7% of CD4+ FCRL3+ cells in low tumor burden patients (SEM: 0.87). The mean expression of FCRL3 on CD4 T cells from MF patients and healthy donors was 13% (SEM: 3.5) and 5.6% (SEM: 2.0), respectively (Figure 3c).
The detailed flow cytometric analysis confirmed the results suggested by the data shown in Figure 3 a-c that CD164 and FCRL3 are mainly expressed on CD4+CD26− T cells. (Figure 3d, Table 1 supplemental)
Among high tumor burden patients, whose CD4 T cells were mainly CD26 negative and expressed a single TCRVβ as defined by antibodies, CD164 and FCRL3 were expressed predominately on CD4+CD26-Vβ+ T cells (Figure 3d, patient 1,upper panel). Similarly, in patients without an identifiable TCRVβ, including low tumor burden patients, CD164 was predominantly expressed on CD4+CD26− T cells. (Figure 3d, patient 2, lower panel). FCRL3 expression was rarely evident on CD4 T cells from medium-to-low tumor burden patients.
CD4+CD164+ cells display cerebriform morphology
CD4+CD164+ cells and CD4+CD164− cells were isolated from PBMC of highly leukemic patients and processed to create 1 μm thick section slides used for the assessment of Sézary cells. Representative photographs are shown in Figure 4a-b. CD4+CD164+, but not CD4+CD164− T cells, manifested a high degree of cerebriform morphology. Additionally, we sorted CD4 T cells into CD4+CD164+ and CD4+CD164−, placed them on glass slides and analyzed them for potential differences in cell size between the two groups. As shown in Figure 4c-d, CD4+ CD164+ T cells were much larger in size compared to the CD4+ CD164− T cells. Although the nuclear morphology cannot be fully assessed using this method, the differences in size between CD4+CD164+ and CD4+CD164− T cells further suggest that CD4 CD164+ T cells may represent the malignant population, a finding consistent with Clark et al., who showed that malignant CTCL cells have a high-scatter profile. (Clark et al., 2011)
Figure 4. CD4+CD164+ demonstrate the phenotype of malignant Sézary cells.

Assessment of cellular morphology, (a-b): CD4+CD164+ cells (5×106, 85% purity) and CD4+CD164− cells (2.9×106, purity 69%) from a high tumor burden patient were recovered using an anti-PE column. Cells were processed to obtain a 1 μm thick section, stained with H&E and assessed for the presence of malignant cells. Cellular morphology was examined using an Olympus BX51 microscope. Images were captured with a Leica DFC 420 camera. Data shown are from one representative patient out of five. Size assessment, (c-d): CD4 T cells from a high tumor burden patient were sorted into CD4+CD164+ and CD4+CD164− (purity of both populations 98%), placed on glass slides, air dried, stained with H&E and analyzed using a Zeiss Axiophot microscope. Images were captured using a Leica DFC 450 camera. Shown images are from one representative patient out of six. Bars =100 μm.
Clinical improvement in disease status of SS patients correlates with a decreasing percentage of CD4+CD26-CD164+FCRL3+ circulating T-cells
We next attempted to determine if clinical improvement in disease status can be monitored by assessing changes in the expression of CD164 and FCRL3 on patients’ CD4 T cells. We focused on three originally highly leukemic patients that had achieved a complete clinical remission with resolution of all skin lesions and lymphadenopathy while being treated with multimodality immune therapy. As shown in Figure 5, upon completion of treatment, two patients with an identifiable Vβ clone experienced a marked decrease in the percentage of Vβ+CD4 T cells (patients 1 and 2), and all three patients had decreases in the percentages of CD26−, CD164+ and FCRL3+ CD4 T cells. These results suggest that the expression of CD164 as well as FCRL3 on SS patients’ CD4 T cells can serve as a surrogate marker of disease progression and circulating tumor burden, and that these molecules may be used to monitor therapeutic efficacy in CTCL.
Figure 5. Clinical remission of disease correlates with the disappearance of CD4+CD26− T cells expressing Vβ, CD164 and FCRL3.
PBMC from three high tumor burden patients were analyzed by multicolor flow cytometry to assess the expression of the molecules on CD3+/CD4+ T cells. Samples of patient’s PBMCs were collected prior to the onset of systemic treatment and during clinical remission. Patient 1 received ECP, IFN-α and PUVA whereas patient 2 and 3 received extracorporeal photopheresis (ECP), IFN-α and total skin electron beam therapy.
Discussion
Our data demonstrates previously unreported high expression of CD164 and FCRL3 on CD4 T cells from Sézary syndrome patients. Importantly, our results show a statistically significant correlation between CD164 acquisition and loss of CD26 expression; high CD164 expression correlates with increased percentages of CD26− T cells.
The potential for CD164 to serve as a marker for malignant cells is underscored by: 1) the presence of CD164 on CD4 T cells of SS patients with a wide range of tumor burdens; 2) the absence of CD164 on CD4 T cells from healthy donors and patients with atopic dermatitis; 3) morphological examination of purified CD4+CD164+ T cells demonstrating the morphology of malignant Sézary cells; 4) disappearance of CD4+CD164+ T cells in three patients that experienced clinical remission as a result of treatment.
Recently, CD164 expression was demonstrated on human prostate cancer cells, and in cell lines derived from human breast carcinoma. It has been also identified as a new marker for acute lymphoblastic leukemia. (Coustan-Smith et al., 2011; Havens et al., 2006; Leccia et al., 2012) There is evidence suggesting an important role for CD164 in prostate cancer cell metastasis and in the development of colorectal cancer. (Havens et al., 2006; Matsui et al., 2000)
It is not yet clear what drives the expression of CD164 on SS CD4 T cells. Based on our preliminary results, TCR stimulation with anti-CD3/28 or polyclonal stimulation with PHA does not play a key role in upregulating CD164 expression on patients’ CD4 T cells. In response to either stimulus, the percent of CD4+CD164+ T cells rose from 0.1% (unstimulated group) to 5.5%, whereas CD25 expression increased from 2% (unstimulated) to nearly 40% (data not shown). It is also highly unlikely that systemic treatment such as interferon or extracorporeal photopheresis (ECP) increases CD164 or FCRL3 expression, as we observed longitudinal decreases, rather than increases, in the percentages of CD4+CD164+/ FCRL3+ T cells among SS patients over their treatment course with these agents.
Interestingly, CXCL12 has been shown to stimulate the expression of both CD164 mRNA and protein in human prostate cancer cells but it remains to be established if it has a similar role in CTCL. CXCL12 was abundantly expressed in the skin of SS patients as demonstrated by Narducci et al. Moreover, inhibition of enzymatic activity of CD26 enhanced the CXCL12-induced migration of the CTCL cell line Hut78. (Narducci et al., 2006) Our preliminary data showed increased CXCL12 mRNA expression in the skin of three high tumor and three medium tumor burden patients, whose CD4 T cells were used in these studies, compared to healthy donors (unpublished data). Thus, it is conceivable that increased levels of CXCL12 in SS patients may contribute to the elevated CD164 expression, although it may not be an exclusive factor. The presence of another factor, presently unknown, contributing to the tumor transformation, loss of CD26 and acquisition of CD164 expression on patients’ CD4 T cells, cannot be ruled out given that patients with a low tumor burden in the circulation have percentages of CD4+CD26− cells comparable to healthy donors, yet still express CD164 on their CD4 T cells.
Considering that absence of CD26 and presence of CD164 on the cell surface enhances CXCL12–mediated cell migration, our findings strongly suggest that CD164 defines a population of malignant CD4 T cells with the ability to invade skin and possibly lymph nodes and bone marrow.
The phenotype of CD4+FCRL3+ T cells in SS patients differs from the described phenotype of naturally occurring Tregs in that the freshly isolated cells from SS patients typically lack Foxp3 expression. We found no correlation between FCRL3 and CD25 expression in high tumor burden patients, as CD4 T cells were highly positive for FCRL3 (>82%) but only marginally so for CD25 (<10%). However, the presence of FCRL3 protein on CD4 T cells from this group of patients and the presence of FCRL3 mRNA in medium tumor burden patients may indicate a gradual loss of responsiveness of patients’ CD4 T cells to cytokines and TCR stimulation, as has been previously suggested. (Fargnoli et al., 1997; Wysocka et al., 2004) We previously showed that some SS patients have an increased expression of programmed death-1 receptor (PD-1) on CD4 T cells, a molecule which has been associated with decreased immune responsiveness. (Samimi et al., 2010; Shimauchi et al., 2007) Recently, Chung et al. demonstrated that association of syndecan-4 (SD-4) on SS patients’ CD4 T cells with dendritic cell-associated heparan sulfate proteoglycan-integrin ligand (DC-HIL) leads to attenuation of TCR-induced proliferation of CD4 T cells. (Chung et al., 2011) We have found significantly elevated levels of mRNA for SD-4 in our cohort of SS patients (data not shown), supporting the notion that SD-4 may also contribute to this unresponsive phenotype.
Currently, we do not have a full understanding of the biological significance of FCRL3 expression on SS patients’ CD4 T cells. Similarly, the functional significance of CD164 expression on patients’ cells awaits a better understanding. However, the statistically significant expression of CD164 on CD4 T cells from patients but not from healthy donors, and the significant correlation between acquisition of CD164 and loss of CD26 expression, all indicate that CD164 may be a potentially interesting marker for malignant SS cells. Furthermore, our preliminary data showing a lack of CD164 expression on CD4 T cells from six patients with severe to moderate atopic dermatitis suggests that CD164 may be restricted to SS but certainly further studies are needed to fully assess this marker specificity. Our future studies will focus on understanding the biological significance of CD164 and FCRL3, particularly in regard to their association with malignant transformation in CTCL and their expression in other inflammatory skin diseases.
In summary, the presence of a positive marker such as CD164 that distinguishes between normal, immunocompetent CD4 T cells and malignant CD4 T cells in Sézary syndrome will facilitate earlier diagnosis as well as therapeutic monitoring of disease status. It will also further our understanding of the underlying mechanisms responsible for the transition from activated normal CD4 T cells to malignant CD4 T cells and may eventually lead to a more targeted Sézary syndrome therapy.
Material and Methods
Patients
Sézary syndrome (SS) patients were diagnosed on the basis of clinical, histopathologic and immunohistologic criteria. (Murphy, 1988) To assess the numbers of circulating malignant T-cells, patients’ PBMC were analyzed by flow cytometry for the presence of CD4+/CD26−/CD7− cells and by examination of one-micron sections of formalin-fixed peripheral blood buffy coats for lymphocytes with atypical ceribriform appearing nuclei. (Introcaso et al., 2005) Patients with erythroderma and circulating malignant T-cells were defined to have Sézary syndrome. (Olsen et al., 2007) Donation of peripheral blood samples by patients and healthy volunteers was according to protocols approved by the University of Pennsylvania Institutional Review Board. The studies were conducted in accordance with the Declaration of Helsinki Principles, and all participants provided written informed consent.
Isolation of CD4 T cells and CD4+CD164+cells
CD4 T cells from patients or healthy volunteers were isolated from freshly collected PBMC as previously described, using Dynal CD4 Positive Isolation Kit (Invitrogen Dynal, Oslo, Norway). (Rook et al., 1995) Pure CD4 T cells were used in microarray and QRT-PCR studies. To assess cellular morphology, CD4 T cells were stained with anti-CD164-PE antibody and recovered using anti-PE microbead columns from Miltenyi Biotec or sorted using BD FACS Aria II SORP into CD4+CD164+ and CD4+CD164−. CD4 T cells were isolated from patients currently undergoing treatment in CTCL clinic. Patients were routinely treated with extracorporeal photopheresis (ECP) combined with Bexarotene, IFN-α or IFN-γ.
Preparation of cells for morphological assessment
A. Assessment of cellular morphology: CD4+CD164+T cells or CD4+CD164-T cells previously separated using anti-PE columns, were processed in JB4 to obtain 1 μm thick sections according to the standardized procedure for analysis of the presence of Sézary cells in peripheral blood buffy coats adapted by the Division of Dermatopathology of the Department of Dermatology at the University of Pennsylvania, Philadelphia, PA. B. Size Assessment: CD4+CD164+T cells or CD4+CD164-T cells, previously sorted, were placed on glass slides, air-dried, H&E stained and microscopically analyzed.
Flow cytometry analysis
To analyze the phenotype of CD4 T cells from patients and healthy donors, PBMCs were stained with antibodies anti-CD3, anti-CD4, anti-CD26, anti-CD164, anti-CD25 purchased from BD Biosciences, San Jose, CA and anti-FCRL3. Production and characterization of anti-FCRL3 antibody, H5, was described previously. (Nagata et al., 2009) To assess the viability of analyzed cells the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit from Invitrogen was used.
Cells were analyzed with the LSRII flow cytometer (Becton Dickinson, San Jose, CA) at the Flow Cytometry and Cell Sorting Core, Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA. 100,000 events were collected to analyze CD4 T-cells. The data was further analyzed by FlowJo software (Tree Star)
Statistical Analysis
Pearson correlation coefficients were estimated to assess the correlation between CD164+ and CD26− expression on CD4 T cells. Student’s t-test was used to assess differences between groups in levels of expression. Tests were considered statistically significant using a two-sided p-value of 0.05. The statistical package R (www.r-project.org) was used for analysis.
Microarray Studies: Preparation of CD4 T cell RNA
Gene expression was analyzed in CD4 T cells from six SS patients and three healthy donors. RNA was processed as previously described and hybridized on Illumina Human WG 8v2 microarray chips. (Showe et al., 2009) All arrays were processed in the Wistar Institute Genomics Facility.
Data Preprocessing
Array data were processed by Illumina’s BeadStudio software and expression levels exported for analysis. The gene-wise, median correlation of each array compared to all other arrays was computed to ensure that no outliers existed within the data. The expression levels were then quantile normalized and non-informative probes (those with detection p-value >0.05 in all samples) were removed to reduce experimental noise. Ultimately, 23,050 probes that targeted known genes were used in our analysis.
Gene Expression Analysis
A one-way ANOVA test was conducted on the data to identify genes differentially expressed between any tumor samples vs healthy donors or high/medium tumor burden samples vs healthy donors separately. False discovery rate (FDR) was estimated using Storey et al. procedure.(Storey and Tibshirani, 2003)The data analysis was conducted using functions of MATLAB 7.2 and significance was defined at p≤0.05 and FDR≤10%.
Quantitative real-time RT-PCR
Total RNA was extracted from CD4 cells using TRIzol Reagent (Ambion). cDNA was synthesized from 1ug of RNA using High Capacity RNA to cDNA Kit (Applied Biosystems). QRT-PCR was performed using Taqman gene expression assays: 18s rRNA, CD164, FCRL3, T-Plastin, SD-4 and NKp46 according to manufacturer’s protocols (Applied Biosystems). Comparison of expression levels of a gene between pairs of groups was done using ΔCt method.
Supplementary Material
Table 1S. Percentages of CD4+CD26− and CD4+CD26+ T cells expressing CD164 or FCRL3 in selected Sezary syndrome patients.
Acknowledgments
We are grateful to Dr. Xuming Mao for his suggestions and valuable discussions. This work was supported by NCI, grant R01CA122569 and Translational Research grant from the Leukemia and Lymphoma Society (AHR); NCI grants RO1 CA 132098 (LS); the Wistar Institute Genomic and Bioinformatics facilities were supported by Cancer Center Support Grant P30 CA010815. SN and TI were recipients of a NIH COBRE grant (1P20RR024219-01A2).
Abbreviations
- SS
Sézary syndrome
- MF
Mycosis Fungoides
- CTCL
cutaneous T-cell Lymphoma
- AD
atopic dermatitis
- PBMC
peripheral blood mononuclear cells
Footnotes
Conflict-of-interest The authors declare no conflict of interest
References
- Bensussan A, Remtoula N, Sivori S, et al. Expression and function of the natural cytotoxicity receptor NKp46 on circulating malignant CD4+ T lymphocytes of Sezary syndrome patients. J Invest Dermatol. 2011;131:969–76. doi: 10.1038/jid.2010.404. [DOI] [PubMed] [Google Scholar]
- Bernengo MG, Novelli M, Quaglino P, et al. The relevance of the CD4+ CD26− subset in the identification of circulating Sezary cells. Br J Dermatol. 2001;144:125–35. doi: 10.1046/j.1365-2133.2001.04014.x. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Clark RA, Watanabe R, et al. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116:767–71. doi: 10.1182/blood-2009-11-251926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Shiue LH, Duvic M, et al. Sezary syndrome cells overexpress syndecan-4 bearing distinct heparan sulfate moieties that suppress T-cell activation by binding DC-HIL and trapping TGF-beta on the cell surface. Blood. 2011;117:3382–90. doi: 10.1182/blood-2010-08-302034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Shackelton JB, Watanabe R, et al. High-scatter T cells: a reliable biomarker for malignant T cells in cutaneous T-cell lymphoma. Blood. 2011;117:1966–76. doi: 10.1182/blood-2010-05-287664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustan-Smith E, Song G, Clark C, Key L, Liu P, Mehrpooya M, et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011;117:6267–76. doi: 10.1182/blood-2010-12-324004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargnoli MC, Edelson RL, Berger CL, et al. Diminished TCR signaling in cutaneous T cell lymphoma is associated with decreased activities of Zap70, Syk and membrane-associated Csk. Leukemia. 1997;11:1338–46. doi: 10.1038/sj.leu.2400745. [DOI] [PubMed] [Google Scholar]
- Ferenczi K, Fuhlbrigge RC, Pinkus J, et al. Increased CCR4 expression in cutaneous T cell lymphoma. J Invest Dermatol. 2002;119:1405–10. doi: 10.1046/j.1523-1747.2002.19610.x. [DOI] [PubMed] [Google Scholar]
- Havens AM, Jung Y, Sun YX, et al. The role of sialomucin CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC Cancer. 2006;6:195. doi: 10.1186/1471-2407-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introcaso CE, Hess SD, Kamoun M, et al. Association of change in clinical status and change in the percentage of the CD4+CD26− lymphocyte population in patients with Sezary syndrome. J Am Acad Dermatol. 2005;53:428–34. doi: 10.1016/j.jaad.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Jones D, Dang NH, Duvic M, et al. Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol. 2001;115:885–92. doi: 10.1309/U1Y6-J4AG-5M4M-7AYV. [DOI] [PubMed] [Google Scholar]
- Kari L, Loboda A, Nebozhyn M, et al. Classification and prediction of survival in patients with the leukemic phase of cutaneous T cell lymphoma. J Exp Med. 2003;197:1477–88. doi: 10.1084/jem.20021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leccia F, Nardone A, Corvigno S, et al. Cytometric and biochemical characterization of human breast cancer cells reveals heterogeneous myoepithelial phenotypes. Cytometry A. 2012;81:960–72. doi: 10.1002/cyto.a.22095. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kurosawa N, Hibi K, et al. The ratio of splicing variants of MGC-24/CD164, a sialomucin, correlates with the metastatic potential of colorectal carcinomas. J Biochem. 2000;127:1103–7. doi: 10.1093/oxfordjournals.jbchem.a022704. [DOI] [PubMed] [Google Scholar]
- Murphy GF. Cutaneous T-cell lymphoma. Adv Pathol. 1988;1:131–56. [Google Scholar]
- Nagata S, Ise T, Pastan I. Fc receptor-like 3 protein expressed on IL-2 nonresponsive subset of human regulatory T cells. J Immunol. 2009;182:7518–26. doi: 10.4049/jimmunol.0802230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narducci MG, Scala E, Bresin A, et al. Skin homing of Sezary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood. 2006;107:1108–15. doi: 10.1182/blood-2005-04-1492. [DOI] [PubMed] [Google Scholar]
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–22. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- Poszepczynska-Guigne E, Schiavon V, D’Incan M, et al. CD158k/KIR3DL2 is a new phenotypic marker of Sezary cells: relevance for the diagnosis and follow-up of Sezary syndrome. J Invest Dermatol. 2004;122:820–3. doi: 10.1111/j.0022-202X.2004.22326.x. [DOI] [PubMed] [Google Scholar]
- Rook AH, Kubin M, Cassin M, et al. IL-12 reverses cytokine and immune abnormalities in Sezary syndrome. J Immunol. 1995;154:1491–8. [PubMed] [Google Scholar]
- Samimi S, Benoit B, Evans K, et al. Increased programmed death-1 expression on CD4+ T cells in cutaneous T-cell lymphoma: implications for immune suppression. Arch Dermatol. 2010;146:1382–8. doi: 10.1001/archdermatol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala E, Abeni D, Pomponi D, et al. The role of 9-O-acetylated ganglioside D3 (CD60) and (Christopherson et al).4(Bernengo et al.)1 (CD49d) expression in predicting the survival of patients with Sezary syndrome. Haematologica. 2010;95:1905–12. doi: 10.3324/haematol.2010.026260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimauchi T, Kabashima K, Nakashima D, et al. Augmented expression of programmed death-1 in both neoplastic and non-neoplastic CD4+ T-cells in adult T-cell leukemia/lymphoma. Int J Cancer. 2007;121:2585–90. doi: 10.1002/ijc.23042. [DOI] [PubMed] [Google Scholar]
- Showe MK, Vachani A, Kossenkov AV, et al. Gene expression profiles in peripheral blood mononuclear cells can distinguish patients with non-small cell lung cancer from patients with nonmalignant lung disease. Cancer Res. 2009;69:9202–10. doi: 10.1158/0008-5472.CAN-09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska-Wojdylo M, Wenzel J, Gaffal E, et al. Circulating clonal CLA(+) and CD4(+) T cells in Sezary syndrome express the skin-homing chemokine receptors CCR4 and CCR10 as well as the lymph node-homing chemokine receptor CCR7. Br J Dermatol. 2005a;152:258–64. doi: 10.1111/j.1365-2133.2004.06325.x. [DOI] [PubMed] [Google Scholar]
- Sokolowska-Wojdylo M, Wenzel J, Gaffal E, et al. Absence of CD26 expression on skin-homing CLA+ CD4+ T lymphocytes in peripheral blood is a highly sensitive marker for early diagnosis and therapeutic monitoring of patients with Sezary syndrome. Clin Exp Dermatol. 2005b;30:702–6. doi: 10.1111/j.1365-2230.2005.01904.x. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–5. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su MW, Dorocicz I, Dragowska WH, et al. Aberrant expression of T-plastin in Sezary cells. Cancer Res. 2003;63:7122–7. [PubMed] [Google Scholar]
- Swainson LA, Mold JE, Bajpai UD, et al. Expression of the autoimmune susceptibility gene FcRL3 on human regulatory T cells is associated with dysfunction and high levels of programmed cell death-1. J Immunol. 2010;184:3639–47. doi: 10.4049/jimmunol.0903943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt SM, Buhring HJ, Rappold I, et al. CD164, a novel sialomucin on CD34(+) and erythroid subsets, is located on human chromosome 6q21. Blood. 1998;92:849–66. [PubMed] [Google Scholar]
- Wysocka M, Benoit BM, Newton S, et al. Enhancement of the host immune responses in cutaneous T-cell lymphoma by CpG oligodeoxynucleotides and IL-15. Blood. 2004;104:4142–9. doi: 10.1182/blood-2004-03-1190. [DOI] [PubMed] [Google Scholar]
- Yawalkar N, Ferenczi K, Jones DA, et al. Yamanaka K, Suh KY, Sadat S, et al. Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood. 2003;102:4059–66. doi: 10.1182/blood-2003-04-1044. [DOI] [PubMed] [Google Scholar]
- Zannettino AC, Buhring HJ, Niutta S, et al. The sialomucin CD164 (MGC-24v) is an adhesive glycoprotein expressed by human hematopoietic progenitors and bone marrow stromal cells that serves as a potent negative regulator of hematopoiesis. Blood. 1998;92:2613–28. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1S. Percentages of CD4+CD26− and CD4+CD26+ T cells expressing CD164 or FCRL3 in selected Sezary syndrome patients.