Abstract
Currently, islet cells are transplanted into the liver via portal vein infusion. One disadvantage of this approach is that it is not possible to adequately biopsy the islets in the liver to assess for rejection. Islet Tx into the gastric submucosal space (GSMS) can be performed endoscopically, and has the potential advantage of histological evaluation by endoscopic biopsy. The aim of this study was to determine whether a representative allograft sample could be obtained endoscopically. We performed islet Tx into the GSMS in non-immunosuppressed pigs using simple endoscopic submucosal injection. Islets were transplanted at 4 sites. Endoscopic ultrasonography and biopsy of the transplanted islets at 2 sites by modified endoscopic submucosal dissection were carried out successfully in all pigs 5 days after islet Tx. Tissue obtained at both biopsy and necropsy (including full-thickness sections of the gastric wall around the sites of the remaining islets and biopsies) were examined by histology and immunohistochemistry to confirm the presence of the islet grafts and any features of rejection. Representative allograft sampling was successfully obtained from all biopsy sites. All biopsies included islets with insulin-positive staining. There was significant CD3+ and CD68+ cell infiltration in the islet masses obtained at biopsy and from sections taken at necropsy, with similar histopathological features. Endoscopic biopsy of islet allografts in the GSMS is feasible, provides accurate histopathological data, and would provide a significant advance if translated into clinical practice.
Keywords: Endoscopy, Gastric submucosal space, Islets, biopsy, Pig, Transplantation, islets
INTRODUCTION
Worldwide, there are many millions of diabetic patients who might benefit from islet allotransplantation (Tx). In the US alone, there are an estimated >2 million people with type I diabetes, and a further approximate 20 million with type 2 diabetes. The number of people developing type I diabetes in the US each year is currently estimated to be more than a quarter of a million (22,41,44).
The liver is the most commonly used site for islet Tx. The Tx of deceased human donor islets into the portal vein (PV) in patients with type I diabetes has been followed by encouraging results (9,32,33,38). However, islet cell Tx into the PV is suboptimal for several reasons. (i) There is a significant incidence of morbidity from hemorrhage and/or thrombosis (28,42). (ii) There is an immediate loss of a large mass of islets (estimated at 60–80%) through an inflammatory response known as the instant blood-mediated inflammatory reaction (IBMIR) (3,6,21,37). (iii) In the majority of patients, there is a steady loss of normoglycemia over the succeeding 5 years, necessitating a return to insulin therapy; this loss could possibly be prevented if IBMIR could be avoided (as only a borderline number of islets survive the IBMIR) (30). (iv) Biopsy of the islets in the liver is not possible, and therefore the cause of loss of islet function, e.g., acute rejection, cannot be fully assessed.
There are several theoretical reasons why the gastric submucosal space (GSMS) may be advantageous as a site for islet Tx (7). (i) It has a similar embryonic origin as the pancreas (10,24). (ii) It has a rich vascular supply of oxygen and nutrients. (iii) Like the pancreas, its venous drainage is into the portal blood stream (38). In addition, islet Tx into the GSMS would avoid (iv) IBMIR and (v) the complications of the percutaneous trans-hepatic catheter procedure necessary when islets are transplanted into the PV (39). Finally, (vi) the GSMS is easily accessible for endoscopic islet Tx (7), and it may be possible to biopsy allografts in the GSMS, which would be an immense advantage over islet Tx into the PV. A pilot report in a large animal model demonstrated that allotransplanted islets can engraft in the GSMS, and that endoscopic islet alloTx can be carried out safely (7).
With regard to biopsy of islets transplanted into the GSMS, endoscopic ultrasonography (EUS) is able to accurately visualize the submucosa (11,17,20,25). EUS can indicate the difference between a tumor and normal healthy tissue. The tumor can be biopsied by endoscopic submucosal dissection (ESD) and/or endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). These methods could be used to identify and biopsy the islet mass after islet Tx into the GSMS.
The aims of the present study were to determine whether (i) the transplant sites could be identified by EUS, and (ii) whether ESD allows submucosal sampling of the allograft for histological assessment.
MATERIAL AND METHODS
Animals
Four large white-Landrace wild-type female non-diabetic non-immunosuppressed pigs (weighing 11.4+/−1.0 kg, aged 8 weeks) were used as recipients (Wally Whippo, Enon Valley, PA), and two female pigs (approximately 250kg, aged 3.5 years), were used as sources of islets. In all recipients, intravascular catheters were inserted into both right jugular veins for blood withdrawal and drug infusion. All received cefazolin 250mg i.v. (x2 daily) and famotidine 2.5mg iv. (x2 daily) throughout the course of the experiment.
All animal care procedures were in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985). All protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Donor pancreatectomy and islet isolation
Under full inhalational anesthesia, the abdomen was opened in the midline. After perfusion of the pancreas in situ with Hank’s Balanced Salt Solution (Mediatech Inc. Manassas, VA), pancreatectomy was performed without warm ischemia. Islet isolation and overnight culture were carried out as described previously (2). Approximately 350,000IEq were obtained from each donor pancreas.
Islet viability
Islet viability was assessed by the double fluorescent calcein-AM and propidium iodide stain (1,2).
Endoscopic islet transplantation
Under full inhalational anesthesia, using an endoscope (GIF-2T160, OLYMPUS MEDICAL SYSTEMS CORP., Tokyo, Japan), endoscopic submucosal injection was performed using a Disposable Injection Needle (needle diameter 23G, MN-200U-0423, Olympus). Specifically, the islets (15,000IEq/kg) were injected into the GSMS at 4 different sites in the posterior and anterior gastric antrum (Figure 1). A 3-way connector (MX4341L Medex, Dublin, OH) was attached to the needle to facilitate delivery of the islets. Immediately before infusion, the islets were re-suspended in 0.5ml CMRL-1066 (Mediatech, Manassas, VA) supplemented with 1% heat-inactivated donor serum. The injection of islets was preceded (to prime the catheter) with the same preparation, but supplemented with 5% heat-inactivated donor pig serum. The islet injection was followed by the injection of 1ml CMRL-1066 supplemented with 1% heat-inactivated donor serum to flush the needle and ensure complete delivery. Two islet masses were injected into the anterior wall and two into the posterior wall of the antrum. To provide some indication of the approximate sites of the islet transplants, two marking clips (HX-201LR-135, Olympus) were applied to the gastric mucosa approximately 20mm on either side of each islet mass, one clip towards the minor curvature of the stomach and the other towards the major curvature.
Figure 1.
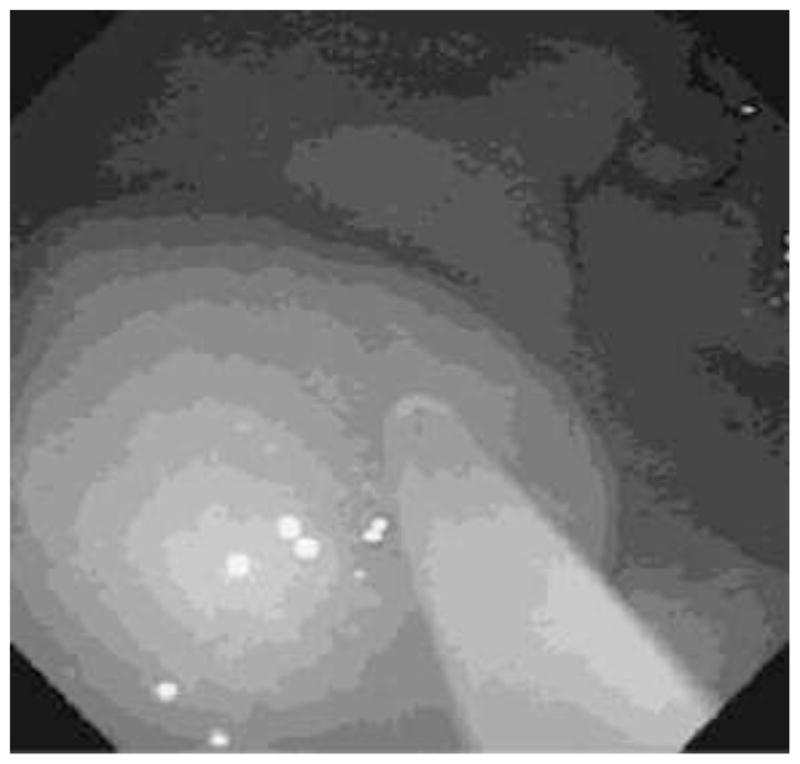
Endoscopic view of the submucosal bleb created as donor islets are injected into the GSMS of a recipient pig.
Follow-up
Although the recipient pigs had not been rendered diabetic, monitoring of blood glucose was carried out twice daily with a True Track system glucometer (Home Diagnostics, Fort Lauderdale, FL) to measure a fasting overnight level and a semi-fasting evening level.
Endoscopic detection and biopsy of transplanted islets
EUS and biopsy of the transplanted islets were carried out in all pigs 5 days after islet Tx (since recipient pigs were not administered any immunosuppressive therapy and therefore acute rejection was likely to develop rapidly). Under full inhalational anesthesia, a therapeutic endoscope (GIF-2T160, Olympus) was used to examine the sites of islet Tx, indicated by elevated lesions similar to submucosal tumors (Figure 2A). EUS was then used to confirm the location of the 4 islet masses. After filling the lumen of the stomach with water, scanning with an ultrasonic miniature probe at 20 MHz (UM-3R, Olympus) showed solid masses of mixed hyperechoic/hypoechoic echogenicity in the submucosal layer (Figure 2B). After identification of the site of the islet mass to be biopsied, saline (2–5ml) was injected around the islets to create a submucosal bleb, and the biopsy was carried out using a modified technique of endoscopic submucosal dissection (ESD). The raised mucosal bleb was incised with a Flexknife (KD-630L, Olympus) to allow access to the submucosal space. An ITknife2 (KD-611L, Olympus) was then used to cut around the site of the graft using a PulseCut slow, 40W (ESG-100, Olympus) (Figure 3A). A biopsy of the graft was taken by snaring the dissected tissue (by electric cutting using a PulseCut slow, 40W), including the submucosal tissue (Figure 3B). The biopsied tissue was then removed with Grasping Forceps (FG-42L-1, Olympus) (Figures 3C and 3D). Two of the 4 islet masses in each pig were biopsied using the modified ESD technique, and the histopathology of the graft was examined.
Figure 2.
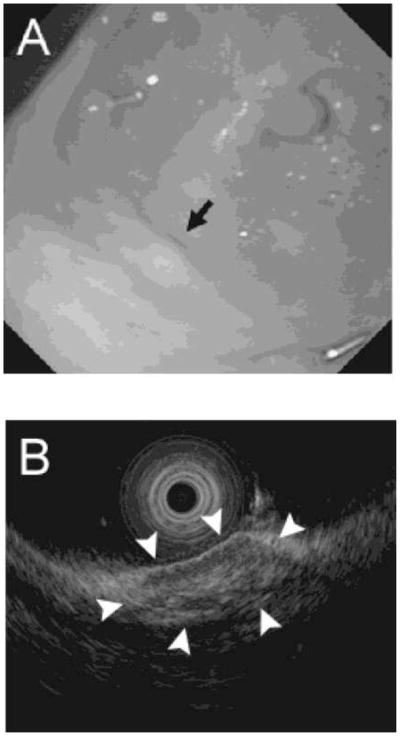
(A) Endoscopic appearance of the gastric submucosa at site of islet mass (black arrow) 5 days after islet Tx. (B) Endoscopic ultrasound identifying the islet mass (white arrows) in the GSMS 5 days after islet Tx.
Figure 3.

Endoscopic view as a biopsy of an islet mass is taken 5 days after islet Tx. (A) After identification of the site of the islet mass to be biopsied, saline (2–5ml) was injected to create a submucosal bleb. The raised mucosal bleb has been incised with a Flex knife to allow access to the submucosal space. An IT2 knife was then used to cut around the site of the graft. (B) A biopsy of the graft is being taken by snaring the dissected tissue, including the submucosal tissue. (C) The biopsied tissue is being removed with forceps. (D) The removed specimen was 23×12 mm.
Euthanasia and necropsy
The recipient pig was immediately euthanized by pentobarbital (200mg/kg i.v.), and the abdomen was opened. The wall of the stomach was carefully examined to determine whether perforation or other complication had occurred. Gastric wall tissue around the 2 sites of biopsy was taken for histological examination to determine the exact depth of the biopsies. Tissue (full-thickness gastric wall) was also taken (that included the 2 islet masses that had not been biopsied) for histopathological examination to compare with the information obtained from the histopathological examination of the biopsied islets.
Histopathologic examination
Tissues obtained at both biopsy and necropsy were fixed in 10% formalin, and paraffin sections were stained with hematoxylin and eosin (H&E), monoclonal mouse anti-human insulin antibody (clone HB125, Biogenex, San Ramon, CA), polyclonal rabbit anti-swine glucagon antibody (Biogenex), polyclonal rabbit anti-human CD3 antibody (Dako), monoclonal mouse anti-human CD20 antibody (clone L26, Dako), and monoclonal mouse anti-pig CD68 antibody (clone BA4D5, AbD Serotec, Oxford, UK). (We were unable to identify antibodies that stained for swine CD4+ and CD8+ cells.) This was followed by blocking, as previously described (36).
RESULTS
Endoscopic Islet Tx
Islets were successfully transplanted in 4 sites in the GSMS of the antrum in each of the recipient pigs. All pigs remained normoglycemic throughout the 5 days of follow-up.
Endoscopic detection and biopsy of transplanted islets
The 4 islet masses in each pig were identified clearly by direct endoscopic vision and confirmed by EUS (Figures 2A and B). Each islet mass was approximately 10±3mm in diameter (Figure 2B). Biopsies of 2 of the 4 masses in each pig were obtained without complication. The tissue obtained from each site (including the islet mass) was approximately 20mm in diameter (Figure 3D).
Euthanasia and necropsy
At necropsy, there was no evidence of perforation or bleeding at the biopsy sites. Examination of the remaining stomach wall at necropsy clearly indicated the sites of endoscopic biopsy by the presence of small ulcers. The remaining islet masses (not biopsied) could also be identified visually.
Histopathology of the biopsied islets and the remaining islet masses
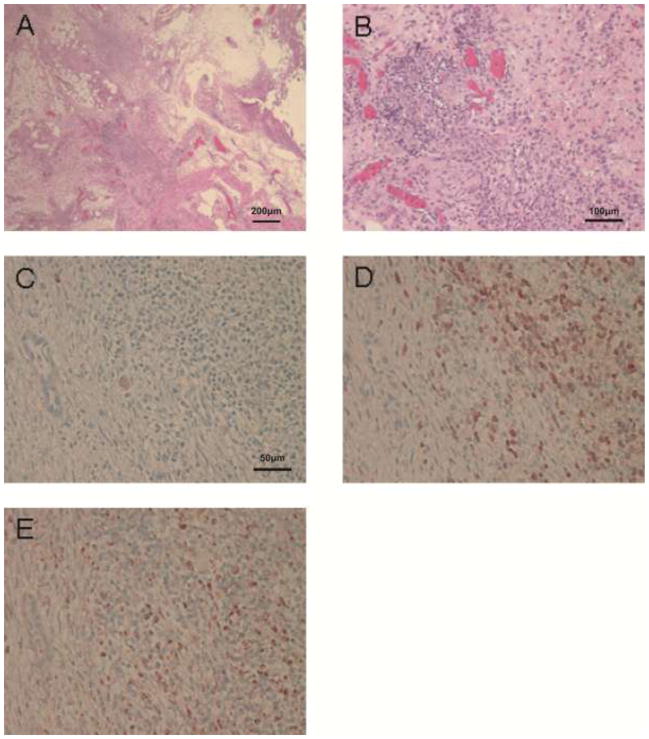
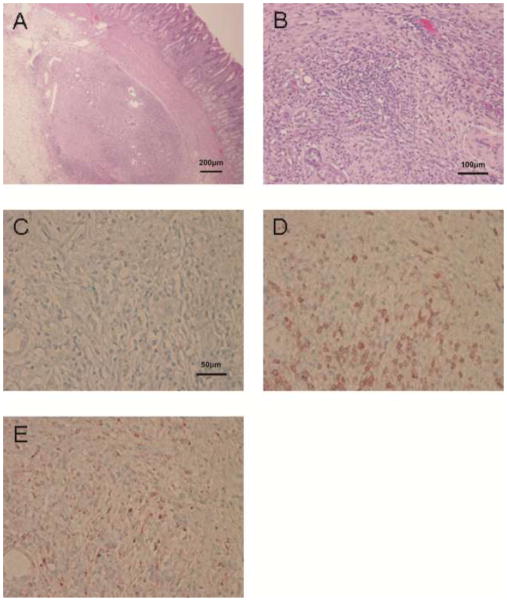
Microscopic examination of all biopsies demonstrated that the tissue obtained included the gastric submucosa (not shown). H-E staining showed significant polymorphonuclear and mononuclear cell infiltration and fibrosis in the islet masses. In addition, early neovasculization of islets was confirmed (Figure 4 and 5). Insulin-positive cells were few in areas of severe cell infiltration of biopsy or of necropsy samples (Figure 4C and 5C). Infiltrates were largely CD3+ T cells (Figures 4D and 5D) and CD68+ macrophages (Figures 4E and 5E), with a smaller number of polymorphonuclear neutrophils. However, in areas with mild cell infiltration, biopsied islets and the remaining islet masses (not biopsied) showed multiple insulin- (Figures 6A and 6C) and glucagon- (Figures 6B and 6D) positive islet cells. Although CD20+ cells could be observed in the mucosal layer (not shown), they were not detected within the islet masses. Appearances in all respects were very similar between biopsied islet masses (Figure 4, Figure 6A and B) and islet masses examined after necropsy (Figure 5, Figure 6C and D).
Figure 4.
Histopathological appearance of an area of severe cellular infiltration of biopsies taken 5 days after islet Tx into the GSMS. (A) Islet mass in the GSMS (H&E, x40). (B) Polymorphonuclear and mononuclear cell infiltrate in islet mass (H&E, x100). (C) Few insulin-positive beta cells were identified in an area of severe cellular infiltration of the islet mass. Islets were infiltrated particularly by (D) CD3+ and (E) CD68+ cells (x200).
Figure 5.
Histopathological appearance of an area of severe cellular infiltration of remaining non-biopsied islets (at necropsy, in the same recipient pig as in Figure 4) 5 days after islet Tx into the GSMS. (A) Islet mass in the GSMS (H&E, x40). (B) Polymorphonuclear and mononuclear cell infiltrate in islet mass (H&E, x100). (C) Few insulin-positive beta cells were identified whenever severe cell infiltration of the islet mass was present. Islets were infiltrated particularly by (D) CD3+ and (E) CD68+ cells (x200). Similar histopathological findings were detected between biopsied islets (Figure 4) and remaining non-biopsied islets.
Figure 6.
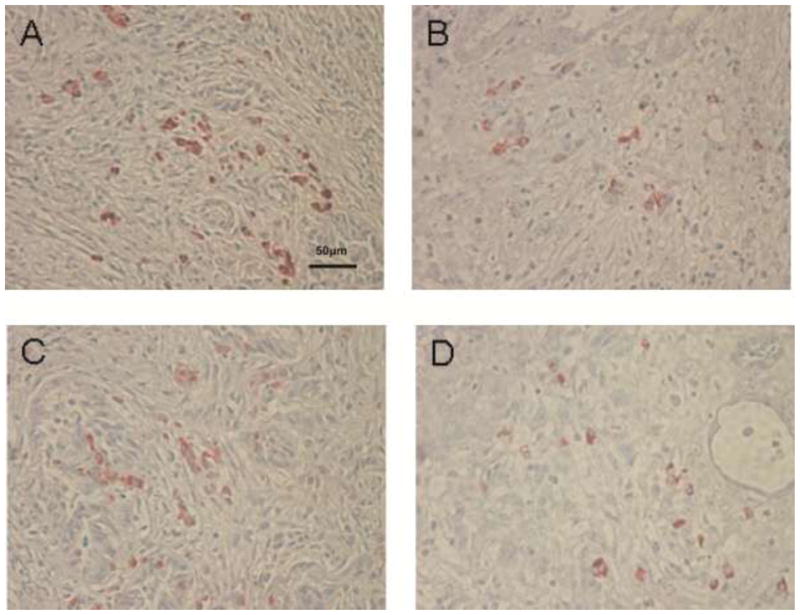
Histopathological appearance of an area of mild cellular infiltration of islet biopsies and non-biopsied islets5 days after islet Tx into the GSMS (x200). (A) Insulin-positive beta cells and (B) glucagon-positive beta cells in an area of mild cellular infiltration in a biopsy of an islet mass in the GSMS. (C) Insulin-positive beta cells and (D) glucagon-positive cells in an area of mild cellular infiltration of an islet mass in the GSMS that had not been biopsied. Multiple insulin- and glucagon-positive cells were present in areas of mild cell infiltration compared to areas of severe cell infiltration.
DISCUSSION
Tx into the liver through the PV is the current technique for islet alloTx (8,29,38). However, following islet Tx into the liver, it is not possible to histologically assess for rejection (38). Previous studies have established the potential value of the GSMS as a site for islet Tx (4,7,43), but no study has yet demonstrated the ability to successfully biopsy islets transplanted into this site. In a previous attempt, endoscopic biopsy using forceps was not successful in identifying any islets (43). The present report, therefore, is the first to describe a successful technique for endoscopic biopsy of islets in the GSMS.
EUS is the procedure of choice for evaluating subepithelial lesions of the gastrointestinal tract (11,17,20,25). Therefore, after initial identification by direct vision through an endoscope, EUS was used to confirm the location of the islet masses in the GSMS. In preliminary experiments, we performed EUS after injection of saline into the GSMS to investigate whether a small mass (0.5ml, 5mm in diameter), which is similar in size to an islet mass, could be detected. EUS clearly detected 0.5ml saline injected into the GSMS (not shown). In the present study, scanning with a 20 MHs miniature probe confirmed the presence of the islet masses (10mm diameter) in the GSMS. EUS showed mixed hyperechoic/hypoechoic masses in the submucosal layer at the site of islet Tx, which probably correlated with the presence of cell infiltration and fibrotic changes. The specific existence of islets could not be confirmed by EUS.
The injection of India ink has been used as a permanent marker of a specific site in the gastrointestinal tract that requires follow-up examination. After injection (1–2 ml) into the submucosa, the ink has been reported to remain for up to 15 months in pigs (34) and 24 months or longer in humans (35). However, in our previous experiments we demonstrated that India ink injected close to islets appeared to be detrimental with regards to islet viability and function both in vitro and in vivo. In in vitro experiments, we noticed that islets exposed to Indian Ink (short exposure, less than 15 minutes) prior to dynamic perfusion, released lower amounts of insulin under basal as well as stimulated conditions, compared to untreated islets from the same donor (data not shown). In a mouse model of islet transplantations, diabetic immunodeficient recipients of islets exposed to Indian ink did not achieve or achieved delayed islet function following kidney capsule islet transplantation, in comparison to animals that received the same number of islets not exposed to Indian ink as control (data not shown). Furthermore, with time, the ink dissipated throughout the submucosa, obscuring identification of the original site of injection.
Following intraportal islet Tx, a histological assessment of the allograft cannot be reliably obtained. In one study, it was only possible to identify insulin-positive cells within the liver parenchyma in 1 of 6 insulin-independent patients (17%) using ultrasound-directed 18-gauge needle liver biopsies (36). EUS-FNA is a technique where intra- and extra-mural lesions can be sampled under direct endoscopic ultrasound guidance. However, EUS-FNA is generally performed using a smaller gauge needle (19 to 25-gauge) compared with that used for liver biopsy (15,40). Therefore, it may be difficult to obtain an adequate cytologic yield to assess for rejection.
Endoscopic mucosal resection (EMR) is currently an accepted method for removal of superficial low-risk gastrointestinal malignancies. A limitation of EMR is the piecemeal resection technique required in the case of large lesions, which leads to difficulty in making an accurate histopathological assessment (19,23). (In a preliminary study by us, EMR provided only mucosa, rather than representative submucosa.)
Because of these limitations with cytological and/or histological sampling, we used a modified ESD technique to biopsy the islet grafts. ESD is frequently performed in Japan for the diagnosis and treatment of early-phase gastrointestinal malignancies. This technique has been developed to enable en bloc endoscopic resection of large tumors (12,26,27,31). A previous report documented the feasibility of ESD in obtaining diagnostic and therapeutic yields of gastric submucosal tumors (14).
In the present study, we performed islet graft biopsy by the ESD technique using an ITknife2 at the site of the islet mass, which was identified by both direct visual means and by EUS. Using the same knife, we dissected around the islet mass. However, to undermine the islet mass, we used a snaring technique (by electric cutting using a PulseCut slow, 40W) which allowed the biopsy to be carried out more rapidly than by classical ESD. The dissection time using the ESD technique is longer than using the snaring technique. Cauterization of the tissue was less when using the snaring technique, thus reducing potential injury to the islets.
Histological examination of the biopsies demonstrated insulin- and glucagon-positive islets infiltrated by CD3+ and CD68+ cells (with peripheral CD20+ cells); the appearances were identical to those in the non-biopsied islet sites excised at necropsy. Early neovasculization of the islets was also seen. ESD therefore appears capable of obtaining representative allograft specimens. The islet grafts in the GSMS were fragmented, with no intact islets, and considerable cellular infiltration. The features suggested rejection of the grafts had developed within 5 days after Tx, correlating with the fact that the pigs had received no immunosuppressive therapy.
Potential complications of ESD include bleeding and perforation (12,26). Significant bleeding can result in termination of the procedure prior to obtaining the appropriate tissue sample. Bleeding might be problematic particularly if a punch biopsy without cauterization had been performed. In the present small study, however, ESD was completed without complication. The absence of perforation and bleeding was confirmed at necropsy immediately following ESD.
There are several reasons why the antrum was chosen as the site for Tx in this study. The stomach has a similar embryonic origin as the pancreas (10,24), and the submucosal space of the antrum is the most common ectopic site for pancreatic tissue (5,18). The upper and middle parts of the stomach may prove more difficult locations to perform ESD (16). Furthermore, the antrum wall is thicker than other gastric sites, increasing the safety of ESD.
In future studies, in order to mimic the clinical situation, diabetes will be induced by streptozotocin in the recipient pigs (13) and longer periods of post-Tx observation will be carried out. We are hopeful that endoscopic biopsy will provide evidence of allograft rejection which correlates with changes in blood glucose levels and insulin requirements after islet alloTx. This ultimately could influence clinical decision-making regarding immunosuppressant dosing and care of the post-Tx patient. However, it is yet to be determined whether islet transplant sites in the GSMS can be identified by endoscopy and/or EUS several weeks or months following Tx. In addition, a comparison of the techniques of ESD and EUS-FNA to investigate whether adequate samples of the islets are obtained to evaluate the histolopathological features will be necessary; ESD has not been accepted worldwide as it is a difficult technique, and specific training is necessary.
In summary, this is the first study to demonstrate successful endoscopic sampling after islet Tx into the GSMS. EUS and ESD proved valuable techniques to allow this aim to be achieved. Further studies are warranted to confirm these preliminary results, specifically with the goal of obtaining biopsies after longer periods of observation post-Tx. We believe these approaches would provide a significant advance if translated into clinical practice.
Acknowledgments
This study was supported by a grant from the Olympus Corporation of the Americas Educational and Research Grant Committee (HH) and in part by NIH grant RO3 (AI096296, HH). Minoru Fujita, MD, PhD, is the recipient of Kawasaki Sukenobu Memorial Fund of Kawasaki Medical School for Research Study, Japan, and Kawasaki Medical School Alumini Association Fund for Foreign Study, Japan. Eefje M. Dons, MD, is the recipient of fellowships from the Ter Meulen Fund of the Royal Netherlands Academy of Arts and Sciences and the Stichting Professor Michael van Vloten Fund, The Netherlands. The authors thank Mr. Masahiro Ashizuka and Mr. Shoichi Matsui for technical help in performing EUS and ESD, and Drs. Martin N. Wijkstrom, Massimo Trucco, David Ayares and Fadi G. Lakkis for support and advice.
ABBREVIATIONS
- EMR
endoscopic mucosal resection
- ESD
endoscopic submucosal dissection
- EUS
endoscopic ultrasonography
- EUS-FNA
endoscopic ultrasound-guided fine needle aspiration
- GSMS
gastric submucosal space
- IBMIR
instant blood-mediated inflammatory response
- PV
portal vein
- Tx
transplantation
Footnotes
Author’s specific contributions
MF1,5 participated in the performance of the research and in the writing of paper. EMD1,4, RB1,3, CL1, GK1, and BE1,7 participated in the performance of the research and in the review of the paper. GJE8, JH6, and KH5 participated in research design and in the review of the paper. DKCC1 participated in research design and in the writing of paper. KMM2 and HH1 participated in research design, in the performance of the research, and in the writing of the paper.
Conflict of interest
The authors have no conflict of interest for this study.
References
- 1.Bertera S, Crawford ML, Alexander AM, Papworth GD, Watkins SC, Robbins PD, Trucco M. Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes. 2003;52(2):387–393. doi: 10.2337/diabetes.52.2.387. [DOI] [PubMed] [Google Scholar]
- 2.Bottino R, Balamurugan AN, Smetanka C, Bertera S, He J, Rood PP, Cooper DK, Trucco M. Isolation outcome and functional characteristics of young and adult pig pancreatic islets for transplantation studies. Xenotransplantation. 2007;14(1):74–82. doi: 10.1111/j.1399-3089.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 3.Cabric S, Sanchez J, Lundgren T, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, Tufveson G, Larsson R, Korsgren O, Nilsson B. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56(8):2008–2015. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- 4.Caiazzo R, Gmyr V, Hubert T, Delalleau N, Lamberts R, Moerman E, Kerr-Conte J, Pattou F. Evaluation of alternative sites for islet transplantation in the minipig: interest and limits of the gastric submucosa. Transplant Proc. 2007;39(8):2620–2623. doi: 10.1016/j.transproceed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Chen SH, Huang WH, Feng CL, Chou JW, Hsu CH, Peng CY, Yang MD. Clinical analysis of ectopic pancreas with endoscopic ultrasonography: an experience in a medical center. J Gastrointest Surg. 2008;12(5):877–881. doi: 10.1007/s11605-008-0476-0. [DOI] [PubMed] [Google Scholar]
- 6.Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation. 1995;59(6):817–820. [PubMed] [Google Scholar]
- 7.Echeverri GJ, McGrath K, Bottino R, Hara H, Dons EM, van der Windt DJ, Ekser B, Casu A, Houser S, Ezzelarab M, Wagner R, Trucco M, Lakkis FG, Cooper DK. Endoscopic gastric submucosal transplantation of islets (ENDO-STI): technique and initial results in diabetic pigs. Am J Transplant. 2009;9(11):2485–2496. doi: 10.1111/j.1600-6143.2009.02815.x. [DOI] [PubMed] [Google Scholar]
- 8.Feng S, Barr M, Roberts J, Oberbauer R, Kaplan B. Developments in clinical islet, liver thoracic, kidney and pancreas transplantation in the last 5 years. Am J Transplant. 2006;6(8):1759–1767. doi: 10.1111/j.1600-6143.2006.01402.x. [DOI] [PubMed] [Google Scholar]
- 9.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8(10):1990–1997. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Koizumi M, Boyer DF, Fujimoto K, Doi R, Kageyama R, Wright CV, Chiba T. Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest. 2006;116(6):1484–1493. doi: 10.1172/JCI27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fusaroli P, Caletti G. Endoscopic ultrasonography. Endoscopy. 2003;35(2):127–135. doi: 10.1055/s-2003-37010. [DOI] [PubMed] [Google Scholar]
- 12.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10(1):1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 13.Hara H, Lin YJ, Zhu X, Tai HC, Ezzelarab M, Balamurugan AN, Bottino R, Houser SL, Cooper DK. Safe induction of diabetes by high-dose streptozotocin in pigs. Pancreas. 2008;36(1):31–38. doi: 10.1097/mpa.0b013e3181452886. [DOI] [PubMed] [Google Scholar]
- 14.Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Endoscopic submucosal dissection for gastric submucosal tumor, endoscopic sub-tumoral dissection. Dig Endosc. 2009;21(4):266–269. doi: 10.1111/j.1443-1661.2009.00905.x. [DOI] [PubMed] [Google Scholar]
- 15.Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K, Rindi G, Bories E, Dogloni C, Bruno M, Dominguez-Munoz JE. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73(6):1189–1196. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 16.Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38(10):987–990. doi: 10.1055/s-2006-944716. [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto K, Yamada Y, Utsunomiya T, Okamura H, Mizuguchi M, Motooka M, Hirata N, Watanabe H, Sakai K, Kitagawa S, Kinukawa N, Masuda K. Gastrointestinal submucosal tumors: evaluation with endoscopic US. Radiology. 1997;205(3):733–740. doi: 10.1148/radiology.205.3.9393529. [DOI] [PubMed] [Google Scholar]
- 18.Kilman WJ, Berk RN. The spectrum of radiographic features of aberrant pancreatic rests involving the stomach. Radiology. 1977;123(2):291–296. doi: 10.1148/123.2.291. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda T, Gotoda T, Saito Y, Nakajima T, Conio M. Our perspective on endoscopic resection for colorectal neoplasms. Gastroenterol Clin Biol. 2010;34(6–7):367–370. doi: 10.1016/j.gcb.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto T, Hizawa K, Esaki M, Kurahara K, Mizuno M, Hirakawa K, Yao T, Iida M. Comparison of EUS and magnifying colonoscopy for assessment of small colorectal cancers. Gastrointest Endosc. 2002;56(3):354–360. doi: 10.1016/s0016-5107(02)70038-2. [DOI] [PubMed] [Google Scholar]
- 21.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, Ostraat O, Salmela K, Tibell A, Tufveson G, Elgue G, Nilsson Ekdahl K, Korsgren O, Nilsson B. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 22.National Diabetes Clearing House. 2011 http://diabetes.niddk.nih.gov/
- 23.Nonaka S, Oda I, Nakaya T, Kusano C, Suzuki H, Yoshinaga S, Fukagawa T, Katai H, Gotoda T. Clinical impact of a strategy involving endoscopic submucosal dissection for early gastric cancer: determining the optimal pathway. Gastric Cancer. 2011;14(1):56–62. doi: 10.1007/s10120-011-0008-6. [DOI] [PubMed] [Google Scholar]
- 24.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122(3):983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 25.Okanobu H, Hata J, Haruma K, Mitsuoka Y, Kunihiro K, Manabe N, Tanaka S, Chayama K. A classification system of echogenicity for gastrointestinal neoplasms. Digestion. 2005;72(1):8–12. doi: 10.1159/000087216. [DOI] [PubMed] [Google Scholar]
- 26.Ono H, Hasuike N, Inui T, Takizawa K, Ikehara H, Yamaguchi Y, Otake Y, Matsubayashi H. Usefulness of a novel electrosurgical knife, the insulation-tipped diathermic knife-2, for endoscopic submucosal dissection of early gastric cancer. Gastric Cancer. 2008;11(1):47–52. doi: 10.1007/s10120-008-0452-0. [DOI] [PubMed] [Google Scholar]
- 27.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48(2):225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajab A, Buss J, Diakoff E, Hadley GA, Osei K, Ferguson RM. Comparison of the portal vein and kidney subcapsule as sites for primate islet autotransplantation. Cell Transplant. 2008;17(9):1015–1023. doi: 10.3727/096368908786991533. [DOI] [PubMed] [Google Scholar]
- 29.Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes. 2010;59(6):1285–1291. doi: 10.2337/db09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, Yoshida S, Ikehara H, Otake Y, Nakajima T, Matsuda T, Saito D. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastrointest Endosc. 2010;72(6):1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 34.Si JM, Sun LM, Fan YJ, Wang LJ. Trial of a novel endoscopic tattooing biopsy forceps on animal model. World J Gastroenterol. 2005;11(12):1859–1861. doi: 10.3748/wjg.v11.i12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun L, Si J, Chen S, Liu W, Zhao L, Wang L. The establishment and clinical appliance of technique of mucosa marking targeting biopsy. Hepatogastroenterology. 2009;56(89):59–62. [PubMed] [Google Scholar]
- 36.Toso C, Isse K, Demetris AJ, Dinyari P, Koh A, Imes S, Kin T, Emamaullee J, Senior P, Shapiro AM. Histologic graft assessment after clinical islet transplantation. Transplantation. 2009;88(11):1286–1293. doi: 10.1097/TP.0b013e3181bc06b0. [DOI] [PubMed] [Google Scholar]
- 37.van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007;14(4):288–297. doi: 10.1111/j.1399-3089.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 38.van der Windt DJ, Echeverri GJ, Ijzermans JN, Cooper DK. The choice of anatomical site for islet transplantation. Cell Transplant. 2008;17(9):1005–1014. [PubMed] [Google Scholar]
- 39.Villiger P, Ryan EA, Owen R, O’Kelly K, Oberholzer J, Al Saif F, Kin T, Wang H, Larsen I, Blitz SL, Menon V, Senior P, Bigam DL, Paty B, Kneteman NM, Lakey JR, Shapiro AM. Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant. 2005;5(12):2992–2998. doi: 10.1111/j.1600-6143.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 40.Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112(4):1087–1095. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 41.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 42.Wilson JT, Chaikof EL. Thrombosis and inflammation in intraportal islet transplantation: a review of pathophysiology and emerging therapeutics. J Diabetes Sci Technol. 2008;2(5):746–759. doi: 10.1177/193229680800200502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wszola M, Berman A, Fabisiak M, Domagala P, Zmudzka M, Kieszek R, Perkowska-Ptasinska A, Sabat M, Pawelec K, Kownacki L, Piotrowska-Kownacka D, Ostrowski K, Januchta M, Klucinski W, Rowinski O, Kwiatkowski A, Chmura A. TransEndoscopic Gastric SubMucosa Islet Transplantation (eGSM-ITx) in pigs with streptozotocine induced diabetes - technical aspects of the procedure - preliminary report. Ann Transplant. 2009;14(2):45–50. [PubMed] [Google Scholar]
- 44.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]