Abstract
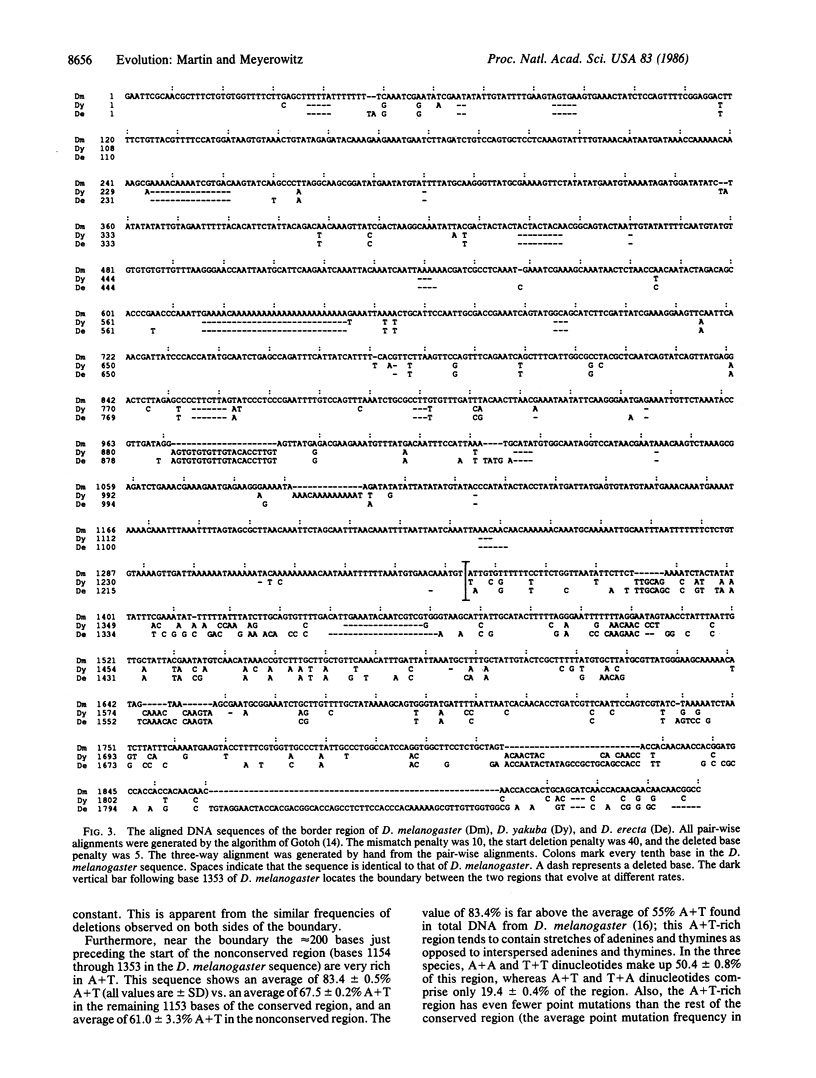
The site of a dramatic change in the rate of DNA sequence evolution exists near the 68C glue gene clusters of several Drosophila species. We have previously determined the approximate location of this transition site by comparison of restriction maps of the regions flanking the 68C-like glue gene cluster of five members of the melanogaster species subgroup. In the present work we report the sequence of the transition region in three of these Drosophila species: D. melanogaster, D. yakuba, and D. erecta. Using a best-fit alignment of these sequences, we find that the site of transition from slowly to rapidly evolving sequences occurs abruptly within a region less than 50 nucleotides in length. Although frequency of nucleotide substitutions changes as much as 10-fold across this boundary, frequency of small insertion/deletion events stays nearly constant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Richards G. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. III. Consequences of ecdysone withdrawal. Dev Biol. 1976 Dec;54(2):241–255. doi: 10.1016/0012-1606(76)90302-x. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. I. Dependence upon ecdysone concentration. Dev Biol. 1973 Nov;35(1):47–61. doi: 10.1016/0012-1606(73)90006-7. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. II. The effects of inhibitors of protein synthesis. Dev Biol. 1974 Jul;39(1):141–157. doi: 10.1016/s0012-1606(74)80016-3. [DOI] [PubMed] [Google Scholar]
- Bodmer M., Ashburner M. Conservation and change in the DNA sequences coding for alcohol dehydrogenase in sibling species of Drosophila. 1984 May 31-Jun 6Nature. 309(5967):425–430. doi: 10.1038/309425a0. [DOI] [PubMed] [Google Scholar]
- Crosby M. A., Meyerowitz E. M. Lethal mutations flanking the 68C glue gene cluster on chromosome 3 of Drosophila melanogaster. Genetics. 1986 Apr;112(4):785–802. doi: 10.1093/genetics/112.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T. E., Bond M. W., Meyerowitz E. M. The structural genes for three Drosophila glue proteins reside at a single polytene chromosome puff locus. Mol Cell Biol. 1983 Apr;3(4):623–634. doi: 10.1128/mcb.3.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T. E., Meyerowitz E. M. Steroid regulation of RNAs transcribed from the Drosophila 68c polytene chromosome puff. Dev Biol. 1984 Mar;102(1):110–121. doi: 10.1016/0012-1606(84)90179-9. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Garfinkel M. D., Pruitt R. E., Meyerowitz E. M. DNA sequences, gene regulation and modular protein evolution in the Drosophila 68C glue gene cluster. J Mol Biol. 1983 Aug 25;168(4):765–789. doi: 10.1016/s0022-2836(83)80074-6. [DOI] [PubMed] [Google Scholar]
- Gotoh O. An improved algorithm for matching biological sequences. J Mol Biol. 1982 Dec 15;162(3):705–708. doi: 10.1016/0022-2836(82)90398-9. [DOI] [PubMed] [Google Scholar]
- Hunt J. A., Hall T. J., Britten R. J. Evolutionary distances in Hawaiian Drosophila measured by DNA reassociation. J Mol Evol. 1981;17(6):361–367. doi: 10.1007/BF01734358. [DOI] [PubMed] [Google Scholar]
- Laird C. D., McCarthy B. J. Magnitude of interspecific nucleotide sequence variability in Drosophila. Genetics. 1968 Oct;60(2):303–322. doi: 10.1093/genetics/60.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Hogness D. S. Molecular organization of a Drosophila puff site that responds to ecdysone. Cell. 1982 Jan;28(1):165–176. doi: 10.1016/0092-8674(82)90386-5. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Martin C. H. Adjacent chromosomal regions can evolve at very different rates: evolution of the Drosophila 68C glue gene cluster. J Mol Evol. 1984;20(3-4):251–264. doi: 10.1007/BF02104731. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze D. H., Lee C. S. DNA sequence comparison among closely related Drosophila species in the mulleri complex. Genetics. 1986 Jun;113(2):287–303. doi: 10.1093/genetics/113.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. C., Kobori J. A., Siu G., Hood L. E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986 Apr;154(1):353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- Zwiebel L. J., Cohn V. H., Wright D. R., Moore G. P. Evolution of single-copy DNA and the ADH gene in seven drosophilids. J Mol Evol. 1982;19(1):62–71. doi: 10.1007/BF02100224. [DOI] [PubMed] [Google Scholar]



